Abstract
Calcium carbonate (CaCO3), which exhibits excellent biocompatibility and bioactivity, is a well-established bone filling material for bone defects. Here, we synthesized CaCO3 microspheres (CMs) to use as an intelligent carrier to load bone morphogenetic protein-2 (BMP-2). Subsequently, drug-loaded CMs and catalase (CAT) were added to methacrylated gelatin (GelMA) hydrogels to prepare a composite hydrogel for differential release of the drugs. CAT inside hydrogels was released with a fast rate to eliminate H2O2 and generate oxygen. Constant BMP-2 release from CMs induced rapid osteogenesis. Results in vitro indicated that the composite hydrogels efficiently reduced the level of intracellular reactive oxygen species, preventing cells from being injured by oxidative stress, promoting cell survival and proliferation, and enhancing osteogenesis. Furthermore, animal experiments demonstrated that the composite hydrogels were able to inhibit the inflammatory response, regulate macrophage polarization, and facilitate the healing of bone defects. These findings indicate that a multi-pronged strategy is greatly expected to promote the bone healing by modulating pathological microenvironments.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The number of patients suffering from bone fractures or defects is continuously increasing, often caused by trauma, tumors, congenital defects, and bone-associated diseases [1]. Bone fractures and defects result in disability worldwide and leave many patients in pain and discomfort [2]. At present, the major strategies for bone repair are achieved through autografts, allografts, and bone tissue engineering. The source of autografts limits their application; allografts are subject to immune rejection, which limits their future use [3]; and tissue engineering offers an alternative treatment and is promising to repair bone defects. Therefore, bone repair materials have attracted increased attention recently, with studies investigating their availability, consistency, and the need for subsequent operations [4].
Among synthetic biomaterials, calcium carbonate (CaCO3) has shown great potential as a bone filling material and has been widely used for bone defect repairs [5–7], due to its favorable biocompatibility, osteoconductivity, and bioresorbability [8–10]. Moreover, CaCO3 is an important calcium supplement and is administered in the treatment and prevention of fractures [11, 12]. There is an abundance of pore structure in porous CaCO3 microspheres (CMs), whose morphology is spherical with a specific surface area, leading to intensive use of CMs for drug delivery systems [13–15]. CaCO3 as a drug carrier has some merits, such as ease of synthesis and preparation processes, sensitivity to pH, as well as being friendly to the environment and non‐toxic to humans [16]. pH sensitivity could be favorable for the release of drugs because of the weak acid environment of bone injury. Therefore, CaCO3 is an ideal drug carrier for bone repair.
Previous research has demonstrated that bone healing is a complex physiological process consisting of a carefully arranged series of bone-induced and conducted biological events, involving numerous cell types and extracellular and intracellular molecular signaling, with a well-defined temporal and spatial sequence to optimize bone repair and restore bone function [17–19]. These include periods of acute inflammation, recruitment of bone marrow mesenchymal stem cells (BMSCs), and primary chondrocallus formation, angiogenesis, and bone remodeling [20]. Prolonged and inadequate inflammatory response often leads to impaired and delayed bone regeneration. In this stage, high reactive oxygen species (ROS) levels are a leading cause to impede bone healing [21]. ROS is one of the major microenvironmental factors in the tissue pathophysiological microenvironment, primarily including hydroxyl radicals (•HO), superoxide anions (O2−), and hydrogen peroxide (H2O2) [22, 23]. This has the capacity to cause oxidative stress and inhibit cell activity [24]. Besides, excess ROS has ability to activate NFκB and p38 MAPK signaling pathways to promoting M1 macrophage activation and up-regulate the expression of pro-inflammatory chemokines/cytokines, thereby aggravating the inflammatory response [25, 26]. These activities could induce osteoclastogenesis; oppositely, the progression of osteoblastogenesis is inhibited [27, 28]. To date, a variety of strategies targeting ROS have been proposed to modulate the pathological microenvironment for achieving effectively tissue repair. For example, A type of ROS-scavenging hydrogel designed by Zhao et al could promote healing of bacteria infected diabetic wounds owing to its powerful ROS-scavenging function [29]. Kumar et al applied MnO2 nanoparticles (NPs) for cartilage protection from oxidative stress damage [30]. Importantly, chitosan-catechol conjugates applied to titanium implants might attenuate cell damage induced by ROS, and promote bone formation [31]. A multifunctional composite hydrogel was developed by Chen et al to rescue ROS-induced pathological microenvironment and facilitate osteogenesis of osteoporotic bone defects [32]. Hence, removal of ROS at the site of bone defects may promote bone healing. Furthermore, hypoxia is also a significant microenvironmental factor that restrains bone formation [33]. What's more, oxygen tension is significantly reduced at the injured site by reason of the damage of the bones and blood vessels, which further increase acidity and causes cell death and defers bone healing [34, 35]. In contrast, a normal pH value and oxygen level is indispensable for cells to perform aerobic metabolism and physiological function. Therefore, the enhancement of oxygen supply to improve the hypoxic condition holds great promise for stimulating bone repair.
Given that ROS scavenging and oxygen release at bone defect sites could function synergistically to facilitate bone formation, catalase (CAT) may have unique advantages in ameliorating the microenvironment because it can rapidly induce the decomposition of H2O2 to produce oxygen, relieving oxidative stress along with hypoxia resolution. CAT is a remarkable antioxidant enzyme and has been recognized as a potential treatment for oxidative stress and inflammation caused by diseases such as cancer [36] and inflammatory bowel disease [37]. A little is known, however, about CAT's role in regulating the microenvironment of bone injury.
It is important to use injectable hydrogels because they can be injected in the target location, accompanied by cells or drugs, without the need to undergo surgery [38]. Hydrogels with physical structures and chemical compositions that have ability to promote cell proliferation and differentiation are highly attractive in bone regeneration [39, 40]. In addition, hydrogels have a hydrophilic surface, which is conducive to more cell attachment [41], and connected porous network structure suitable for drug delivery and substance exchange [42]. In the near future, therefore, there's no doubt hydrogels will act as a powerful tool for the clinical treatment of bone defects. Currently, methacrylated gelatin (GelMA) hydrogel is generally applied to tissue engineering because of its remarkable biocompatibility and injectability.
In this work, we aimed to improve the microenvironment at the bone injury site and effectively facilitate bone formation. We prepared porous CMs and mixed with GelMA hydrogels to form a composite drug delivery system. Bone morphogenetic protein-2 (BMP-2), a bioactive protein that stimulates osteogenesis, and CAT were encapsulated in the CMs and hydrogels, respectively, to achieve differential delivery of the two drugs. This drug platform could result in rapid CAT release for improving the pathophysiological microenvironment, including oxidative stress and hypoxia, while controlled BMP-2 release enhances bone regeneration. We have revealed that composite hydrogels significantly promote cell proliferation and reduced cell senescence and oxidative stress injury. What's more, the released CAT and BMP-2 were found to be beneficial for cell survival and osteogenic differentiation, and efficiently promoted bone repair (scheme 1). In general, our work presents a convenient approach toward bone regeneration by regulating the injury microenvironment.
Scheme 1. Schematic illustration of preparation of a composite hydrogel containing CAT and BMP-2-loaded CMs and its implantation in a rat calvarial defect model. Abbreviation: FR, Fast release; CR, Constant release.
Download figure:
Standard image High-resolution image2. Methods and materials
2.1. Synthesis of CMs
The CMs were prepared as previously described [32]. Briefly, a solution of 0.33 M anhydrous sodium carbonate (Na2CO3, SCRC, Beijing, China) was added rapidly into a solution of 0.33 M calcium chloride (CaCl2, SCRC, Beijing, China) with an equal volume under stirring for 3 min at room temperature. Afterwards, the CMs were formed and centrifuged at 6 000 rpm for collection. The obtained CMs were subsequently washed with deionized water and ethanol before drying in air.
2.2. Preparation of CMs-embedded hydrogels
CM-Gel was fabricated by adding 5 mg CMs into 1 ml 5% w/v GelMA solution with 0.25% w/v photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), and then exposed to a blue light source with a wavelength of 405 nm for 30 s.
2.3. Scanning electron microscopy observation and elemental analysis
The surface morphology of CMs and CM-Gel was examined using a scanning electron microscopy (SEM, Quanta 250, FEI, Hillsboro, OR) at an accelerating voltage of 30 kV. Elemental analysis was carried out using energy dispersive spectrometer (EDS).
2.4. Property of drug loading of CMs
To assess the property of drug loading of CMs, bovine serum albumin (BSA) and FITC-BSA (Solarbio, Beijing, China) were loaded into CMs, respectively. After encapsulation, images were observed under a fluorescence microscope (Carl Zeiss Inc, Thornwood, NY).
2.5. Ca2+ release of from CM-Gel in vitro
The CM-Gel was immersed in 2 ml of PBS with different pH values (6.4 and 7.2) to investigate the release of Ca2+ from the hydrogels. After supernatant collected at predetermined intervals, an equal amount of fresh PBS is added to the sample for continuing the release process. A calcium colorimetric assay kit (Beyotime, Shanghai, China) was used to test Ca2+ concentration in collected solutions.
2.6. Encapsulation of BMP-2 and CAT into CMs and hydrogels
For CAT and BMP-2 loading, 5 mg of CMs were added to 1 ml of PBS (pH 7.4) containing BMP-2 (15 μg, R&D Systems, Minneapolis, MN, USA). After stirred for 24 h, the samples were centrifuged, and the microspheres were collected. The obtained microspheres and CAT (1 mg; Sigma-Aldrich, St. Louis, MO, USA) were added to the 5% Gel-MA solution to prepare the composite hydrogels which were grouped into CMs-embedded hydrogels (CM-Gel), single loading of CAT (C/CM-Gel), single loading of BMP-2 (BCM-Gel) and double loading of CAT and BMP-2 (C/BCM-Gel), respectively.
2.7. In vitro drug release
The BSA-loaded CMs (5 mg) and BSA (1 mg) were added into 1 ml of GelMA solution, respectively, to prepare composite hydrogels after blue light crosslinking. Using a bicinchoninic acid (BCA) protein assay kit (Beyotime, Shanghai, China), the release of BSA from composite hydrogels was quantified at predetermined intervals in 2 ml of PBS solution with different pH values (6.4 and 7.2).
2.8. Cytoskeleton staining
After fixed in 4% paraformaldehyde and permeabilized with 0.3% Triton X-100 in PBS, rat BMSCs were stained with TRITC-phalloidin solution (Yeasen, Shanghai, China) at room temperature for 30 min, and then the nuclei were counterstained with DAPI solution (Beyotime, Shanghai, China) for 10 min. Images were observed via a fluorescence microscope.
2.9. Cell viability measurement
BMSCs (3 × 103 cells per well) were seeded and cultured on different hydrogels in a 96-well plate. The pure GelMA hydrogel (Gel) was used as a control group (Ctrl group). Cell viability was assessed using a CCK-8 assay at one, three, and five days.
2.10. EdU assay
A BeyoClick™ EdU cell proliferation kit (Beyotime, Shanghai, China) was used to assess cell proliferation. Briefly, rat BMSCs (3 × 104 cells per well) were seeded and cultured on the surface of different hydrogels in a 24-well plate. After 24 h, immediately before cell treatment with H2O2, EdU solution was added into medium in order to label cells. After 6 h incubation, fixation was performed with 4% paraformaldehyde, followed by permeabilization with 0.3% Triton X-100 in PBS. Then, a click reaction solution was applied to the cells at room temperature for 30 min, and Hoechst 33 342 dye was used to counterstain nuclei.
2.11. Intracellular ROS assay
For measuring accumulated intracellular ROS, the rat BMSCs were seeded on different hydrogels in the presence of 100 μM H2O2. After 24 h, cells were incubated with DCFH-DA solution (10 μM, Beyotime, Shanghai, China) at 37 °C for 1 h. Afterwards, the cells were observed by a fluorescence microscope.
2.12. Mitochondrial membrane potential assay
To detect mitochondrial activity, Mito-Tracker red staining was performed. After 24 h of rat BMSCs seeded and cultured on different hydrogels in the presence of 100 μM H2O2, Mito-Tracker Red CMXRos working solution (200 nM, Beyotime, Shanghai, China) was added into each well and the samples were incubated at 37 °C for 30 min. Then, the working solution was removed and the cells were observed by a fluorescence microscope.
2.13. Senescence-associated β-galactosidase (SA-β-Gal) staining
BMSCs (3 × 103 cells per well) were seeded and cultured on different hydrogels in a 24-well plate. The attached cells were treated with 100 µm H2O2 for three days, followed by fixation with 4% paraformaldehyde, and then stained with β-galactosidase staining solution (Beyotime, Shanghai, China) overnight under a dry incubator at 37 °C without CO2. The cells were observed by a light microscope.
2.14. ALP staining and ALP activity assay
BMSCs were seeded and cultured on different hydrogels in an osteogenic induction medium supplemented with 100 μM H2O2 for 7 d. Cells were subsequently fixed with 4% paraformaldehyde and stained with a solution of ALP staining (Beyotime, Shanghai, China). Images were acquired by a light microscope. An ALP assay kit (Beyotime, Shanghai, China) was used to test ALP activity according to manufacturer's instructions.
2.15. Alizarin red staining
BMSCs were seeded and cultured on different hydrogels in an osteogenic induction medium supplemented with 100 μM H2O2 for 14 d. Cells were subsequently fixed with 4% paraformaldehyde and stained with a solution of alizarin red (Beyotime, Shanghai, China). The images were acquired by a microscope.
2.16. Transwell migration assay
Different hydrogels were placed in the lower chamber of 24-well transwell plates with 8 μm pore size, and BMSCs were seeded on the top chamber. After co-cultured for 12 h in the presence of 100 μM H2O2, cells were fixed and stained with a crystal violet staining solution. Migrated cell number was counted by ImageJ software.
2.17. Quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted from rat BMSCs using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA). After RNA concentration measured, 5X All-In-One RT MasterMix (abm, Vancouver, Canada) was used to reverse transcribe RNA into cDNA according to the manufacturer's protocol. Then, qPCR was performed to compare the gene changes of different treatments. The primer sequences of the genes (Sangon Biotech, Shanghai, China) are listed in table 1. In order to determine the relative changes in mRNA level, each gene expression was normalized by GAPDH and calculated using the 2−ΔΔCT method.
Table 1. Primers for qPCR.
| Species | Gene | Forward(5ʹ-3ʹ) | Reverse(5ʹ-3ʹ) |
|---|---|---|---|
| Rat | OPN | GCGGTTCACTTTGAGGACAC | TATGAGGCGGGGATAGTCTTT |
| Rat | Runx2 | ATCCAGCCACCTTCACTTACACC | GGGACCATTGGGAACTGATAG |
| Rat | Col-I | CAGGCTGGTGTGATGGGATT | CCAAGGTCTCCAGGAACACC |
| Rat | OCN | AACGGTGGTGCCATAGATGC | AGGACCCTCTCTCTGCTCAC |
| Rat | GAPDH | GGTTGTCTCCTGCGACTTCA | TGGTCCAGGGTTTCTTACTCC |
| Mouse | TNF-α | CCACCACGCTCTTCTGTCTACTG | TGGTTTGTGAGTGTGAGGGTCTG |
| Mouse | IL-1β | CTCGCAGCAGCACATCAACAAG | CCACGGGAAAGACACAGGTAGC |
| Mouse | IL-6 | TCACCAGCATCAGTCCCAAGAAG | CCACCACGCTCTTCTGTCTACTG |
| Mouse | IL-8 | AGACGGACATGGCTGCTCAAG | GTCACAGGGACGGACGAAGATG |
| Mouse | GAPDH | GGTTGTCTCCTGCGACTTCA | TGGTCCAGGGTTTCTTACTCC |
2.18. Immunofluorescent staining
After fixation and permeabilization, commercial blocking buffer (Beyotime, Shanghai, China) was applied to prevent non-specific binding and then primary antibodies were incubated at 4 °C overnight (rabbit anti-Col-I, rabbit anti-OCN, rat anti-F4/80, rabbit anti-CD80, rabbit anti-CD206, Abcam, Cambridge, UK). Cells were subsequently treated with second antibodies (Abcam, Cambridge, UK) at room temperature for 1 h followed by nuclei counterstained with DAPI (Beyotime, Shanghai, China). Images were acquired using a fluorescence microscope. Using the ImageJ software (NIH, Bethesda, MD, USA), a semi-quantitative fluorescence analysis was carried out.
2.19. Animal study
We followed the NIH Guide for the Care and Use of Laboratory Animals and obtained approval from the Institutional Animal Care and Use Committee of Soochow University (SUDA20220830A01). After skull defects in SD rats established, different hydrogels were implanted into the defects to evaluate their effects on bone formation in vivo.
Two weeks after implantation, half of the cranial tissues were fixed in 10% formalin for 24 h and decalcified in 10% EDTA in PBS for 4 weeks. Following that, harvested samples were embedded in optimum cutting temperature compound (OCT, SAKURA, Japan), and horizontally cut into 10 μm thick sections, which were stained with CD80, CD206 and Runx2 (Abcam, Cambridge, UK). The secondary antibodies were used with Cy3-labeled goat anti-rabbit for CD80 and Runx2, and Alexa Fluor 488-labeled goat anti-rabbit for CD206. Tissue sections were then stained with DAPI mounting solution. Images were acquired with a fluorescent field microscope and quantitative analysis was done with the aid of Image J (NIH, Bethesda, MD).
Explanted bone grafts from the remaining half of the cranial tissues were placed in 200 μl lysis buffer (Sangon Biotech, Shanghai, China) without phosphatase inhibitors and homogenized using TissueLyser II (QIAGENE). After that, a centrifuge was used for 15 min at 12 000 g to collect the supernatant from the samples. Half of the supernatant was used to measure iNOS and IL-8 level by ELISA kits (NCM Biotech, Suzhou, China). The other half of the supernatant was used to measure ALP activity using the ALP assay kit (Beyotime, Shanghai, China).
At four and eight weeks after surgery, rat skulls were collected for micro-CT scanning and H&E staining.
2.20. Statistical analysis
The data are presented as mean ± standard deviation (n ⩾ 3). A one-way analysis of variance (GraphPad Software, San Diego, CA, USA) was performed along with Tukey's multiple comparison test to assess statistical differences between groups. P values < 0.05 were considered statistically significant.
3. Results
3.1. Synthesis and characterizations of CMs and CM-Gel
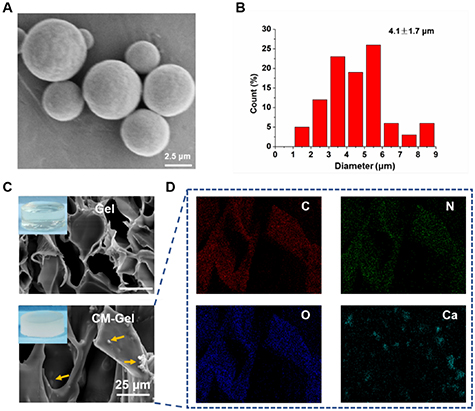
The CMs were successfully synthesized by mixing Ca2+ and CO3 2− ions together rapidly and then used as a carrier for drug delivery. The spherical morphology and some pores could be observed in the SEM images of the CMs (figure 1(A)). The average diameter of the product was 4.1 ± 1.7 μm (figure 1(B)). Subsequently, CMs were added into the Gel-MA solution to obtain composite hydrogels (CM-Gel) with 0.5% (w/v) CMs. The SEM images of hydrogels showed that the CMs were distributed randomly in the hydrogels (figure 1(C)). The EDS mapping proved the presence of C, N, O, and Ca elements in the CM-Gel (figure 1(D)).
Figure 1. Synthesis and characterization of CMs and CM-Gel. (A) SEM images of CMs. (B) Size distributions of CMs. (C) SEM images of Gel and CM-Gel. (D) EDS mapping of CM-Gel.
Download figure:
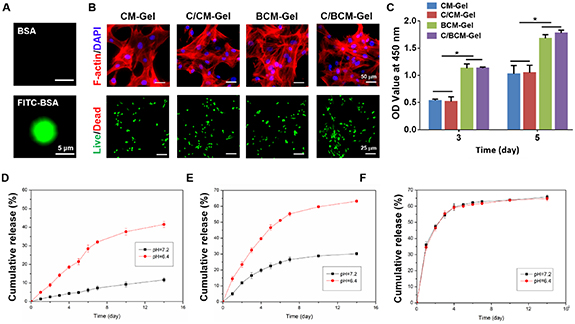
Standard image High-resolution imageNext, FITC-BSA was loaded into the CMs and a high fluorescent signal was observed, indicating the great drug loading properties of the CMs (figure 2(A)). Furthermore, results of cytoskeletal staining showed that the cells adhered and spread well on the surface of the composite hydrogels, indicating that the composite hydrogels had excellent biocompatibility. Consistent with the results of cytoskeletal staining, live/dead staining found few dead cells (figure 2(B)). Subsequently, a CCK-8 assay showed that CAT supplementation could not further promote cell activity while the addition of BMP-2 could increase the cell viability compared with the CM-Gel and C/CM-Gel groups (figure 2(C)). What's more, in vitro Ca2+ and the drug release characteristics of the hydrogel were investigated. The cumulative release curve showed that Ca2+ from the CM-Gel could be sustainably delivered for at least 14 d, and the release rate of Ca2+ was higher in the acidic medium compared with the neutral medium (figure 2(D)). Compared with BSA, which was released directly from GelMA hydrogels (BSA-Gel) for 4 d to 60%, the system of BSA-loaded CMs mixed with GelMA hydrogels (BSA/CM-Gel) can be released BSA for 14 d. Moreover, BSA/CM-Gel rather than BSA-Gel was able to accelerate the release of BSA in the acidic medium (figures 2(E) and (F)).
Figure 2. Drug behavior and biocompatibility of composite hydrogels. (A) Representative images of FITC-BSA-loaded CMs. (B) Representative images of F-actin staining and live/dead staining of BMSCs in the CM-Gel, C/CM-Gel, BCM-Gel and C/BCM-Gel groups, respectively. (C) Relative viabilities of BMSCs in the CM-Gel, C/CM-Gel, BCM-Gel and C/BCM-Gel groups, respectively, at three and five days. (D) In vitro release of Ca2+ from CM-Gel under different pH values (7.4 and 6.4). (E), (F) The cumulative release curve of BSA from BSA/CM-Gel and BSA-Gel, respectively. *p < 0.05.
Download figure:
Standard image High-resolution image3.2. Protection of BMSCs from oxidative damage by C/BCM-gel
To evaluate the protective capacity of the C/BCM-Gel on BMSCs with H2O2 treatment, live/dead staining and EdU staining were carried out. The results of live/dead staining found that increased the numbers of dead cells (red) in the CM-Gel group. Treatment with C/BCM-Gel, rather than C/CM-Gel and BCM-Gel groups, could importantly suppress cell death induced by H2O2 (figure 3(A)), with C/BCM-Gel treatment contributing to the greatest level in the percentage of live cells (figure 3(B)). The EdU assay further showed that the BMSCs seeded on the C/BCM-Gel exhibited cell proliferation well under oxidative condition, as indicated by the highest percentage of EdU-positive cells (figure 3(C)). The cell proliferation rate was low in the CM-Gel group and this number was increased in the C/CM-Gel and BCM-Gel groups (figure 3(D)).
Figure 3. Protective effect of C/BCM-Gel on BMSCs in the presence of H2O2. (A) Representative images of live/dead staining of BMSCs in the CM-Gel, C/CM-Gel, BCM-Gel and C/BCM-Gel groups, respectively. (B) Quantification of the percentage of live cells in all groups. (C) Representative images of EdU staining of the CM-Gel, C/CM-Gel, BCM-Gel and C/BCM-Gel groups, respectively. Green represents the EdU-labeled proliferating cells and blue represents the nuclei. (D) Quantification of the percentage of EdU-positive cells in all groups. *p < 0.05.
Download figure:
Standard image High-resolution image3.3. Antioxidant properties of C/BCM-gel
The antioxidant activity of the C/BCM-Gel was assessed by investigating the ROS-scavenging activity, and the DCF fluorescence intensity was detected using an ROS assay kit. The ROS content decreased after C/CM-Gel and C/BCM-Gel treatment, whereas treatment with CM-Gel and BCM-Gel had high ROS level (figures 4(A) and (B)). Indeed, mitochondria are a potential source of ROS, and overproduction of ROS could lead to mitochondrial dysfunction which, in turn, deteriorates oxidative stress. Mito-Tracker Red CMXRos assays were conducted to investigate mitochondrial function. As a result, cells cultured on the C/CM-Gel and C/BCM-Gel had higher fluorescence intensity than those seeded on the CM-Gel and BCM-Gel in the present of H2O2 (figures 4(C) and (D)). Results from the ATP test found that the ATP levels were significantly increased in cells treated with C/CM-Gel and C/BCM-Gel compared to cells treated with CM-Gel and BCM-Gel (figure 4(E)). In addition, SA-β-Gal staining indicated that adding H2O2 to the culture medium for three days could induce more cell senescence in the groups of CM-Gel and BCM-Gel. However, the cells in the C/CM-Gel and C/BCM-Gel groups exhibited lower SA-β-Gal activity (figures 4(F) and (G)).
Figure 4. Antioxidant activity of C/BCM-Gel. (A) Representative images of BMSCs stained with DCFH-DA in the CM-Gel, C/CM-Gel, BCM-Gel and C/BCM-Gel groups in the presence of 100 μM H2O2, respectively. (B) Quantification of fluorescence intensity of DCFH-DA in all groups. (C) Representative images of BMSCs stained with Mito-Tracker in the CM-Gel, C/CM-Gel, BCM-Gel and C/BCM-Gel groups in the presence of 100 μM H2O2, respectively. (D) Quantification of fluorescence intensity of Mito-Tracker in all groups. (E) Evaluation of ATP level of BMSCs in the CM-Gel, C/CM-Gel, BCM-Gel and C/BCM-Gel groups in the presence of 100 μM H2O2, respectively. (F) Representative images of BMSCs stained with SA-β-Gal in the CM-Gel, C/CM-Gel, BCM-Gel and C/BCM-Gel groups in the presence of 100 μM H2O2, respectively. (G) Quantification of the percentage of SA-β-Gal-positive cells in all groups. *p < 0.05.
Download figure:
Standard image High-resolution image3.4. Osteogenesis of BMSCs cultured on composite hydrogels in vitro
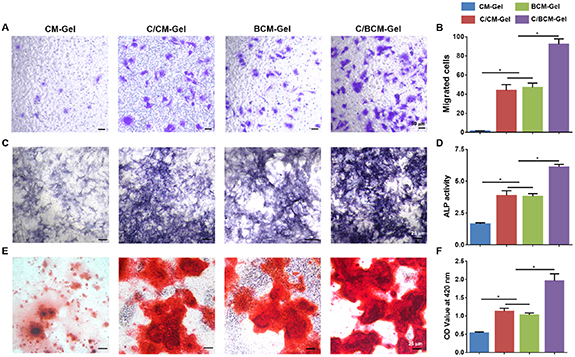
The migration of BMSCs to the site of bone defect is an important part of osteogenic process. The results of cell migration assay implied that the number of migrated cells was increased in the C/CM-Gel, BCM-Gel and C/BCM-Gel group compared with CM-Gel group. Importantly, migration activity of cells treatment with C/BCM-Gel was dramatically induced among all groups (figures 5(A) and (B)). Moreover, after BMSCs cultured on composite hydrogels in the osteogenic induction medium with 100 μM H2O2, ALP staining and ALP activity were performed to determine ALP production, and alizarin red staining was taken to observe calcium deposition. The results showed that ALP production was dramatically enhanced in the group treatment with C/BCM-Gel (figures 5(C) and (D)), and a similar trend was observed in the alizarin red staining analysis (figures 5(E) and (F)). In addition, qPCR analysis was performed to examine the expression of osteogenic genes, such as osteopontin (OPN), runt-related transcription factor 2 (Runx2), collagen type I (Col I) and osteocalcin (OCN). The C/BCM-Gel group significantly promotes the expression level of these osteogenic genes (figures 6(A)–(D)). Consistent with the qPCR results, immunofluorescence further showed that the expression of osteogenic related protein Col-I and OCN was higher in the C/BCM-Gel group compared with the other groups (figures 6(E)–(H)).
Figure 5. Migration activity and osteogenesis of BMSCs cultured with composite hydrogels in vitro. (A) Migration activity of BMSCs treatment with composite hydrogels in the present of 100 μM H2O2. (B) Quantitative analysis of migrated cells. ALP staining (C) and ALP activity (D) of BMSCs treatment with composite hydrogels in the present of 100 μM H2O2 after osteogenic induction for 7 d. (E) Alizarin red staining of the BMSCs treatment with composite hydrogels in the presence of 100 μM H2O2 after osteogenic induction for 14 d. (F) Quantitative analysis of alizarin red staining. *p < 0.05.
Download figure:
Standard image High-resolution imageFigure 6. Expression of osteogenesis-related genes and proteins in BMSCs cultured with composite hydrogels. (A)–(D) Relative gene expression of Runx2, OPN, OCN, and Col I of BMSCs in the CM-Gel, C/CM-Gel, BCM-Gel, and CBCM-Gel groups, respectively, in the presence of 100 μM H2O2. GAPDH was used as the housekeeping gene. (E) Immunofluorescence staining of Col I in CM-Gel, C/CM-Gel, BCM-gel, and C/BCM-gel groups, respectively, in the presence of 100 μM H2O2. (F) Quantification of the positive signal intensity of Col I. (G) Immunofluorescence staining of OCN in CM-Gel, C/CM-Gel, BCM-gel, and C/BCM-gel groups, respectively, in the presence of 100 μM H2O2. (H) Quantification of the positive signal intensity of OCN. *p < 0.05.
Download figure:
Standard image High-resolution image3.5. Effects of composite hydrogels on macrophages in vivo and in vitro
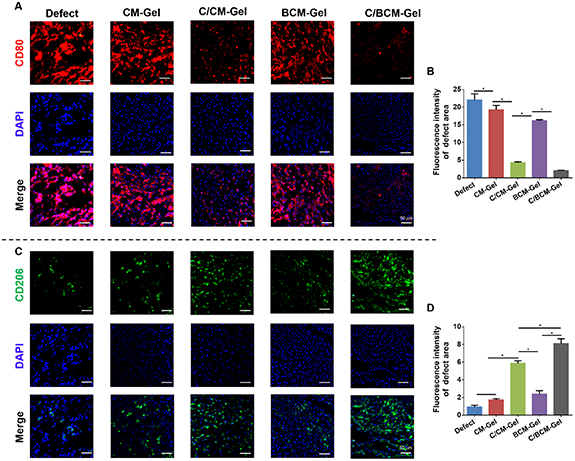
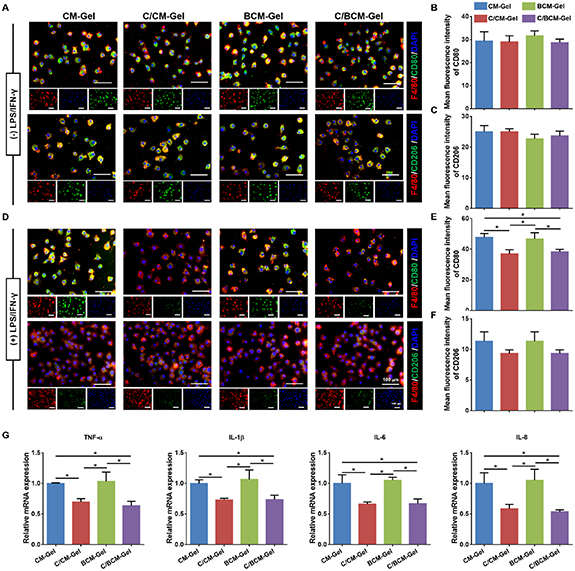
Critical-size defects on calvarial bone defects were created in rats to investigate the osteoimmunomodulation and osteogenesis of the composite hydrogels. There was a significantly higher expression of CD80 (M1 macrophage) and a lower expression of CD206 (M2 macrophage) in the defect and CM-Gel groups at two weeks after implantation. The BCM-Gel group showed a slight difference in the expression of the two different macrophage markers, compared with the defect group. CD80 expression was inhibited in the C/CM-Gel and C/BCM-Gel groups, while CD206 was promoted (figure 7). Moreover, in vitro experiment found that RAW 264.7 cells, a type of mouse mononuclear macrophage, cultured on different composite hydrogels showed no difference on polarization direction (figures 8(A)–(C)). When LPS and IFN-γ was used to induce RAW 264.7 cells to differentiate into M1 type macrophages, interestingly, C/CM-Gel and C/BCM-Gel treatment could inhibit M1 macrophage activation compared with CM-Gel and BCM-Gel (figures 8(D)–(F)). qPCR analysis further showed the expression of pro-inflammatory (TNF-α, IL-1β, IL-6, and IL-8) induced by LPS and IFN-γ was blocked in the C/CM-Gel and C/BCM-Gel groups (figure 8(G)).
Figure 7. Effects of composite hydrogels on macrophage polarization in vivo at two weeks. The representative images of cross sections were stained with CD80 (A) with quantification of its expression level (B), and CD206 (C) with quantification of its expression level (D). *p < 0.05.
Download figure:
Standard image High-resolution imageFigure 8. Effects of composite hydrogels on macrophage polarization in vitro. (A) Immunofluorescent staining of CD80 and CD206 without LPS and IFN-γ treatment. (B), (C) Quantification of CD80 and CD206 expressions, respectively. (D) Immunofluorescent staining of CD80 and CD206 with LPS and IFN-γ treatment. (E), (F) Quantification of CD80 and CD206 expressions, respectively. (G) Relative gene expression of TNF-α, IL-1β, IL-6, and IL-8 of RAW 264.7 cells. GAPDH was used as the housekeeping gene. *p < 0.05.
Download figure:
Standard image High-resolution image3.6. Effects of composite hydrogels on early osteogenesis and cytokine secretion
Runx2, a marker for early osteogenesis, was investigated at two weeks after implantation. The bone grafts of the C/BCM-Gel group induced a significantly higher Runx2 expression (figures 9(A) and (B)), as well as ALP activity (figure 9(C)) compared with other groups. In addition, the results of the ELISA to assess proinflammatory cytokine secretion at the defect sites showed that the grafts of the C/CM-Gel and C/BCM-Gel groups triggered significantly lower production of iNOS and IL-8 at two weeks among all the groups.
Figure 9. Early osteogenesis and cytokine secretion by composite hydrogels at two weeks. (A) The representative images of cross sections were stained with Runx2 in the Defect, CM-Gel, CCM-Gel, BCM-Gel, and CBCM-Gel groups, respectively. (B) Quantification of fluorescence intensity of Runx2 in all groups. (C)–(E) The level of ALP, iNOS, and IL-8 in the defect sites after implantation. *p < 0.05.
Download figure:
Standard image High-resolution image3.7. Bone formation by composite hydrogels in vivo
To investigate the effects of composite hydrogels on bone formation, the entire skull was harvested for micro-CT examination at four and eight weeks. In the defect group, new bone was barely observed at four weeks post-surgery. A small amount of new bone tissue was found at the defects in the CM-Gel, C/CM-Gel, and BCM-Gel groups. In contrast, vast new bone formation was observed in the C/BCM-Gel group (figure 10(A)). The percent of neobone volume (BV/TV) demonstrated that new bone formation in the C/BCM-Gel group was highest at the fourth week post-surgery. After eight weeks, newly formed bone in the C/BCM-Gel group completely covered the defects, and the BV/TV value reached 70% (figure 10(B)). H&E staining further showed that new bone formation in the C/BCM-Gel group exceeded that observed in other groups (figure 10(C)).
Figure 10. Neobone formation detected at four and eight weeks after implantation using micro-CT and H&E staining. (A) Representative images of the 3D construction from micro-CT. (B) Quantitative analysis of BV/TV values. (C) Representative images of H&E staining of neobone tissue at four and eight weeks. *p < 0.05.
Download figure:
Standard image High-resolution image4. Discussion
In this study, we prepared porous CMs through a simple synthesis method and embedded them into GelMA hydrogel to fabricate multifunctional drug platform loaded with CAT and BMP-2 inside the hydrogel and CMs, respectively. The composite hydrogels were injected into the site of bone defect, and the fast release of CAT could decrease ROS levels and, especially, decompose H2O2 into H2O and O2 to improve oxidative stress damage and hypoxia. Furthermore, controlled BMP-2 release recruited stem cells and enhanced osteogenic differentiation in response to the slightly acidic environment of bone injury. In recent years, many biomaterials have been developed for bone repair, while the effective repair of bone defects is still a huge challenge for clinicians. Prolonged hospitalization times and the increase of economic cost caused by bone fracture place a great burden upon patients [43]. With the characteristics of the bone injury microenvironment being clarified, researchers have increasingly focused on developing biomaterials targeting the bone injury microenvironment to promote bone regeneration.
Previous studies have reported that the inflammatory stage is the first phase of bone injury, which mainly takes place in the initial 5 d. Apart from inflammation, low oxygen saturation, excessive ROS generation, and increased acidity also hindered bone healing during the early phage [44]. Basal ROS levels contribute to normal cell homeostasis and functions and the increase in ROS production breaks the balance between their number and antioxidant defenses, resulting in the occurrence of oxidative stress [45, 46]. Almeida et al demonstrated that high ROS levels impair osteoblast lifespan and ECM deposition, suggesting that oxidative stress could potentially be the main inductor of bone decay effect [47]. Therefore, rescuing the ROS microenvironment to promote bone repair may be a promising avenue to pursue. Zheng et al reported that lignin-carbohydrate complexes is a biomacromolecule that can effectively reduce ROS amount and activate cellular antioxidant activities, thereby enhancing osteoblast differentiation in an inflammatory environment [48]. Moreover, the inhibition of ROS is able to restrain osteoclastogenesis in vitro [49], and promote the healing of osteoporotic bone defects in vivo [50]. Therefore, early intervention strategies targeting oxidative stress microenvironment are of great importance for bone healing. CAT, an enzyme that can decompose H2O2 to generate O2, was used as an ROS scavenger in our study. A unique property of CAT is not only scavenging ROS, but also relieving hypoxia, both of which exist in the microenvironment of bone injury. We supplemented CAT in GelMA to avoid topical overdose, while CAT was released at a faster rate to remove excess ROS and protect cells from H2O2-induced damage, represented by lower cell senescence and death, and higher cell proliferation, mitochondrial membrane potential and ATP level.
Stem cell migration is initiated by the early inflammatory response, followed by osteogenic differentiation and bone formation. BMP signaling is involved in major procession of bone regeneration and mainly facilitate mesenchymal condensation in the later stage. To achieve it, hence, we decided to encapsulate BMP-2 into porous CMs for constant release. CaCO3 has been extensively used as a drug delivery system due to its excellent biocompatibility and degradability [51]. The pH-sensitive properties of CaCO3-based materials, along with its osteoinductive activity, can offer a novel possibility to control drug delivery and osteogenesis. The materials could decompose slowly in a physiological pH, and display a faster decomposition in the acidic pH of bone-injury environments. Our results showed that synthetic CMs had a spherical and porous structure, exhibited great drug loading efficiency with no obvious toxicity toward the BMSCs, and was able to precisely and controllably release the drug and Ca2+ in acidic environments. In vitro experiments further demonstrate that increased migrated cells, ALP production and calcium deposition of cells in the presence of H2O2 in the C/BCMs-Gel group among all groups. Moreover, expression of osteogenic genes and proteins was enhanced most markedly in the group of C/BCM-Gel compared to other groups. We hypothesized that the enhanced effects of the C/BCM-Gel may be related to the elimination of H2O2 by CAT, resulting in a better environment for osteogenesis induced by released BMP-2.
To further investigate the role of in vivo C/BCM-Gel, calvarial bone defects of 5.0 mm in diameter, considered critical-size defects, were established in rats [52]. Our in vivo results showed that the level of M1 macrophage-specific biomarker (CD80) significantly increased in the defect, CM-Gel, and BCM-Gel groups, along with low expression of CD206, a M2 macrophage-specific biomarker, compared with C/CM-Gel and C/BCM-Gel groups at two weeks after post-implantation. Consistent with in vivo results, the experiments of Raw 264.7 cells treated with composite hydrogels have also found that C/CM-Gel and C/BCM-Gel were able to inhibit macrophage differentiation to M1 type under the treatment of LPS and IFN-γ. Additionally, evaluation of the local concentration of inflammatory cytokines showed that the expression of iNOS and IL-8 was lower in the C/CM-Gel and C/BCM-Gel groups than other three groups, indicating that the addition of CAT may modulate local overactive oxidative stress and inflammation. Growing evidence suggests that the ROS is essential for M1 macrophage activation and local excessive ROS could induce persistent M1 macrophage activation [53, 54], leading to uncontrollable inflammation and undesirable healing. Therefore, the biomaterial scaffolds are capable of modulating the host immune response to provide an appropriate microenvironment for osteogenesis. This is vital in determining the quality and efficiency of endogenous bone regeneration. Macrophages, a major constituent of the innate immune system, act in a critical role in the material-induced immune response, and their flexibility allows them to shift to different phenotypes under specific microenvironments [55, 56]. Previous reports revealed that M2 macrophages could upregulate the expression of Runx2 and BMP-2 of MSCs, which in turn promotes osteogenesis [57, 58]. Indeed, the highest level of Runx2 and ALP was observed in the C/BCM-Gel group at two weeks after implantation. Consequently, neobone formation in the C/BCM-Gel group exceeded that observed in other groups, both at four and eight weeks.
5. Conclusions
Overall, we propose a precise CAT-mediated ROS removal strategy that contributes to a favorable condition for BMSC osteogenesis. Considering clinical application, BMP-2-loaded porous CMs were chosen to construct a drug carrier. CAT was encapsulated into GelMA for a fast initial release according to the sequential steps of bone fracture healing. Balanced inflammatory response was established and macrophage polarization was accurately modulated. Subsequently, controlled BMP-2 release accelerated the process of osteogenesis, mainly involving MSCs recruitment and differentiation in the later stage. These findings from this study imply that appropriate and timely modulation of bone healing process, such as early inflammatory stage and later osteogenic stage, was crucial to the healing of bone defects.
Data availability statement
The data that support the findings of this study are available upon request from the authors.
Authorship contribution statement
Yongming Sun and Kun Lu conceived and designed the study. Kun Lu, Dongliang Wang, Guoyou Zou, Ya Wu, Feng Li and Qunshan Song performed the experiments and data analysis. Kun Lu, Dongliang Wang, and Yongming Sun wrote the manuscript with input from all coauthors. All authors have read and approved the final version of the manuscript.
Conflict of interest
The authors declare that no competing interest exists with this study.