Abstract
Biocompatibility is one of the key issues for implants, especially in the case of stainless steel with medium to low biocompatibility, which may lead to a lack of osseointegration and consequently to implant failure or rejection. To precisely control preferential cell growth sites and, consequently, the biocompatibility of prosthetic devices, two types of surfaces were analyzed, containing periodic nanogrooves laser induced periodic surface structure (LIPSS) and square-shaped micropillars. For the fast and efficient production of these surfaces, the unique combination of high energy ultrashort pulsed laser system with multi-beam and beamshaping technology was applied, resulting in increased productivity by 526% for micropillars and 14 570% for LIPSS compared to single beam methods. In vitro analysis revealed that micro and nanostructured surfaces provide a better environment for cell attachment and proliferation compared to untreated ones, showing an increase of up to 496% in the number of cells compared to the reference. Moreover, the combination of LIPSS and micropillars resulted in a precise cell orientation along the periodic microgroove pattern. The combination of these results demonstrates the possibility of mass production of functionalized implants with control over cell organization and growth. Thus, reducing the risk of implant failure due to low biocompatibility.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Despite many polymers with excellent biocompatibility, metallic prosthetic parts are still essential in bio-applications sensitive to toughness and durability [1, 2]. The high reliability and mechanical performance of metallic prosthetic parts make them suitable for hard tissue replacements such as artificial hip joints or bone plates. The most common metallic materials are Ti-based alloys and stainless steel with applications in orthopedy, dentistry or dental implantology [3]. Compared to Ti-based alloys, stainless steel offers medium to low biocompatibility, which may lead to a lack of osseointegration and consequently to implant failure or rejection from the human body [4].
To overcome these issues steel surfaces can be functionalized in order to improve surface interaction with biological tissues. It has been shown that precisely fabricated topographic features at the micro and nanoscale levels can enhance the overall performance of implants and prostheses [5, 6]. These features can affect the interactions between cells and the surface to optimize the biocompatibility of implanted biomaterials through improved osseointegration [6].
Generally, the tissue biological response to the implant depends on the cell type, surface topography and chemistry [7]. Additionally, wettability modification has been proven to play an important role in bio-recognition and acceptance by the surrounding tissue [8]. In this regard, a large variety of fabrication methods have been developed to adjust the surface properties of biomaterials, including coating and deposition techniques [9], chemical etching [10], plasma treatments [11], or laser surface micro and nanostructuring [12, 13]. However, most of these techniques are not environmentally friendly due to the use of chemicals or too slow to be implemented in an industrial environment. Among these, laser micro- and nanostructuring is a non-contact method which does not contaminate the surface with additional materials or gasses [14]. Thus, it provides an environmentally friendly and flexible solution for precise fabrication of micro and nano geometries on a large variety of materials [15, 16].
Several groups reported improved biological response on laser structured surfaces. Kenar et al reported increased adhesion of endothelial cells and an increased bone formation rate on the laser nanostructured stainless steel plates [17]. Improved osseointegration was observed by Oberringer et al on stainless steel nanostructured by femtosecond laser [18]. Cunha et al found that laser induced periodic surface structures (LIPSSs) may improve human mesenchymal stem cells differentiation into an osteoblastic lineage [19].
However, in all these studies single beam approaches with productivity only up to a few cm2 min−1 have been utilized. To increase the productivity in laser micro and nanostructuring, multi-beam [20–22] or beamshaping technologies have been recently employed [23, 24]. Despite their advantages for large-scale production and potential industrial use, the combination of these technologies has never been used for the efficient functionalization improving biocompatibility of stainless steel.
Hence, the objective of this work is to study culturing capabilities of a stainless steel surface containing combinations of micro- and nanostructures made by fast and efficient production technologies, including a unique combination of multi-beam technology for microstructuring and dynamic beam shaping for rapid production of nanostructures. Thus, introducing an efficient way to enhance the biocompatibility of stainless steel prosthetics.
2. Materials and methods
Stainless steel plates AISI 316L were used for the purpose of this study due to its common use in biomedical field including prosthesis parts requiring good mechanical properties. All samples were cleaned in ethanol prior to laser processing and treated in open atmosphere by multifunctional multi-beam processing station utilizing ytterbium based diode pumped solid-state laser system Perla 100 (HiLASE, Czech Republic) emitting 1.7 ps pulses with M2 of 1.15 and wavelength of 1030 nm. The laser system generates pulses with up to 2 mJ pulse energy with a repetition rate of 50 kHz. To increase the efficiency during the microstructuring process the incident laser beam was guided through the diffractive optical element (DOE) splitting the input beam into the line of 6 beams. Similarly, nanostructuring efficiency was increased by the dynamic beam shaping unit FBS G3 (Pulsar Photonics GmbH, Germany) equipped with a spatial light modulator (SLM, Hamamatsu Photonics, Japan) which is able to shape the beam according to the pre-calculated computer-generated hologram (phase-mask) uploaded to the SLM (see figure 1). The output beam of DOE and SLM was guided into the galvanometric scanner IntelliScan 14 (Scanlab, Germany) and focused on the sample by 100 mm telecentric F-theta lens. The beam could be displaced on a sample with the maximum scanning speed of 2 m s−1.
Figure 1. Schematics of the beamshaping setup with insets of input beam, computer generated hologram (CGH) and final beam at the image plane.
Download figure:
Standard image High-resolution imageFor the cell culture cultivation materials were placed in a 24-well plate and sterilized using low-temperature ethylene oxide (Anprolene, USA). After a week of airing at room temperature, the materials were seeded with mouse fibroblasts of the 3T3 cell line (ATCC, USA; passage 12, viability 89%) at a concentration of 7 · 103 cells per well (the cell concentration was chosen based on the preliminary experiment—data not shown). Cell activity was subsequently monitored together with adhesion and proliferation using the following methods: metabolic Cell Counting Kit-8 (CCK-8) test; scanning electron microscopy (SEM); fluorescence microscopy.
The surface morphology, roughness and z-profile was investigated by laser scanning confocal microscope, Olympus OLS5000 and scanning electron microscope Tescan MIRA at electron energy of 15 keV. The static contact angle was analyzed for a water droplet volume of 10 µl utilizing contact angle measuring device OCA 15EC (Data Physics Instruments, Germany). The test results were acquired through an average of 5 measurements on different locations for every textured surface.
The visualization of the cells was carried out using the fluorescence microscope Nikon Eclipse-Ti-E (Nikon Imaging, CZE). The structure of the fixed cells (with 2.5% glutaraldehyde in phosphate-buffered saline) was evaluated via the scanning electron microscope. The fixed cells were dehydrated via increasing ethanol concentration series (60%, 70%, 80%, 90%, 99%, 100% ethanol). The samples were sputter coated with 7 nm of gold by using QUORUM Q50ES (Quorum Technologies, GB) and subsequently assessed via scanning electron microscopy TESCAN VEGA 3 (SB Easy Probe, CZE).
3. Results and discussion
Aiming to study cell behavior on different surface morphologies, optimal laser and processing parameters had to be determined for micro and nanoprocessing of stainless steel surface. For the production of microstructures, fluence of 3 J cm−2 was used to produce square-shaped micropillars by deflecting the laser beam in a rectangular grid with hatch distance of 60 µm (see figure 2), which is comparable to the NIH/3T3 fibroblast cell size of ∼50 µm. As depicted in figure 2(c) the micropillar height reached ∼8 µm, increasing the areal average roughness Sa to 3.59 µm.
Figure 2. Analysis of square shaped microstructures by the 3D profile from confocal microscope (a), optical microscope image (b) and profilometry (c).
Download figure:
Standard image High-resolution imageThe productivity of microstructuring reached only 3.8 cm2 min−1 for a standard single beam process. To improve the process efficiency, DOE beamsplitter was placed before the galvanometric scanner to split the input beam into 6 sub-beams. The utilization of this setup resulted in increased productivity of 20 cm2 min−1 for the structures depicted in figure 2.
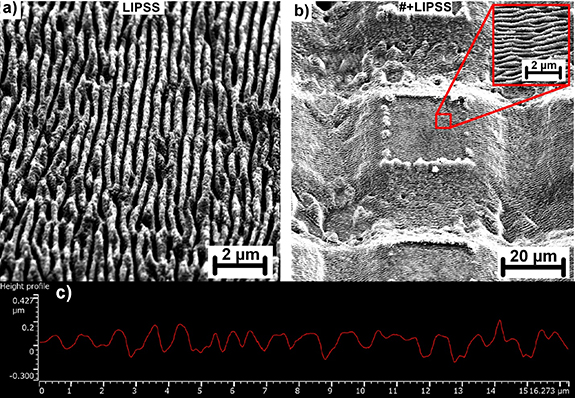
In the second step, the optimal processing window for production of periodic nanostructures was determined. The best quality nanostructures in a form of periodically arranged ridges were observed for the fluence of 0.7 J cm−2 (see figure 3). Similarly to the previous case, productivity of nanostructuring was significantly increased by utilization of advanced processing technique. By shaping the beam into the line with dimensions of 500 µm × 30 µm (see figure 1), and scanning perpendicular to the line axis, the nanostructuring productivity was improved from original 3 cm2 min−1 for the standard single beam approach to 102 cm2 min−1. The resulting nanostructures could be fabricated either on plane surface (figure 3(a)) or on the top of previously fabricated microstructures (figure 3(b)).
Figure 3. SEM analysis of nanostructures; (a) detail on nanostructures on a plane surface, (b) nanostructures made on the top of microstructures (#+LIPSS) with inset of higher magnification SEM picture, (c) profilometry of nanostructures.
Download figure:
Standard image High-resolution imageThe orientation of periodically arranged ridges was perpendicular to the beam polarization with the lateral spacing of ∼420 nm which is approximately half of the input beam wavelength. The areal average roughness Sa of nanostructured surface reached 0.05 µm. Considering the characteristic size and orientation of these structures and parameters of the laser source, these structures can be identified as high spatial frequency LIPSS [25, 26].
To study culturing capabilities of structured stainless steel surface, the cell metabolic activity was assessed using a colorimetric metabolic assay based on converting the tetrazolium salt into a yellow formazan soluble in the culture medium. Thus, it was not necessary to dissolve the formed crystals and the testing accuracy is increased. By using the spectrophotometer the absorbance of resulting solutions were analyzed after 3 h of contact with the CCK-8 solution (10% v/v) in medium at 37° and 5% CO2. The metabolic CCK-8 assessment of cell activity after 4, 7 and 14 d of cultivation is depicted in figure 4. The amount of formazan is directly proportional to the number of viable cells.
Figure 4. (a) The metabolic CCK-8 assessment of cell activity after 4, 7 and 14 d of cultivation with 3T3 mouse fibroblasts; n = 3 (Analysis of Variance, Bonferroni).
Download figure:
Standard image High-resolution imageBased on the results of in vitro experiment, the materials supported the cell proliferation, as the measured absorbance values increased within the testing days. The highest metabolic activity was detected during the last testing day (14D), see figure 4. The obtained data were statistically analyzed, the normality of the distribution was verified and parametric two-way Analysis of Variance (Bonferonni) test was performed resulting in no statistical differences between surfaces.
To visualize cells after 2, 7 and 14 d of cultivation in vitro, the double staining with phalloidin and DAPI (4',6-diamidino-2-phenylindole) were applied, allowing visualization of the cytoplasm (binding of phalloidin to actin fibers of the cytoskeleton—green color in figure 5) and nuclei (binding of DAPI to DNA—blue color in figure 5).
Figure 5. Fluorescence microscopy images (cytoskeleton—green; nuclei—blue) after 2, 7 and 14 d of cultivation in vitro. SEM image insets show the LIPSS orientation (d)–(f).
Download figure:
Standard image High-resolution imageThese results are consistent with the viability measurement. The cells have reached the full confluency on the last testing day and were homogenously spreaded over the material's surfaces without any preferential areas. However, during the gradual growth, the cells were following the patterns on microstructured surface leading to the formation of a grid-like structure (see figure 5(h)). In addition, a mild influence of the nanostructured surface on the cell growth direction was detected on the surface covered by LIPSS (see figures 5(e) and (f)). To support these findings SEM analysis were performed, as depicted in figure 6. During the first testing day only isolated cell clusters were observed, which were gradually growing into full confluency reached on 14D. Thus, material's surfaces were fully covered with cell colonies on the last testing day.
Figure 6. SEM images of NIH/3T3 cells on materials. A fully confluent layer is found on the materials during the 14-days testing the cells grew over the entire surface of the materials.
Download figure:
Standard image High-resolution imageIn the next step, the number of the cells on the materials surface was quantified by using MATLAB software (count from DAPI stained nuclei), the results are shown in figure 7.
Figure 7. Results of number of calculated cells on the surfaces per 1 mm2; n = 10 (Analysis of Variance, Bonferroni). *** p < 0.
Download figure:
Standard image High-resolution imageAs shown in figure 7, statistically significant difference was revealed in a number of calculated cells. The number of cells was increasing with the cultivation time. However, this increase was influenced by surface structures. This phenomenon is especially visible in the gradual growth phase (7D) where the number of cells on laser structured surfaces was significantly increased. Compared to the reference sample with 808 cell/1 mm2 the number of cells increased by 236% to 1908 cell/1 mm2 and by 496% to 4006 cell/1 mm2 for surface covered by LIPSS and LIPSS on microstructured surface, respectively.
In the final state of the cell grow analysis (14D) material's surfaces were fully covered by cell colonies reaching 8410 cell/1 mm2 for the reference surface, 5596 cell/1 mm2 for surface covered by LIPSS and 11 043 cell/1 mm2 for LIPSS on microstructured surface. Thus, interestingly number of cells on LIPSS surface is smaller by 67% compared to the reference. However, on the surface combining LIPSS and microstructures the number of cells is higher by 131% compared to the reference.
The phenomena of advanced cell growth and orientation could be explained by additional sites provided by LIPSS nanopatterns for protein adsorption compared to the untreated reference sample. It was shown that these precursors may act as sites for the cell processes attachment increasing the probability of cell growth and proliferation [27]. Thus, increasing the number of cells on LIPSS structured surfaces. Additionally, microscale grooves and LIPSS nanopatterns may act as scaffold, providing directional cues guiding cell alignment and orientation [28]. This effect is usually more pronounced in the case of microscale patterns [29, 30] and further enhanced by contact guidance phenomenon [28] which amplify the contact inhibition effect and make the cells difficult to grow over the ridges resulting in cell orientation along the periodic microgroove pattern. Thus, the demonstrated combination of micro and nanogroove patterns enables tailoring the osteointegrative properties, which may potentially lead to successful mimicking of the native morphology of human tissues and open novel possibilities in regenerative medicine and reduce the probability of implant failure.
Additionally, the control of surface wetting is essential for surface adhesion and biocompatibility. Wettability was shown to play a significant role in success of any bio-implant [31]. It influences cell attachment, tissue response, and protein adsorption [32]. Hence, to further support the claim of enhanced biocompatibility wettability measurements were carried out as depicted in figure 8.
Figure 8. Contact angle analysis of reference and laser structured surfaces.
Download figure:
Standard image High-resolution imageAs can be observed in figure 8, samples which experienced enhanced cell number were more hydrophilic compared to reference sample. These results are in accordance with other authors [32, 33] pointing out that surfaces with hydrophilic nature are favorable for cell adhesion and proliferation. The enhanced hydrophilic character was confirmed by Energy-dispersive X-ray spectroscopy (EDS) analysis (not shown) revealing an increased amount of metal oxides with high surface energy formed during the laser treatment [34].
4. Conclusion
The unique combination of high energy ultrashort pulsed laser system with multi-beam and beamshaping technology resulted in efficient production of functional micro and nanostructures. By combining both of these technologies the production speed of rectangular shaped micropillars were increased by 526% from 3.8 cm2 min−1 to 20 cm2 min−1 and production speed of LIPSS nanogrooves were increased by 14 570% from 0.7 cm2 min−1 to 102 cm2 min−1 by the means of beamshaping.
Fabricated micro- and nanostructured surfaces and their combination demonstrated enhanced cell culturing capabilities affecting the cell proliferation and orientation. The effect of different surface topography was most visible during the gradual growth phase after 7 d of cultivation. During this phase, cell orientation was mostly influenced by directional cues guiding cell alignment enhanced by contact guidance phenomenon resulting in cell orientation along the periodic microgroove pattern. Therefore, it was possible to control cell growth using the created structure, which may lead to successful mimicking of the native morphology of human tissues and novel possibilities in regenerative medicine. Additionally, in vitro tests indicated that micro and nanostructured surfaces provide better environment for cell attachment and proliferation compared to untreated ones during the initial adhesion. Compared to the reference sample during the gradual growth phase with 800 cell/1 mm2 the number of cells increased by 236% to 2000 cell/1 mm2 and by 496% to 4300 cell/1 mm2 for surface covered by LIPSS and LIPSS on microstructured surface, respectively. Moreover, in the final state of the cell grow analysis the number of cells for LIPSS on microstructured surface was higher by 131% compared to the reference.
The combination of these results shows that the proposed method can be scaled-up for the mass production of implants with tailored osteointegrative properties reducing the probability of implants failure. We hope these findings will help to develop productive technology for surface functionalization of implant materials that will enable to control cell organization and growth and open novel possibilities in regenerative medicine to lower the risk of implant failure due to low biocompatibility.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Funding
This work was co-financed by the European Regional Development Fund and the state budget of the Czech Republic (Project HiLASE CoE: Grant No. CZ.02.1.01/0.0/0.0/15_006/0000674) and from the European Union's Horizon 2020 research and innovation programme (Grant Agreement No. 739573).
Author contributions
Conceptualization, P H, M K, S M; Methodology, P H, M K, S M, R B, M P, J B, M C, T M, M S; Validation, P H, M K, S M, R B, M P, J B, M C, T M, M S; Investigation, P H, M K, S M; Writing—Original Draft, P H, M K; Writing—Review & Editing, P H, M K, S M, R B, M P, J B, M C, T M, M S; Visualization, P H, M K; Project administration, P H, M K; Funding acquisition, T M.
Conflict of interest
The author(s) declare no competing interests.