Abstract
Collagen has been used as a common template for mineralization and assembly of inorganic particles, because of the special arrangement of its fibrils and the presence of charged residues. Streptococcal bacterial collagen, which is inherently secreted on the surface of Streptococcus pyogenes, has been progressively used as an alternative for type I animal collagen. Bacterial collagen is rich in charged amino acids, which can act as a substrate for the nucleation and growth of inorganic particles. Here, we show that bacterial collagen can be used to nucleate three different inorganic materials: hydroxyapatite crystals, silver nanoparticles, and silica nanoparticles. Collagen/mineral composites show an even distribution of inorganic particles along the collagen fibers, and the particles have a more homogenous size compared with minerals that are formed in the absence of the collagen scaffold. Furthermore, the gelation of silica occurring during mineralization represents a means to produce processable self-standing collagen composites, which is challenging to achieve with bacterial collagen alone. Overall, we highlight the advantage of simply combining bacterial collagen with minerals to expand their applications in the fields of biomaterials and tissue engineering, especially for bone regenerative scaffolds.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Animal collagen is an abundant protein with a variety of applications in the fields of biomaterials, tissue engineering, and drug delivery, due to its fascinating characteristics such as being biocompatible [1]. As a protein template, it has been combined with natural, synthetic, or inorganic materials to enhance its properties for tissue engineering, orthopedic, and dental applications [2, 3]. More specifically, type I collagen is known as the universal template for bio-inspired mineralization of the inorganic particles, such as hydroxyapatite (HAP) [4], calcium carbonate [5], silica [6], and metal nanoparticles [7, 8].
To synthesize inorganic minerals, the use of proteins, particularly collagen, can help with the nucleation of mineral ions, growth, and stabilization of the materials, as well as providing a structural framework for mechanical support [9]. The special arrangement of fibrils in type I animal collagen and a high content of carboxylic groups make them proper templates for nucleation and growth of minerals such as HAP [10]. They assemble in a parallel arrangement, in such a way to form a repeated 40 nm gap, called the 'hole zone', which is known to be a critical site for minerals nucleation [10]. In addition, the presence of the charged residues in the collagen can enhance the colloidal stability of nanoparticles, such as silver, during the nucleation and growth stages [11, 12].
The broad application of animal collagen in the field of biomaterials and tissue engineering has motivated investigations for alternative sources of collagen with lower immunogenicity and inflammatory response compared to animal sources [13], such as recombinant collagen-like proteins [14, 15]. For instance, bacterial collagen-like proteins such as Scl2, which inherently are expressed on the surface of Streptococcus pyogenes, have been recombinantly produced in Escherichia coli (E. coli). These proteins are non-cytotoxic and non-immunogenic [16]. They have a stable triple helical structure, with mechanical and thermal stability similar to type I animal collagen [14, 17], with the exception that they lack hydroxyproline, the main amino acid contributing to the stabilization of the triple helix in animal collagen. Instead, the stability of bacterial collagen is a result of its high content of proline and charged amino acids, which participate in electrostatic interactions and support interchain hydrogen bonding [17, 18].
Previously, the extracellular secretion of bacterial collagen through a modified curli secretion pathway in E. coli was reported, and the purification methods were simplified for scalable isolation of bacterial collagen and subsequent fabrication of recombinant collagen-based biomaterials [19]. Although bacterial collagen is structurally similar to type I animal collagen, its potential for forming fibrillar structures is more limited compared with type I animal collagen [17]. The consequence of this limited aggregation is that the protein does not form stable networks, and the dried samples become difficult to handle, limiting their uses in coating applications. Bacterial collagen has been modified genetically, physically, and chemically [20] to modulate its mechanical properties and processability (e.g. spinnability, gelation, and injectability) [21]. Alternatively, mineralization is an easy approach to improving the structural and mechanical properties of collagen scaffolds [22]. Since bacterial collagen contains several positively (lysine and arginine) and negatively (glutamate and aspartate) charged amino acids, we hypothesized that they could serve as a template for mineralization, by stabilizing precursor ions and providing nucleation and growth sites. Therefore, in this study, we used this feature to nucleate three different inorganic components–silver nanoparticles (AgNps), HAP crystals, and silica particles–on a bacterial collagen substrate, and we characterized their structures and physicochemical properties. Due to their antibacterial properties, AgNps have been applied for biotechnology applications such as biomaterials coatings [23], wound dressings [24], and bone and dental composites [25]. The size and distribution of AgNps can affect their release profile and antibacterial properties, which can be controlled by their nucleation rate. HAP and silica are also minerals with unique properties, such as their biocompatibility, biodegradability, and mechanical strength, and have been combined with the proteins such as collagen. The use of animal collagen with these minerals has previously shown a synergistic osteoconductive effect [4]. The mineralization mechanisms of these three minerals proceed via different steps, and we show that bacterial collagen is a suitable substrate for all three of them. We believe that the mineralization of bacterial collagen is a new route toward its application in the fields of biomaterials and tissue engineering by improving its processability and mechanical properties.
2. Materials and methods
2.1. Expression and purification of bacterial collagen
Previously, an adapted secretion pathway, used natively by E. coli to secrete curli fibers, was reported for extracellular secretion of bacterial collagen (V'CL), which consists of the N-terminal variable domain plus N22, secretion signal peptide, (V'), and a collagen domain (CL) [19]. We transformed an engineered plasmid (pET21d-v'cl-csgCEFG), containing the genes coding for V'Cl, CsgC, CsgE, CsgF, and CsgG, into E. coli PQN4 (a curli operon deletion mutant strain) for expression of bacterial collagen as the sole extracellular product. We then used a filtration-based purification method to isolate the extracellularly secreted collagen, as previously described [19]. Briefly, we digested the non-collagen impurities in the collected supernatant of the bacterial culture using pepsin (0.01 mg ml−1, BioBasic, PB0688) and concentrated and isolated the bacterial collagen using crossflow filtration.
2.2. Synthesis and characterization of collagen-templated silver nanoparticles (CL/AgNp)
We added 100 µl of bacterial collagen solution (at different concentrations: 0 mg ml−1 (No CL/AgNp), 0.5 mg ml−1 (CL0.5X/AgNp), 1 mg ml−1 (CL1X/AgNp), and 4 mg ml−1 (CL4X/AgNp) in water) to 100 µl of 30 mM AgNO3 (BioShop Life Science Products) and agitated at 500 rpm at room temperature for 10 min. We added sodium citrate (BioShop Life Science Products) as a reducing agent, and coumalic acid (Sigma-Aldrich) as a capping agent [26], to the collagen/AgNO3 mixture, with final concentrations of 27.6 mg ml−1 and 2.18 mg ml−1, respectively. The mixtures were agitated at 37 °C and 60 °C for 4 h. To remove excess salts and reagents, we centrifuged samples for 2 min at 13 000 rpm and washed them with water (3 times). We obtained the UV spectra of the prepared samples in the range of 350–650 nm by ultraviolet-visible (UV–Vis) absorption using a NanoDropTM One Microvolume Spectrophotometer.
Using scanning electron microscopy (SEM), we studied the morphology and size of the particles and their interaction with the bacterial collagen fibers. We prepared the samples by depositing 50 µl of the CL/AgNp solution on 0.2 µm polycarbonate filter membranes (Whatman ® Nuclepore from Millipore Sigma). We washed the membranes with 0.1 M sodium cacodylate buffer (Electron Microscopy Sciences), fixed with 2% (v/v) glutaraldehyde (BioBasic) and 2% (v/v) paraformaldehyde (Electron Microscopy Sciences) for 2 h at room temperature, solvent-exchanged sequentially in 0%, 25%, 50%, 75% and 100% (v/v) ethanol (for 15 min in each solvent), and dried the membranes in a critical point dryer (CPD). We sputtered until they were coated with a 5 nm layer of Pt. We performed the imaging using field electron and ion company (FEI) Quanta 450 environmental scanning electron microscope (ESEM) at 5 kV equipped with an energy-dispersive x-ray spectroscopy (EDS) detector.
We examined the antibacterial properties of the CL/AgNps mixture against E. coli K12 ER2738 (Gram-negative) by monitoring the optical density of the bacteria at 600 nm (OD600). To evaluate the effect of the CL/AgNp on the optical density of E. coli, we prepared exponentially growing bacterial suspensions by inoculating a single colony in 5 ml lysogeny broth (LB), growing overnight, diluting 1:250 in LB, and incubating at 37 °C to reach OD600 of 0.6. We treated 1 ml of the E. coli suspension with 10 µl of the CL/AgNp solutions, incubated at 37 °C, and measured the OD600 after 1 h, 2 h, and 18 h. We defined 100% viability for bacteria grown in the absence of CL/AgNp samples. In addition, using the colony counting method, we examined the antibacterial properties of the CL/AgNp samples. To do so, we prepared the bacterial logarithmic-phase culture by suspending a single colony in 5 ml LB and incubating at 37 °C to reach OD600 of 0.6, then diluting 100 times (1 × 106 cfu ml−1) and resuspending 15 µl of the diluted suspension into 45 ml LB. After overnight incubation at 37 °C, the concentration of bacteria went up to 108 CFU ml−1 and we diluted it to a working concentration of 106 CFU ml−1. We added 10 µl of CL/AgNp solutions to 1 ml of the inoculum and incubated for 20, 40, and 60 min, 120 min at 37 °C. At the predetermined contact times, we removed 90 µl of the suspension, diluted it serially with PBS (101, 102, 103, 104, 105, and 106), and spread 5 µl of each of the diluted suspension onto four zones of an LB agar plate and incubated at 37 °C for 18 h. We used a bacterial collagen solution (4 mg ml−1) and a bacterial suspension only as controls.
2.3. Synthesis and characterization of collagen-templated hydroxyapatite nanoparticles (CL/HAP)
To mineralize the bacterial collagen with HAP, we dissolved lyophilized collagen (with a final concentration of 0 mg ml−1 (HAP only) 1 mg ml−1 (CL1X/HAP), and 4 mg ml−1 (CL4X/HAP) in the mixture of 100 µl of 100 mM CaCl2 and 100 µl of 50 mM Na2HPO4 and incubated the mixtures overnight at 37 °C. We removed the excess salts by washing the samples with water and centrifuging for 2 min at 13 000 rpm (3 times). After fixing and drying using the same protocols used for CL/AgNp samples we studied the morphology of the CL/HAP samples by SEM coupled with an EDS detector. We sputtered the samples until they were coated with a 5 nm layer of carbon. We also carried out x-ray diffraction (XRD) using Cu Kα radiation (λ = 1.5418 Å) on a diffractometer (Bruker D8 Discovery) and recorded the pattern from 20° to 60° with a step size of 0.02°.
2.4. Synthesis and characterization of collagen-templated silica (CL/Silica)
To synthesize the CL/Silica nanoparticles, we carried out the reaction by hydrolyzing 100 µl tetramethyl orthosilicate (TMOS) (Sigma-Aldrich) containing 1 ml of a bacterial collagen solution (at 0 mg ml−1 (Silica only), 1 mg ml−1 (CL1X/Silica), and 4 mg ml−1 (CL4X/Silica) with 100 µl 50 mM Tris–HCl buffer, pH 6.8, while placed on ice, vortexed for 1 min, and then incubated the mixture at room temperature for 1 h. We rinsed the resulting gels by soaking them in ethanol 99% (5 min, 3 times) followed by a 5 min rinse in water.
We conducted characterization of the CL/Silica samples by SEM observation along with elemental analysis by EDS. To better observe the interaction of the bacterial collagen and silica particles through SEM, we dissolved 0.5 mg of the formed hydrogels in 100 µl water to obtain a less entangled network. Fourier transform infrared (FTIR) spectra were collected in a transmission mode on an FTIR spectrometer (Spectrum II from Perkin Elmer) from 400 to 4000 cm−1 at a resolution of 2 cm−1. We measured the water absorption of the samples by soaking the dried samples in water for 12 h and measured the weight difference before and after absorbing water.
The hardness and elastic modulus of the composites was assessed using a Nanovea nanoindenter at room temperature. We cold-mounted the samples in epoxy resin and polished them before testing to decrease surface heterogeneity. A Berkovich diamond indenter with an approach speed of 2.5 µm min−1, contact load of 0.3 mN, maximum load of 3 mN, and a 10 s hold time at maximum load (to minimize thermal drift and creep effects) was used for all the measurements.
2.5. Synthesis and characterization of collagen/HAP/AgNp/Silica composite
To fabricate the four-component composite based on bacterial collagen, we added 100 µl of TMOS to 1 ml of bacterial collagen solution (4 mg ml−1) and thoroughly mixed it. Then, we added the 4 mg of the synthesized CL4X/AgNp composite (washed and dried) and 4 mg of the CL4X/HAP composite (washed and dried) to the collagen/TMOS mixture and vigorously stirred using a vortex mixer. We added 100 µl of 50 mM Tris–HCl buffer, pH 6.8 dropwise to the mixture while stirring.
We conducted characterization of the CL/HAP/AgNp/Silica sample by elemental analysis using EDS. We sputtered the samples until they were coated with a 5 nm layer of carbon. We assessed the hardness and elastic modulus of the composites using a Nanovea nanoindenter at room temperature. We also examined the antibacterial properties of the CL/Silica/HAP/AgNp sample against E. coli K12 ER2738 using the zone of inhibition test. We spread the bacterial suspension (106 CFU ml−1) over an agar plate using a sterile swab and incubated it overnight at 37 °C in the presence of the CL/HAP/AgNp/Silica sample and CL/HAP/Silica as a control.
2.6. Statistical analysis
Statistical analyses were performed via IBM SPSS Statistics program (Statistical Package for the Social Sciences). Comparison of obtained data for different samples was performed with One-Way analysis of variance (ANOVA) with Tukey post-hoc test. The significance level was set at p < 0.05.
3. Results and discussion
3.1. Mineralization of bacterial collagen with AgNps
We first used bacterial collagen as a template to synthesize AgNps, hypothesizing that bacterial collagen could serve as a stabilizing scaffold for the nucleation of AgNps, because of its high content of charged residues. We used a combination of a chemical reductant and temperature to drive the synthesis of the nanoparticles. To confirm the reduction of silver ions, we first obtained the UV-visible spectrum of the samples. A typical silver surface Plasmon resonance at a wavelength of around 400 nm, due to the oscillations of free electrons on the AgNps surface AgNps [27], confirmed the formation of AgNps (figure 1(B)). In this synthesis, sodium citrate serves both as a reducing agent and as a stabilizer for the colloidal particles. It was found that the reducing reaction with only sodium citrate at low temperature (room temperature and lower) takes a long (∼1 week) and shows a wide size distribution of AgNps [28]. Thus, sodium citrate, as a reducing agent, is often used in a boiling solution or combined with other capping agents (such as coumalic acid in the present case). In the presence of sodium citrate and coumalic acid along with the temperature, we observed the reduction of AgNO3 to Ag.
Figure 1. Bacterial collagen as a template for the synthesis of AgNps. (A) The scheme shows the interactions between the functional groups of the collagen and the silver ions as the driving force for the nucleation of silver nanoparticles. (B) UV-visible spectra of the composites with different concentrations of collagen. Higher collagen content increased the reduction of silver ions and the formation of AgNps. (C) UV-visible spectra of the composites at different temperatures. At higher reaction temperatures, the reduction rate of silver ions increases. (D) SEM images of composites in the presence and absence of bacterial collagen at different temperatures. In the absence of collagen AgNps, tended to aggregate and form larger particles. At both temperatures AgNps were uniformly distributed along the fibers, confirming the effect of bacterial collagen in controlling the reduction reaction. The scale bar is 500 nm. (E) and (F) EDS map and elemental analysis of silver and nitrogen. The CL4X/AgNp showed a strong elemental signal of silver and its uniform distribution throughout the composite. The scale bar is 500 nm. (G) OD600 of the E. coli in the presence of different formulations of composites. CL/AgNp samples showed slower bacterial reduction compared with the sample without bacterial collagen (ns: not statistically significant).
Download figure:
Standard image High-resolution imageAt pH values higher than the isoelectric point of bacterial collagen (∼5.5), negatively charged residues serve as stabilizing substrates to cap the silver ions. We studied the effect of bacterial collagen concentration on the synthesis of AgNps. At higher concentrations of collagen, the intensity of the absorbance peak increased at both 37 °C and 60 °C (figure 1(B)). The specific interactions between the functional groups of the bacterial collagen template and the silver ions could thus promote the formation of the nanoparticles (figure 1(A)) [29]. The higher intensity of the absorption peak of the samples containing bacterial collagen compared with the No CL/AgNp sample indicates that the bacterial collagen has a synergic effect with sodium citrate on the reduction of the silver ions and formation of the AgNps (figure 1(B)).
In addition, as the concentration increases, there is a clear peak position shift to the higher wavelength, indicating a different nucleation behavior of silver ions in the presence of protein templates. Theoretically, particle formation could involve two mechanisms: (a) the formation of particles through the interaction of silver seeds that are homogeneously nucleated in solution and the protein template or (b) the formation of particles through adsorption of silver ions onto the nucleation sites and functional groups of the proteins, resulting in heterogeneous nucleation [30]. Based on the UV-visible spectra (figure 1(B)), at the lower concentration of bacterial collagen (No CL/AgNp and CL0.5X/AgNp samples), silver ions are homogeneously seeded in the solution phase and form AgNps. This observation indicated that the protein is less involved in the particle formation process. However, in the presence of collagen templates, silver ions are adsorbed on the surface of the proteins—they interact with their side chains and are heterogeneously nucleated. In addition, monitoring the growth of AgNp at different incubation times (figure S1), showed that the silver ions are able to penetrate the protein network over time and have access to more nucleation sites. Thus, at a longer time, they form smaller particles inside the network compared with the surface particles, as a shoulder around 400 nm has appeared at t = 4 h. In particular, due to the nanoscale dimensions of the bacterial collagen fibers, they can adjust the size of the inorganic particles during nucleation, and they possess abundant binding sites which bind to metal ions [31]. As stated previously, bacterial collagen contains negatively charged moieties (aspartic acid and glutamic acid) which serve as capturing and nucleating sites for the silver ions before the reduction step.
We also studied the effect of temperature on the formation of AgNps (figure 1(C)). We obtained the UV-visible absorbance spectra of the samples after 4 h of incubation at 37 °C and 60 °C. The AgNps prepared at 37 °C without bacterial collagen substrate showed a single peak at around 410 nm and the ones prepared at 60 °C showed a wider peak at around 425 nm with higher intensity (figure 1(C)). At higher temperatures, the reducing activity of the sodium citrate acid increases and result in more nucleation sites for the formation of the AgNps, therefore, the peak corresponding to 60 °C showed higher intensity than 37 °C. In addition, Liu et al [32] showed that, at sufficiently high concentrations of precursors, the growth rate of nanoparticles increases linearly with reaction temperature, thus, the particle size increases at higher temperatures [28, 32, 33]. The broader peaks corresponding to AgNps synthesized at 60 °C correlate with the large size of AgNps. The CL4X/AgNp sample also showed absorbance intensity at 60 °C compared with 37 °C. However, the width of the two peaks is comparable, with no distinct shift. Although the reaction at 60 °C is faster than 37 °C due to the higher activity of reducing agents (faster nucleation), the growth rate remains controlled by the presence of collagen.
We also studied the morphology of the silver-mineralized samples using SEM (figure 1(D)). In the absence of the collagen, silver ions were reduced, but the reaction was less controlled and the obtained AgNps were more polydispersed, compared with the collagen-templated samples at both 37 °C and 60 °C. AgNps formed at 60 °C showed larger particle size (98 ± 30 nm) compared with the particles formed at 37 °C (45 ± 23 nm). The higher temperature increased the reaction rate and silver ions reduce faster with less control. In some areas, we observed aggregated silver structures after synthesis at 60 °C. However, the collagen-templated AgNps showed uniformly distributed AgNps along the collagen fibers at both 37 °C and 60 °C. From the obtained SEM images, the particle size difference between these two samples is indistinguishable. This observation indicates that increasing the reaction temperature did not significantly affect the particle size, which can be due to the stabilizing effect of the collagen. At higher reaction temperatures, although the silver ions tend to reduce quickly, as we observed in UV-visible absorbance spectra, the bacterial collagen molecules provide enough nucleating sites for them and consequently prevent aggregation and uncontrolled reduction. However, further investigation would be needed to study the average size and distribution of the nanoparticles. AgNp-based composites have been used for different applications from antibacterial to conductive biomaterials, which makes it useful to prepare them simply and effectively with controlled size and shape [34]. The SEM results clearly showed the importance of bacterial collagen in modulating the morphology and size of the AgNps.
To be applied as a biomaterial, bacterial collagen needs to retain its structure and functionality at the processing temperature of interest (often body temperature), therefore we chose the CL4/AgNp (37 °C) for further analysis. We studied the distribution of the elements using EDS elemental analysis (figures 1(E) and (F)). AgNps are known to display the binding energies of Ag between the ranges of 2.5–3.5 KeV which is due to their surface Plasmon resonance [35]. We confirmed the presence of AgNps which displayed a strong signal of elemental silver around 3 keV with additional energy peaks in this range (figure 1(F)) [36, 37]. The EDS elemental map obtained for the CL4X/AgNp (37 °C) sample showed the uniform distribution of the silver atoms consistent with the distribution of the nitrogen atoms throughout the composite (figure 1(F)).
To assess the potency of the collagen–AgNp composites in bacterial growth inhibition, we measured the optical density of E. coli, when growing in the presence of the composites (figure 1(G)). We started the measurement at OD600= 0.83 for the control (t = 0). We subtracted OD600 of the AgNp in LB (0.20) from the absorbance values of the samples. By increasing the incubation time, the OD600 of both controls increased (cells and bacterial collagen only), which indicated that the presence of collagen did not impede cell growth. However, the OD600 for all of the AgNp-containing samples decreased, starting at t = 1 h, which indicated both growth inhibition of the cells and oxidation of the AgNp to the Ag+ form, the active form for antibacterial activity of the AgNps. AgNps can continually release silver ions and due to the electrostatic interaction with the bacterial membrane, they can adhere to the cytoplasmic membrane, therefore, enhancing the permeability of the cytoplasmic membrane and disrupting the bacterial envelope [38]. This interaction can prevent further cell growth. It is worth noticing that the bacterial inhibition of the collagen-containing samples was slower than the sample without. For example, the bacterial reduction of the CL4X/AgNp sample was two times less than the AgNp sample after 18 h. This slow release may be due to the stabilization of the AgNps by bacterial collagen that prevents burst release. The controlled and sustained release of an antibacterial agent can potentially lead to a prolonged antimicrobial efficacy and be beneficial for wounds that would require a longer healing process, such as chronic diabetic wounds and bone defects [39–41]. In addition, we assessed the antibacterial activity of the AgNp composites by colony counting methods (figure S2), which revealed that all the AgNp-containing samples inhibited the growth of E. coli within 60 min.
3.2. Mineralization of bacterial collagen with HAP
We also used bacterial collagen to mineralize HAP crystals using CaCl2 and Na2HPO4 solutions containing Ca2+ and PO4 3− precursors. In the formation of natural bone, type I collagen and non-collagenous component are known to play a critical role in initiating the growth of HAP minerals [42]. Non-collagenous proteins, such as bone sialoprotein, osteonectin, and osteopontin often consist of a high content of negatively charged residues, such as aspartic acid (Asp, D) and glutamic acid (Glu, E), with a high affinity for calcium ions [10, 12]. While bacterial collagen lacks the periodicity and the hole zone feature that is specific for type I collagen, we postulated that the presence of charged and polar residues could make it a proper scaffold for the nucleation of the HAP particles. After the negatively charged amino acids (D and E), with a total content of ∼17% in bacterial collagen, glutamine (Gln) has the highest affinity constant for HAP crystal [12]. Gln is the most abundant uncharged polar amino acid in the structure of bacterial collagen (∼8%) and can make a huge impact on the mineralization potential of bacterial collagen.
To analyze the formation of the HAP minerals we used SEM to observe the mineralized scaffolds. (figure 2(B)). The formation of HAP without the bacterial collagen substrate resulted in the formation of the large particles (up to 1 µm) with random shapes, due to the fast and uncontrolled reaction between the ion precursors (Ca2+ and PO4 3−). However, the presence of the bacterial collagen as a template directed the formation of HAP crystals with uniform shape and size (less than 200 nm). After the mineralization, bacterial collagen retained its fibrillar and highly entangled micro-strand structure, which is key for supporting cell seeding and in vitro growth of bone tissue in tissue engineering applications [43, 44]. Both mineralized collagen samples (CL1X/HAP and CL4X/HAP) showed dense nucleation of HAP along the fibers with platelet morphology, which is the dominant morphology for the HAP crystals [10]. The EDS results demonstrated that Ca and P element peaks in addition to N and O peaks were detected within the CL4X/HAP composite scaffold. The Ca/P ratio of the deposited particles was 1.6, very close to the theoretical ratio in HAP ((Ca10(PO4)6(OH)2): 1.67) (figure 2(C)) [45, 46]. Moreover, the XRD spectrum of the CL4X/HAP sample indicated the main characteristic peaks for crystalline HAP at around 32°, 26°, and 40° 2θ, corresponding to the (211), (002), and (310) diffraction planes of crystalline HAP (figure 2(D)), comparable to what has been observed for animal collagen/HAP composite [46, 47]. Two broad peaks at 32° and 50° are respectively corresponding to the crystallographic planes (211), (121), (300) and (213), (321), and (402) combined into the broad peaks, indicating a relatively low degree of crystallinity and nanosized dimensions [47, 48].
Figure 2. Bacterial collagen as a template for synthesis of hydroxyapatite. (A) The scheme shows the initiation of HAP nucleation by positively and negatively charged groups on the collagen. (B) SEM images of the different formulations of the HAP composites. In the presence of bacterial, collagen smaller crystals were formed with a more uniform distribution compared with the sample with no collagen. (C) Elemental analysis using EDS confirmed the formation of hydroxyapatite. (D) XRD identified the main characteristic peaks for crystalline HAP. (E) EDS map showed that collagen prevents the local aggregation of the HAP crystals (scale bar is 2 µm).
Download figure:
Standard image High-resolution imageEDS elemental map of the CL4X/HAP further indicated that the distribution of Ca and P (from HAP) was homogenous and consistent with the distribution of the N (from proteins), indicating a uniform interaction of the ion precursors (Ca2+ and PO4 3−) with the charged residues in the bacterial collagen backbone (figure 2(E)).
3.3. Mineralization of bacterial collagen with silica
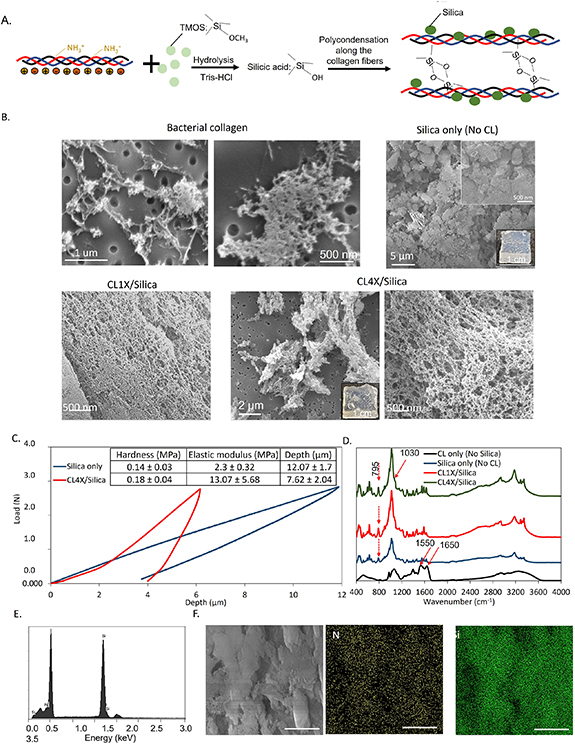
To fabricate a collagen/silica composite we used TMOS as a precursor and Tris–HCl as a hydrolyzing agent. After 10–15 min of TMOS treatment, the composites started gelating and forming aggregates. Immediately after the treatment, we cast the solutions in a silicone mold and let them solidify (figure 3(B)). When the TMOS (the source of alkoxide) is mixed with Tris–HCl, the hydrolysis takes place followed by the condensation reaction of silanol groups that produce oligomers [49]. The gelation of the silica is pH-dependent. The pH of the reaction mixture was 6.8, higher than the isoelectric point of silicic acid (pH = 2), therefore, gelation can be catalyzed by the anionic form of the silicic acid [50]. This mechanism supports the fact that the processability of bacterial collagen, such as hydrogel formation, can be improved by silica-induced gelation. Also, gelation of this composite at around pH = 7 (close to the physiological pH) can be beneficial to activate in situ gelation of hydrogels after injection to the bone defects. To further study the microstructure of the collagen/silica composites we first used SEM (figure 3(B)). Starting with the pure silica gel, in addition to the aggregation of the silica particles, the SEM images showed relatively smooth topography of the surface with no porosity and an interconnected network. The presence of fibrillar bacterial collagen significantly changed the structure of the hydrogels. The bacterial collagen retained its 3D porous and fibrillar structure after silicification. Besides, silica particles formed along the collagen fibers. The surface of silica contains a high density of silanol groups, thus, electrostatic interactions between the negatively charged silanol groups and positively charged groups present on collagen, such as the amine groups, enhance the interactions between the SiO2 particles and the collagen matrix [51].
Figure 3. Silicification of bacterial collagen. (A) The scheme shows the role of positively charged residues in the polycondensation of silicic acid on the collagen substrate. (B) SEM and optical images of the composites with different formulations. In the presence of collagen, a porous and interconnected network of silica and collagen was formed. (C) Relationship between the loads and the maximum indention depth obtained by indentation test. Collagen increased the hardness and elastic modulus of the collagen/silica composites. (D) FTIR identified the characteristic peaks after silicification. (E) and (F) EDS detected the Si and its uniform distribution throughout the composites. The scale bar is 5 µm.
Download figure:
Standard image High-resolution imageWe also observed a higher water absorption for the CL4X/Silica hydrogel (100. 9%  22.4%) compared with silica hydrogel (6.1%
22.4%) compared with silica hydrogel (6.1%  2.6%). This difference was likely due to the 3D porous structure of the bacterial collagen and its higher hydrophilicity compared with silica. High porosity and water absorption are beneficial for cell growth and allow adhesion and proliferation of the cells when using the composite as a regenerative scaffold [52]. Bacterial collagen also affected the hardness and elastic modulus of the silica composite. Figure 3(C) presents the relationship between the load and the maximum indention depth for the silica samples with and without collagen. After adding collagen to the silica, the maximum indention depth under the same load decreased, indicating that the hardness and elastic modulus increased. Bacterial collagen can penetrate the submicron features of the silica hydrogel and through interaction with its charged residues, it can organize the silica particles in a more controlled and organized manner to strengthen the surface.
2.6%). This difference was likely due to the 3D porous structure of the bacterial collagen and its higher hydrophilicity compared with silica. High porosity and water absorption are beneficial for cell growth and allow adhesion and proliferation of the cells when using the composite as a regenerative scaffold [52]. Bacterial collagen also affected the hardness and elastic modulus of the silica composite. Figure 3(C) presents the relationship between the load and the maximum indention depth for the silica samples with and without collagen. After adding collagen to the silica, the maximum indention depth under the same load decreased, indicating that the hardness and elastic modulus increased. Bacterial collagen can penetrate the submicron features of the silica hydrogel and through interaction with its charged residues, it can organize the silica particles in a more controlled and organized manner to strengthen the surface.
In the FTIR analysis (figure 3(D)), the bacterial collagen sample exhibited characteristic infrared (IR) bands at 1650 cm−1 attributed to amide I C=O stretching and 1550 cm−1 to amide II N–H bending [53]. The silica sample showed the characteristic absorptions at 795 cm−1 arising from the bending motion of Si–O–Si bridges, 1020 cm−1 assigned to Si–O–Si stretching with a shoulder at around 910 cm−1 attributed to the stretching vibration of Si-OH groups, and 3200 cm−1 related to the stretching vibration of the silanol groups (Si–OH) [54, 55]. In the collagen/silica samples (CL1X/Silica and CL4X/Silica) the peak at 795 cm−1 corresponding to Si–O–Si bridges appeared, confirming the silicification. The band shift from 1650 cm−1 to 1635 cm−1 of amide groups in both CL1X/Silica and CL4X/Silica samples was indicative of the interactions between hydroxyl groups on the surface of silica and amide groups in the side chains of collagen.
Further, after fabrication of the CL4X/Silica composite and adequate washing to remove the unreacted TMOS and remaining Tris–HCl buffer, we analyzed it by EDS for the detection and distribution of silicon. The EDS profile illustrated the strong silicon peak at approximately 2 keV after TMOS treatment (figure 3(E)) [56]. To further elucidate the interaction of the silanol group with the bacterial collagen substrate, we obtained the distribution of the nitrogen and silicon atoms by EDS. N (from collagen) and Si (from silica) are generally uniformly dispersed throughout the collagen/silica and their signals overlap, because of the uniform interaction of the silanol groups with the bacterial collagen substrate. In some areas, the intensities of elemental signals vary, due to small thickness variations in the sample, as seen in the representative SEM image (figure 3(F)). Through silica mineralization, the bacterial collagen composite can gel, which enhances its potential uses for biomaterials applications. More specifically, the collagen-silica composite could be a potential injectable scaffold for bone tissue engineering [57]. The physiological pH is higher than the pI of silicic acid, therefore, the composite could undergo in situ gelation after injection.
3.4. Mineralization of bacterial collagen with silica, HAP, and AgNp
The healing process of bone defects is extremely long and complicated and can pose a big risk of bacterial contamination. Therefore, the development of bone grafts that are capable of simultaneously controlling infections and promoting bone regeneration would be advantageous [58, 59]. As we showed in the previous sections, the three minerals are uniformly dispersed through the bacterial collagen network, and a combination of all three with bacterial collagen could result in a multifunctional composite. We hypothesized that bacterial collagen has the potential to control the nucleation and mineralization of the different minerals while preventing their aggregation throughout the composites. To fabricate a composite of bacterial collagen with HAP, silica, and AgNp, we used prefabricated collagen/HAP and collagen/AgNp composites that we then mixed with silica precursors (figure 4(A)). This strategy allowed us to avoid the uncontrolled nucleation of the minerals, and to ensure that nucleation sites were available on the collagen template to mineralize each type of nanoparticle.
Figure 4. Collagen as a template to nucleate three inorganic particles (HAP, AgNp, and silica). (A) Optical images of the collagen composites, which were formed into the shape of the molds. (B) Maximum depth, hardness, and elastic modulus of different formulations obtained by indentation test. The four-component composite showed superior mechanical properties compared with silica only and collagen/silica samples (ns: not statistically significant). (C) The distribution map of the main elements from the inorganic particles and collagen obtained by EDS shows the uniform throughout the composite. The scale bar is 10 µm. (D) Zone of inhibition test. Silver nanoparticles inhibited the growth of bacteria and provided antibacterial properties for the four-component composite.
Download figure:
Standard image High-resolution imageUsing indentation, we compared the hardness and elastic modulus of the CL/HAP/AgNp/silica composite with the CL/silica sample (figure 4(B)). After the addition of HAP and AgNp to the CL/silica composite, the hardness increased from 0.18 MPa to 0.86 MPa. This increment, in addition to the increase of elastic modulus, can be attributed to an increase in resistance against deformation in the presence of HAP crystals and AgNps, as the maximum depth also decreased from 7.62 µm to 2.76 µm. Although the mechanical properties of mineralized composites depend on several parameters, such as mineralization condition, the form of collagen matrix (film, sponge, crosslinked, etc), organic content, the concentration of precursors, and the use of additives, the elastic modulus value that we obtained in this study was in the range of elastic modulus of human trabecular bone (ranges between 10 and 3000 MPa) [60] and some reported mineralized animal collagen [61, 62].
In composites consisting of several minerals, the particles are often prone to aggregate, which makes it important to study the distribution of each element in the composite. Using EDS, we observed a uniform distribution of the characteristic elements (N, Si, Ag, Ca, and P) throughout the composite (figure 4(C)). There were areas where the population of Si is slightly higher than others, which can be because of the fast gelation reaction and can be controlled by the slower addition of Tris–HCl. The overall uniformity of the distribution comes from the controlled nucleation and interaction of the ions with functional groups in bacterial collagen.
We further qualitatively tested the antibacterial efficacy of the CL/HAP/AgNp/silica composite (figure 4(D)). The release of AgNp from the composite into the agar exerts a bacterial growth-inhibiting effect and a clear zone of inhibition (around 3 mm) appeared around the sample, compared with the control (CL/HAP/silica) that showed no zone of inhibition.
Overall, combining the three minerals in a single composite brought together the advantageous properties of each mineral. The presence of the AgNps provided antibacterial properties and increased hardness along with HAP crystals. Silica, with its gelation, served as a useful component to broaden the processability and applicability of bacterial collagen/mineral composites as biomaterials.
4. Conclusion
In a composite biomaterial consisting of nanoparticles, uniform interaction between substrate and particles and controlled particle size are key parameters. Bacterial collagen contains a high content of charged amino acids that can support the controlled nucleation and uniform distribution of the inorganic particles. In addition, the nano-sized bacterial collagen fibrils can direct the nucleation and growth of the particles, and its 3D structure provides a porous network structure of the protein/mineral composite, with potential for bone tissue engineering. Collagen/AgNp composites exhibited slower bacterial inhibition compared with the sample without collagen, which indicated a sustained release of AgNps when using a scaffold. Collagen/HAP composites showed uniformly synthesized HAP platelets along the collagen fibers, compared with HAP crystals synthesized without collagen that formed aggregated structures. Further investigation is needed to illuminate the clear mineralization mechanism of HAP on a bacterial collagen template for in vivo applications. Silicification of the collagen resulted in a hydrogel with a porous network that could be easily molded into macroscopic self-standing structures and was more water-absorbent compared with silica-only gels. Since bacterial collagen lacks active biological sites for cell interaction, growth, and differentiation, further genetic modifications will be needed if using bacterial collagen for in vivo applications. Together, the lack of cytotoxicity and immunogenicity, the ease of production, and purification would be sufficient to indicate the promise of bacterial collagens for biomedical material applications. We showed that the three different minerals can provide bacterial collagen with new features, AgNps with antibacterial properties, HAP as the main bone mineral, and silica as both processable and bone cement components. All three were dispersed uniformly along the bacterial collagen fibers. Therefore, the combination of all three types of nanomaterials with bacterial collagen can pave the way for designing new bone regenerative scaffolds.
Acknowledgments
The authors thank the Facility for Electron Microscopy and Research (FEMR) at McGill for assistance with SEM. This research was funded, in part, by the Natural Sciences and Engineering Research Council of Canada (NSERC) through a Discovery Grant (NSERC RGPIN-2017-04598), by the Fonds de Recherche du Québec—Nature et Technologies (FRQNT), and it was undertaken, thanks to the funding from the Canada Research Chairs Program and the support by the Canadian Foundation for Innovation (Project #37524). Z A is grateful for financial support via a McGill Engineering Doctoral Award (MEDA) and an FRQNT Doctoral Research Scholarship.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary data (2.3 MB DOCX)