Abstract
Pure gelatin hydrogels lack antibacterial function and have poor mechanical properties, which restrict their application in wound dressings. In this study, nanosized silver bromide-doped mesoporous silica (AgBr@SiO2) microspheres with hollow structures were prepared by a modified Stober method. The novel microspheres can not only release silver ions to treat bacteria but also release drugs to treat skin wound. Furthermore, AgBr@SiO2 microspheres were modified with propyl methacrylate, incorporated into methacrylated gelatin (GelMA), and crosslinked by UV light to prepare AgBr@SiO2/GelMA dressings consisting of composite hydrogels. The results showed that the AgBr@SiO2 microspheres could enhance the mechanical properties of the hydrogels. With the increase in the AgBr@SiO2 concentration from 0.5 to 1 mg ml−1, the dressings demonstrated effective antimicrobial activity against both Staphylococcus aureus and Escherichia coli. Furthermore, full-thickness skin wounds in vivo wound healing studies with Sprague–Dawley rats were evaluated. When treated with AgBr@SiO2/GelMA containing 1 mg ml−1 AgBr@SiO2, only 15% of the wound area left on day 10. Histology results also showed the epidermal and dermal layers were better organized. These results suggest that AgBr@SiO2/GelMA-based dressing materials could be promising candidates for wound dressings.
Export citation and abstract BibTeX RIS
1. Introduction
Bacterial infection, which may impede the healing process and cause life-threatening complications, is emerging as one of the main problems threatening wound care worldwide [1, 2]. Wound dressings with antibacterial agents possess a broad spectrum of activity and a high kill rate and are generally used to treat wound infections [3, 4]. However, the rising risk of antibiotic-resistant bacterial infections caused by antibacterial agents has attracted burgeoning attention [5]. In recent years, extensive research has been conducted to produce silver-based substances like silver nanoparticles (AgNPs), silver halides nanoparticles, etc. Due to their broad antibacterial spectrum and long-term antibacterial activity [6, 7]. Various approaches, including chemical and physical methods, have been reported to produce AgNPs and other silver-based substances, which have been shown as promising visible-light active disinfectants [8]. Although the antibacterial mechanism of AgNPs is not clear, some reports have shown that it is the silver ions (Ag+) that are released from AgNPs rather than the particles themselves that play a key role [9, 10]. Among them, silver bromide (AgBr) have the potential antibacterial properties by controlling the release of biocidal Ag+ in aqueous environments [Ksp (AgBr), 5 × 10−13] [11], and the water-insoluble coatings on substrate exhibited long-lasting antibacterial potential toward both airborne and waterborne bacteria [12]. In addition, the rate of release of Ag+ can be tailored by the appropriate choice of the encapsulating substrate and the size of the embedded silver-based substances [13, 14]. It was reported that Ag+ and other drugs, such as antibiotic combinations, can provide obvious synergistic effects when killing drug-resistant bacterial strains [13, 15]. AgNPs-doped mesoporous silica microspheres were synthesized, indicating a sustained release of Ag+ through numerous mesopores [16, 17]. AgNPs or other silver halides nanoparticles encapsulated in silica microspheres also sustain the release of Ag+ and other drugs at the same time, which is beneficial in treating wound infections [13, 18]. Therefore, a drug delivery system containing AgBr and other drugs would generate promising therapeutic dressings to kill bacteria and accelerate wound repair. To load more drugs into silica microspheres, mesoporous microspheres with hollow structures are promising [19]. Therefore, artificial dressing imitating the skin combined with AgBr NPs-doped mesoporous silica (AgBr@SiO2) microspheres with hollow structures is effective in preventing infection, protecting the wound, and accelerating healing.
Hydrogels are widely used to provide a long-lasting moist environment for wounds [20, 21], which promotes the epithelialization of shallow wounds [22, 23]. Gelatin, a hydrolysate of collagen, is a typical gel material that contains a rich arginine-glycine-aspartate sequence that promotes cell adhesion and the target sequence of matrix metalloproteinase [24, 25]. Compared with collagen, gelatin has better solubility and lower antigenicity. Meanwhile, the gelatin solution itself has unique properties of gelation at low temperature to form physically crosslinked hydrogels [26, 27]. In particular, methacryloyl substituents were introduced into gelatin (methacrylated gelatin, GelMA) to endow it with photopolymerization properties [28]. This photocrosslinking reaction can be carried out rapidly under mild conditions, such as room temperature, neutral pH, and aqueous environments, which provides convenience for the rapid generation of wound dressings [29]. However, pure GelMA hydrogels lack antibacterial function and have poor mechanical properties, which restricts their application in wound dressings [30, 31]. AgBr@SiO2 microspheres have abundant silicon hydroxyl groups on the surface, so the functional surface of AgBr@SiO2 microspheres can be prepared with reactive acrylate groups. Following the method to prepare thiol-silica by Tian [32], 3-(trimethoxysilyl) propyl methacrylate (TMSPMA)-modified silica was obtained. Therefore, AgBr@SiO2 microspheres with functional surfaces that react with GelMA can not only enhance mechanical properties but also serve as a drug release system to develop new wound dressings.
In this work, novel mesoporous SiO2 microspheres with hollow structures and AgBr NPs incorporation were prepared first. Then, AgBr@SiO2 microspheres were modified with TMSPMA, incorporated into GelMA, and crosslinked by UV light to prepare AgBr@SiO2/GelMA composite dressings. The antimicrobial activity of the dressings was studied in vitro and in vivo.
2. Materials and methods
2.1. Reagents
Cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), silver nitrate (AgNO3), 3-(trimethoxysilane) propyl propionic acid (TMSPMA), type A gelatin, methacrylic anhydride, 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylphenyl-acetone (Irgacure 2959) and phosphate buffer solution (PBS) were purchased from Aladdin Chemical Co., Ltd (Shanghai, China). Methanol, anhydrous alcohol and ammonia were obtained from Kelong Chemical Co., Ltd (Chengdu, China). The above reagents were all analytically pure and used without further purification. The deionized water was made by our laboratory.
2.2. Sample preparation
2.2.1. Preparation and modification of mesoporous AgBr@SiO2 microspheres with hollow structures
First, two kinds of solutions were prepared. Ammonia water (1.5 ml), deionized water (150 ml), and ethanol (30 ml) were mixed under stirring conditions. CTAB (0.48 g) was completely dissolved in the solution to obtain solution A. TEOS (3.0 ml), 60 ml of ethanol and 1 ml of deionized water were mixed, and 0.2 g of AgNO3 was added to obtain solution B. At 25 °C, solution B was dropped into solution A with magnetic stirring at 400 rpm. Afterward, the reaction system was sealed for 24 h. Then, the slurry was filtered, washed and mixed with deionized water at a ratio of 1 g/500 ml for 48 h. This mixed solution was filtered, washed, and freeze-dried again to obtain a powder sample.
The freeze-dried material was mixed with acetone and refluxed for 2 × 12 h at 75 °C with stirring to remove the CTAB. Finally, mesoporous AgBr@SiO2 microspheres with hollow structures were obtained. Meanwhile, hollow mesoporous SiO2 microspheres without AgBr NPs were prepared as controls.
Then, TMSPMA-modified AgBr@SiO2 microspheres were prepared according to a previous report [32]. AgBr@SiO2 microspheres (0.04 g), 10 ml of methanol, and 1.2 ml of TMSPMA were stirred at room temperature for 5 h. The microspheres were centrifuged and cleaned with methanol three times. After vacuum drying for 24 h, TMSPMA-modified AgBr@SiO2 microspheres were prepared.
2.2.2. Preparation of AgBr@SiO2/GelMA composite hydrogels
GelMA was prepared according to our previous report [33]. Briefly, 10 g of type A gelatin from pig skin was dissolved in 100 ml of PBS at 50 °C, and stirred until completely dissolved. About 10 ml of methacrylic anhydride solution was slowly dropped into the gelatin solution, and the sealed reaction was continued for 3 h at 50 °C. Then, the solution was diluted with 200 ml of PBS. The diluted solution was placed into a dialysis bag with a cut-off molecular weight of 8000–12 000 D and dialyzed with deionized water at 60 °C for seven days, during which the deionized water was changed more than 5 times per day. After dialysis was completed, the obtained gelatin solution was freeze-dried to obtain GelMA with UV sensitivity.
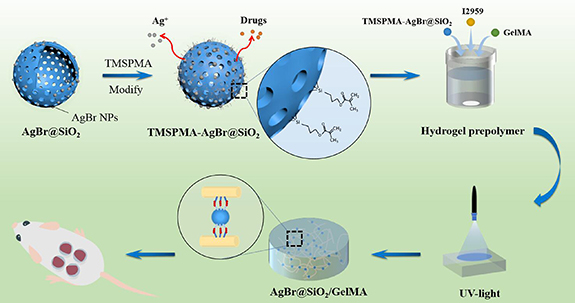
The prepared GelMA was dissolved in PBS at a fraction of 8% (w/V) to obtain the hydrogel prepolymer solution. As shown in table 1, the TMSPMA-modified AgBr@SiO2 microspheres and the hydrogel prepolymer solution were mixed uniformly with a certain mass/volume ratio, and 1% (w/v) photoinitiator Irgacure 2959 was added to the prepolymer solution. After the above solution was repeatedly pipetted and mixed, 150 µl of the solution was added dropwise to a homemade model and crosslinked under ultraviolet light (wavelength: 320–480 nm, power: 7.0 mW cm−2) for 30 s. Finally, an AgBr@SiO2/GelMA composite hydrogel was obtained. The entire preparation flow chart of the composite hydrogel is shown in figure 1.
Figure 1. Schematic illustration of AgBr@SiO2/GelMA composite hydrogel.
Download figure:
Standard image High-resolution imageTable 1. Compositions of AgBr@SiO2/GelMA hydrogel samples.
| Sample | AgBr@SiO2 (mg) | GelMA (mg) | I2959 (mg) | PBS (ml) |
|---|---|---|---|---|
| S0 | 0 | 80 | 10 | 1 |
| S1 | 0.05 | 80 | 10 | 1 |
| S2 | 0.5 | 80 | 10 | 1 |
| S3 | 1 | 80 | 10 | 1 |
Note: PBS—phosphate buffer;I2959—2-Hydroxy-4ʹ-(2-hydroxyethoxy)-2-methylpropiophenone.
2.3. Physical and chemical analysis of AgBr@SiO2 microspheres and composite hydrogel
The morphology of the microspheres and composite hydrogels was observed and analyzed by a JSM-7800F field emission scanning electron microscope (SEM, JEOL, Japan). A JEM-2100F transmission electron microscope (TEM, JEOL, Japan) was used to characterize the microstructure of hollow AgBr@SiO2 microspheres and the distribution of AgBr NPs. X-ray diffraction (XRD, SmartLab, Rigaku, Japan) and Fourier transform infrared (FT-IR, Nicolet-6700) spectroscopy were used to examine the composition of the sample [12]. The specific surface area and pore size distribution of AgBr@SiO2 microspheres were calculated and analyzed by a nitrogen isothermal adsorption-desorption curve, measured with the Autosorb IQ system (BET, Quantachrome, USA).
2.4. Drug control release of AgBr@SiO2 microspheres
Rhodamine B (RhB) was selected as a drug model to evaluate the adsorption and release of AgBr@SiO2 microspheres. Different concentrations of RhB (100, 80, 50, 20, 10 μg ml−1) were prepared first, and SiO2 microspheres (5 mg) with or without incorporation of AgBr NPs were added to the above solution (5 ml). The adsorption system was co-cultured for 2 h at 37 °C and a rotation speed of 200 rpm. After centrifugation at 2000 rpm for 3 min, the absorbance of the supernatant was measured at 555 nm with ultraviolet light (Multiscan GO-1510, Thermo, USA) to obtain the amount of adsorbed RhB in microspheres. Microspheres with an initial RhB adsorption concentration of 80 μg ml−1 were selected for the subsequent release test. Microspheres loaded with RhB (1 mg) were added to 1 ml of PBS and shaken at 37 °C and 120 rpm. At predetermined times, 200 μl of supernatant was removed after centrifugation, and the same amount of PBS was added. The absorbance of released RhB was tested at 555 nm with ultraviolet light, and the cumulative release of RhB was calculated with the standard curve. The drug's cumulative release rate was recorded as the percentage of the cumulative release amount in the adsorbed RhB of microspheres.
2.5. Mechanical test
The mechanical properties of the composite hydrogel were characterized by stress–strain curves. A cylindrical hydrogel sample with a diameter of approximately 9.7 mm and a thickness of approximately 4.2 mm was prepared [34]. The hydrogel material was compressed in the axial direction using a universal mechanical test machine (CMT-1202, SUST Electrical Equipment, Zhuhai, China) with a 200 N load cell at a crosshead rate of 1 mm min−1, and the compression rate was reduced to 90% when the strain or fracture was stopped. Seven samples were taken from each group for testing. The compressive modulus of the hydrogel is calculated by the first 10% of the stress–strain curve of the sample, and the expression of the obtained elastic modulus is the mean value of the standard deviation.
2.6. Water absorption
After freeze-drying, the dry antibacterial gel was weighed and recorded as W0, and it was then soaked in PBS (pH = 7.4) and placed in an incubator at 37 °C. They were taken out at the predetermined time, respectively, and the surface moisture was removed and weighed (W1). The number of samples was six hydrogels in each group. The swelling ratio of antibacterial hydrogel under PBS condition is calculated as follows:
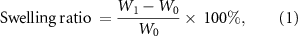
where W0 (g) is the mass after drying and W1 (g) is the mass after water absorption.
2.7. Cytotoxicity evaluation
MC3T3-E1 cells were cultured in 10 cm petri dishes with complete medium (αMEM (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 mg ml−1 streptomycin, and 100 U ml−1 penicillin (Biosharp) at 37 °C in a 5% CO2 humidified incubator. The medium was replaced every three days. When the cells were 80%–90% fused, they were digested with trypsin (Biosharp) for subsequent use.
To quantitatively estimate the biocompatibility of our composite hydrogel, we conducted a cytotoxicity study with MC3T3-E1 cells. The four groups of hydrogels were incubated with complete medium at 37 °C in a 5% CO2 incubator for 24 h (7.5 mg ml−1, n = 5). The extract was collected and diluted with a fresh complete medium. Confluent MC3T3-E1 cells were trypsinized and seeded in a 96-well plate at 1 × 103 cells/well and incubated for 24 h. After 24 h, the medium was replaced with diluted composite hydrogel extracts and incubated for 1, 4, and 7 days. Cell viability was detected utilizing the Cell Counting Kit-8 (CCK-8, Abcam) at each timepoint. The absorption values were read with an automated microplate reader (Perkin Elmer, USA) at a wavelength of 450 nm.
2.8. Antibacterial property evaluation
2.8.1. Bacterial cultivation
Escherichia coli (ATCC25922) and Staphylococcus aureus (ATCC6538) were purchased from the Shanghai Bioresource Collection Center (SHBCC, China). The bacteria were grown at 37 °C in Luria Bertani (LB) broth (Difco, Sparks, MD, USA) overnight in an orbital shaker, and the optical density (OD) was measured at 600 nm using a spectrophotometer. Then, the bacteria were seeded on a solid LB medium.
2.8.2. Antibacterial properties of AgBr@SiO2/GelMA composite hydrogel
AgBr@SiO2/GelMA composite hydrogels with different AgBr@SiO2 microsphere concentrations were placed on the culture medium with bacteria and then cultivated in an incubator at 37 °C. After 12 h, samples were removed for observation and imaging. To investigate the lasting antibacterial effect of the material, the dishes were taken for photographic imaging at 24 h intervals for 5 days.
2.9. Scalded skin model and would healing
2.9.1. Animals
Adult Sprague–Dawley (SD) rats (female, 240–260 g) were purchased from the Laboratory Animal Center at the Army Medical University. All experimental procedures were approved by the Army Medical University Animal Care and Use Committee (SYXK-PLA-20120031) and performed according to institutional animal welfare guidelines. In this experiment, to minimize animal suffering, SD rats were maintained on a 12 h light/dark cycle with free access to food and water at 25 °C–28 °C. All in vivo experiments were conducted following the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.9.2. Scalded skin induction and would healing
SD rats were anesthetized with 2.0% (w/v) pentobarbital through intraperitoneal injections. Four areas 2 cm in diameter on the back of each rat were depilated with a depilatory agent. At Day zero, a stainless steel stick preheated in boiling water was placed on each area for 10 s to produce deep second-degree burns (1 cm in diameter each). Each rat was kept separately in a cage. On day 1, the wounds were first photographed and then disinfected with iodophor before hydrogel treatments began. Gauze with 0.9% saline (control) and composite hydrogel with 0.05%, 0.5% and 1% AgBr@SiO2 (w/v) were placed on the depilated areas of each rat and fixed with medical adhesive tape. The treatment was continued for ten days. On days 4, 7, and 10, the wounds were photographed, and the wound healing process was analyzed via ImageJ.
2.9.3. Histopathology examination
After ten days, all the rats were sacrificed via anesthesia, and the skin tissue from each rat was cut along the wound-healing perimeter. The samples were fixed in 4% (w/v) paraformaldehyde and embedded in paraffin. The samples were sectioned at a thickness of 5 µm and mounted onto poly-L-lysine-coated slides for hematoxylin and eosin staining method (H&E) staining (Servicebio, Wuhan, China). Digital photographs of the sections were recorded with an upright Nikon microscope (Eclipse Ci-L, Tokyo, Japan).
2.10. Statistical analysis
The data were analyzed by one-way or two-way ANOVA as appropriate followed by Tukey's multiple comparison test.
3. Results and discussion
3.1. Characterization of AgBr@SiO2 microspheres
3.1.1. Morphology of AgBr@SiO2 microspheres
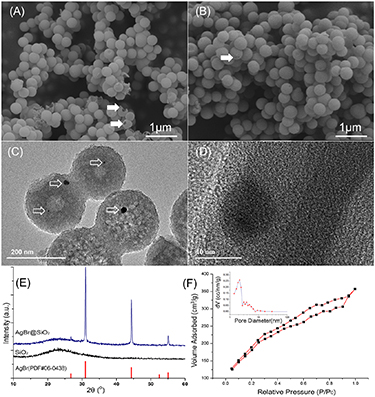
SEM images showed that AgBr@SiO2 (figure 2(A)) and pure SiO2 microspheres (figure 2(B)) have similar spherical shapes, smooth surfaces, and uniform particle sizes. Further statistical analysis revealed that the average particle size of the microspheres was approximately 300 nm. It was also found that the damaged spheres had obvious hollow structures (black arrowhead). Furthermore, the internal structure of the microspheres was observed by TEM. Figure 2(C) shows that the inside and outside of the microspheres exhibited obvious boundaries, which confirmed that the AgBr@SiO2 microspheres had a cavity and shell structure. The shell layer had a uniform thickness of approximately 50 nm. At the same time, the silver nanoparticles were embedded inside the shell of the microsphere (hollow arrowhead). Further high-resolution TEM scanning of a single AgBr nanoparticle in the shell (figure 2(D)) showed typical crystal surface stripes and a diameter of approximately 10 nm.
Figure 2. SEM images of AgBr@SiO2 (A) and SiO2 (B), TEM images of AgBr@SiO2 (C) and (D), XRD pattern of microspheres (E) and typical N2 isothermal adsorption curves (F) and mesopore distribution analysis (inset) of AgBr@SiO2 microspheres. The symbol 'black arrowhead' denotes the shell of the hollow structure, and the symbol 'hollow arrowhead' denotes AgBr NPs.
Download figure:
Standard image High-resolution imageThe reason for the formation of the hollow structure of AgBr@SiO2 microspheres maybe that SiO2 solid microspheres with differential internal and external silica condensation were formed in the first reaction. At the same time, the added AgNO3 was transferred to AgBr nanoparticles in a solution with abundant bromine ions from CTAB and was uniformly wrapped by SiO2 inside the spheres, preventing the agglomeration of nanoparticles. When solid SiO2 was immersed in deionized water, water molecules penetrated the inside core of the microspheres through the spherical shell. The low degree of SiO2 condensation inside the microspheres is beneficial for dissolution in deionized water at room temperature [35]. By the modified Stober method, the specific yield of our microspheres can reach about 90%. Therefore, AgBr NPs-doped AgBr@SiO2 microspheres with hollow structures were successfully prepared by the modified Stober method.
3.1.2. Phase composition of AgBr@SiO2 microspheres
The phase composition was detected and analyzed by XRD. The x diffraction peak of pure SiO2 in figure 2(E) was relatively diffuse, with only a wide diffraction peak of 22°, corresponding to amorphous SiO2. In the XRD pattern of the silver-doped SiO2 microspheres, 2θ values equal to 31.1° and 44.3° corresponded to the typical diffraction peak of (200) and (220) for AgBr, respectively, which was consistent with the standard peak of AgBr (PDF#06–0438) [12, 36], indicating that Ag+ was transformed into crystal AgBr in bromine ions environment with good crystallinity. The existence of AgBr NPs in the microspheres was further confirmed.
3.1.3. Pore structure of AgBr@SiO2 microspheres
The N2 adsorption-desorption results of AgBr@SiO2 microspheres are shown in figure 2(F). The overall curve showed a typical type IV curve, indicating that a large number of mesopores were in the sample, which may have strong adsorption capacity. The specific surface area of hollow microspheres was 793.363 m2 g−1. The pore size distribution of the sample was also calculated based on the BJH method. As shown in figure 2(F) (inset), the pore size of the hollow microspheres was concentrated within 4 nm, confirming a typical mesoporous structure.
The above results suggest that AgBr@SiO2 mesoporous microspheres with good dispersion and hollow structures were prepared successfully. The incorporation of AgBr NPs into hollow microspheres was beneficial to the release of Ag+ and drugs in the following experiment, which may ensure high bactericidal efficiency and low cytotoxicity of the Ag-based substrate.
3.2. Characterization of composite hydrogels
3.2.1. Microstructure analysis of composite hydrogels
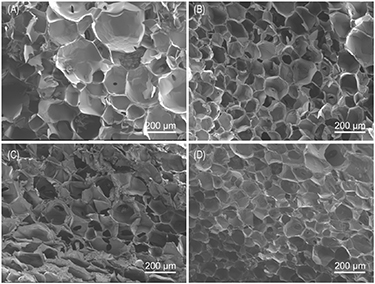
The as-prepared AgBr@SiO2 microspheres were evenly mixed with GelMA hydrogel precursor solution and a photoinitiator. Through UV light cross-linking (table 1), composite hydrogels with different AgBr@SiO2 microsphere concentrations (0, 0.05, 0.5, and 1 mg ml−1) were obtained, and the specific yield of the composite hydrogel can exceed 95%. Figure 3 shows that the four groups were all porous sponge-like structures with uniform pore diameters and good connectivity. The pore size of the hydrogel was approximately 100 nm, and the incorporation of AgBr@SiO2 microspheres did not change the porous structure. This porous structure could give the hydrogel higher water content, and it is beneficial for Ag+ and drugs to penetrate through the waterborne channel, thereby fulfilling a positive antibacterial role.
Figure 3. SEM images of AgBr@SiO2/GelMA hydrogels with different mass volume fractions (A:S0, B:S1, C:S2, D:S3).
Download figure:
Standard image High-resolution image3.2.2. Drug control release of AgBr@SiO2 microspheres
RhB was chosen as a drug model to evaluate the adsorption and release of AgBr@SiO2 microspheres in this study. It is clearly shown that the RhB loading amount increased with an increasing concentration of the initial drug solution (figure S1(A) available online at stacks.iop.org/BMM/17/025005/mmedia). The absorbed RnB reached 59.88 and 74.09 μg mg−1 for SiO2 and AgBr@SiO2, respectively, when the initial concentration of RhB was 100 μg mg−1. The superior RhB loading capacity of SiO2 was mainly attributed to its more ordered mesopores and hollow structure. Samples with an initial loading concentration of 80 μg mg−1 for both SiO2 and AgBr@SiO2 were chosen for the next evaluation experiments except in specific cases.
The in vitro release behaviors of RhB from SiO2@RhB and AgBr@SiO2@RhB were tested in PBS (pH = 7.4) at 37 °C (figure S1(B)). The release profiles showed a quick release during the first 48 h, reaching 30.91% and 29.65% of the loaded RhB amount for SiO2@RhB and AgBr@SiO2@RhB, respectively. In the following stage, prolonged-release profiles over 504 h (21 days) were observed, with cumulative percentages of 64.94% and 61.37% for SiO2@RhB and AgBr@SiO2@RhB, respectively. The results showed that mesoporous and hollow structures ensure AgBr@SiO2 microspheres can not only adsorb more drugs but also control the release of drugs, which is beneficial to accelerating wound repair if some drugs are loaded.
3.2.3. Modification analysis of AgBr@SiO2 and composite hydrogel
The FT-IR spectra of AgBr@SiO2 microspheres in figure 4 showed that the absorption peak at 1050 cm−1 was the antisymmetric contraction vibration peak of Si–O–Si bonds, and the peak at 550 cm−1 was the symmetric contraction vibration peak of O–Si–O bonds in the framework of hollow microspheres. By comparing the infrared spectra of the unmodified and modified hollow microspheres, modified microspheres displayed an absorption characteristic peak at 1730 cm−1 assigned to the C=O stretching frequency from the acrylic ester groups of TMSPMA [18], indicating that TMSPMA was successfully grafted on the AgBr@SiO2 surface, which can act as a bridge to further react with GelMA [18]. Meanwhile, the FT-IR spectra of the GelMA hydrogel before and after the addition of AgBr@SiO2 microspheres showed little change, which may be because the characteristic peak of AgBr@SiO2 was covered by the characteristic peak of the GelMA matrix hydrogel.
Figure 4. FT-IR spectra of AgBr@SiO2 modified with TMSPMA and AgBr@SiO2/GelMA hydrogel.
Download figure:
Standard image High-resolution image3.2.4. Mechanical test
The representative stress–strain curves of the hydrogel with AgBr@SiO2 prepolymer ratios are shown in figure 5(A). The compressive modulus of the hydrogel for all formulations was calculated from the curves. As shown in figure 5(B), the modulus of the hydrogels was significantly higher after introducing AgBr@SiO2 of S2 and S3 (p < 0.05); however, there was no statistically significant difference in the compressive modulus between S0 and S1 because the incorporation concentration was too low. The digital photos of the samples showed that the composite hydrogel better maintained the original shape after it was pressed by a mechanical testing machine (figure S2). Compared to this, pure GelMA was more likely to be crushed, suggesting that TMSPMA-modified AgBr@SiO2 provides better mechanical properties via covalent bonding to GelMA. Sufficient mechanical strength ensures the integrity of wound dressing and avoid the intrusion of external bacteria resulting from the breakage of dressing [37]. Therefore, GelMA hydrogels modified with AgBr@SiO2 show better mechanical properties and are more suitable for dressing.
Figure 5. (A) Representative stress–strain curves of the composite hydrogel. (B) The calculated compressive modulus from the slope of the 0%–10% strain of the stress–strain curve. The symbol '*' denotes a significant difference (*p < 0.05), and the symbol 'o' denotes no significant difference (0 p > 0.05).
Download figure:
Standard image High-resolution image3.3. Water absorption
Figure S3(A) shows water absorption with an immersion time of PBS. During the first 12 h, water sorption quickly increased over time. At approximately 48 h, the saturated adsorption rate of four kinds of samples was reached. The saturated adsorption rates of S2 and S3 was obviously exceeded those of S0 and S1 (figure S3(B)). The hollow microspheres have abundant hydroxyl groups, which contribute to excellent hydrophilicity for the composite hydrogel. The higher water absorption is beneficial for the recovery of wounds because it can absorb tissue exudation, and provide a moist environment.
3.4. Cytotoxicity evaluation
Too much Ag+ released from AgBr@SiO2/GelMA hydrogels is harmful to cells, so the extract was collected and cocultured with MC3T3-E1 cells to evaluate their cytotoxicity. As shown in figure S4, compared with pure GelMA hydrogel (S0) and tissue culture plate (control), the OD value of three types of hydrogels incorporating different amounts of AgBr@SiO2 had no obvious significant difference, suggesting that AgBr@SiO2/GelMA hydrogels have good cell compatibility and can be used following wound care.
3.5. Evaluation of antibacterial properties
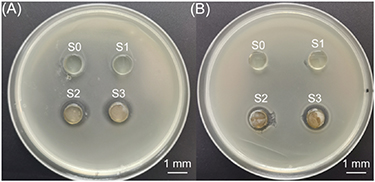
The antibacterial properties of AgBr@SiO2/GelMA hydrogels with different AgBr@SiO2 concentrations on gram-negative bacteria (E. coli, figure 6(A)) and gram-positive bacteria (S. aureus, figure 6(B)) are shown, which is consistent with previous reports [38]. For both kinds of bacteria, the pure GelMA group did not show any bacteriostatic ability (figure 6). At the low concentration of 0.05 mg ml−1, there was little difference in the bacteriostatic circles. Furthermore, with the increase in the AgBr@SiO2 concentration from 0.5 to 1 mg ml−1, the bacteriostatic circles became larger, suggesting that antibacterial effects can be adjusted according to the concentration of AgBr@SiO2.
Figure 6. Typical digital images of AgBr@SiO2/GelMA hydrogel cocultured with E. coli (A) and S. aureus (B) after 12 h.
Download figure:
Standard image High-resolution imageFigure S5 shows the antibacterial effect of the AgBr@SiO2/GelMA hydrogel over time. From day 1 to day 5, the bacteriostatic circles of the composite hydrogel remained similar and showed no obvious decreasing trend, while the hydrogel gradually shrank and dehydrated. Therefore, the composite hydrogel showed a lasting bacteriostatic effect, indicating that Ag+ demonstrated a stable and sustained release rate in this complex system, which may have resulted in a sustained antibacterial effect.
3.6. Would healing studies
Various hydrogel samples with 0.05, 0.5, and 1 mg ml−1 (w/v, S1, S2, S3, respectively) were chosen in this study. As shown in figure 7(A), the wound healing performance in the scalded skin model was observed at different time intervals (1, 4, 7, and 10 days). The wound area compared to the area on the first day was calculated and is shown in figure 7(B). On the first day (day 1), no visible difference in wound area was observed for any of the groups. However, for S2 and S3, the white blisters made by scalding disappeared, which did not happen for the control samples of saline (figure 7(A)). On day 4, the wound area treated with S1 and S2 was reduced to almost the same as that of the control (80%∼90%), while the S3-coated dressing showed 67% of the remaining area. On day 7, all four groups demonstrated better healing, with 73%, 65%, 64%, and 44% of the area left, respectively (figure 7(B)). On day 10, wound reduction was much faster in comparison to the control, with 65%, 40%, 44%, and 15% of the area left, respectively. These results suggest that the wound heals faster when the Ag+ concentration is increased, and the group with the highest concentration (1 mg ml−1) showed the best performance.
Figure 7. Digital photographs of the wound appearance (A) and quantified variation (B) in the wound reduction treated with different samples vs recovery time. * denotes a significant difference (*p < 0.05).
Download figure:
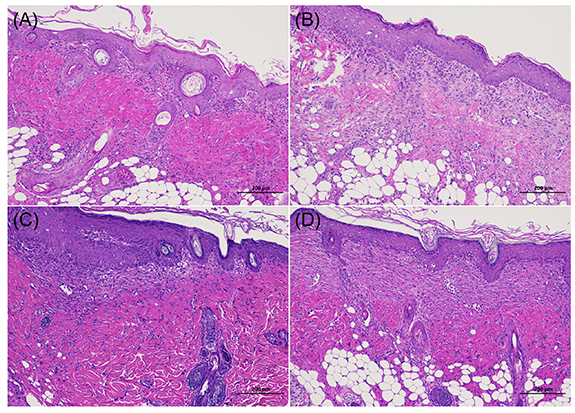
Standard image High-resolution imageA histological examination was further conducted on healed tissues to analyze the formation of reepithelialization and inflammation at day 10. In figure 8, all four groups showed almost normal skin with low-grade inflammatory cell infiltration and adequate collagen deposition. As AgBr@SiO2 increased to 1 mg ml−1 in S3, the epidermal and dermal layers were better organized. Consistent with the above results, the thickest dermis with clear hypodermis was observed in S3-treated wounds, indicating the efficient healing ability of the composite hydrogel.
Figure 8. Histopathology results of rat skin after wound healing at D10. (A) Saline, (B) S1, (C) S2, (D) S3.
Download figure:
Standard image High-resolution imageMany nanoparticles, in particular, silver-based substances can regulate the behavior, metabolism, and growth of bacterial species [39]. At present, the antibacterial mechanism of AgNPs is mainly understood in the following two aspects [5]: on one hand, the antibacterial effect of silver nanoparticles is mainly produced by the nano effect. Some nanoparticles can adhere to the surface of the bacterial cell membrane, significantly disrupting the normal physiological functions of the cell membrane, such as permeability and respiration [40]. Some nanoparticles infiltrate bacterial cells and react with the sulfur and phosphorus of DNA and some proteins to inhibit bacterial replication, resulting in the inhibition and destruction of bacterial growth and reproduction. These AgNPs finally lead to bacterial death. On the other hand, due to the active chemical properties of AgNPs, Ag+ will form via contact with water and penetrate into bacteria. The sustained release of Ag+ will induce the production of superoxide radicals and other reactive oxygen radicals, which can lead to cell oxidation and damage to the cell membrane, thus leading to cell apoptosis or necrosis. In this work, AgBr NPs were doped into the shell of AgBr@SiO2 microspheres, so the antibacterial effect was mainly attributed to the release of Ag+.
Because of the high water content, hydrogels can be shaped easily and even fill wound sites, which is almost suitable for any kind of wound infection, providing necessary conditions for effective healing. At the same time, their biocompatibility and drug release ability are better than those of dry dressings [37, 41]. The antibacterial test in this paper indicates that the AgBr@SiO2 microspheres had good antibacterial properties. After the combination of hydrogels, the internal porous structure and water-borne channels of hydrogels enhanced the sustained release of Ag+ and maintained a longer-lasting antibacterial effect. In addition, hollow microspheres can carry a variety of drugs to promote wound healing, achieving multiple functions, which can effectively improve wound healing speed and anti-infection ability.
4. Conclusion
In this work, AgBr@SiO2 microspheres with mesoporous and hollow structures were prepared by a modified Stober method. The microspheres and gelatin were successfully modified to connect active groups and photosensitive groups, respectively, and crosslinked by UV light to prepare composite hydrogels. Functionalized AgBr@SiO2 microspheres reacted with GelMA not only enhanced the mechanical properties but also served as a drug release system to develop a new wound dressing. The antibacterial experiment results showed that the AgBr@SiO2/GelMA hydrogel had a durable, highly effective, and stable antibacterial effect. This composite hydrogel has broad application prospects in the fields of tissue infection, skin application, and drug transportation.
Acknowledgments
The Natural Science Foundation Project of CQ (cstc2018jcyjAX0711, cstc2021jcyj-msxmX0360, cstc2021jcyj-msxmX0707), Chongqing Graduate Education and Teaching Reform Research Project (yjg202036), Graduate Education and Teaching Reform Research Project of CQUST (YJG2019y003), Graduate Science and Technology Innovation Project in Chongqing University of Science and Technology (YKJCX2020228, YKJCX2020514).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.