Abstract
Perfusion-decellularization was an interesting technique to generate a natural extracellular matrix (ECM) with the complete three-dimensional anatomical structure and vascular system. In this study, the esophageal ECM (E-ECM) scaffold was successfully constructed by perfusion-decellularized technique through the vascular system for the first time. And the physicochemical and biological properties of the E-ECM scaffolds were evaluated. The bone marrow mesenchymal stem cells (BMSCs) were induced to differentiate into myocytes in vitro. E-ECM scaffolds reseeded with myocytes were implanted into the greater omenta to obtain recellular esophageal ECM (RE-ECM), a tissue-engineered esophagus. The results showed that the cells of the esophagi were completely and uniformly removed after perfusion. E-ECM scaffolds retained the original four-layer organizational structure and vascular system with excellent biocompatibility. And the E-ECM scaffolds had no significant difference in mechanical properties comparing with fresh esophagi, p > 0.05. Immunocytochemistry showed positive expression of α-sarcomeric actin, suggesting that BMSCs had successfully differentiated into myocytes. Most importantly, we found that in the RE-ECM muscularis, the myocytes regenerated linearly and continuously and migrated to the deep, and the tissue vascularization was obvious. The cell survival rates at 1 week and 2 weeks were 98.5 ± 3.0% and 96.4 ± 4.6%, respectively. It was demonstrated that myocytes maintained the ability for proliferation and differentiation for at least 2 weeks, and the cell activity was satisfactory in the RE-ECM. It follows that the tissue-engineered esophagus based on perfusion-decellularized technique and mesenchymal stem cells has great potential in esophageal repair. It is proposed as a promising alternative for reconstruction of esophageal defects in the future.
Export citation and abstract BibTeX RIS
1. Introduction
Esophageal cancer was the seventh leading cancer in the world and the sixth leading cause of cancer death, surgical resection was the main treatment method [1, 2]. Anastomotic fistula was a serious postoperative complication, with an incidence of 5%–20% and a fatality rate of 60% [2–5]. Serious anastomotic leakage not only prolonged hospital stay and decreased quality of life, but also was the main cause of postoperative death. Doctors used patients' own tissues or organs to replace the damaged esophagus in the early stage, such as the stomach [6], jejunum and pectoralis major myocutaneous flap [7, 8]. However, surgery was an extensive and complex procedure and postoperative complications were frequent, so researchers had been trying to find an ideal replacement for the artificial esophagus.
In order to reduce the occurrence of complications and preserve the function of the esophagus to the greatest extent. Much attention had been paid to tissue-engineered esophagus. Tissue-engineered esophageal materials mainly included synthetic materials and natural materials. Synthetic materials such as polylactic acid, polyglycolic acid and polyethyl lactone had poor biocompatibility, low biological activity and tended to cause aseptic inflammation [9]. The natural materials such as gelatin, hyaluronic acid, chitosan and extracellular matrix (ECM) had no immunogenicity and their degradation products were non-cytotoxic [9]. ECM had been used in the tissue engineering and regenerative medicine, such as the regeneration of tendons, dermal tissues, heart valves, bladders, among others [10–15].
Perfusion-decellularization was a mature technique to completely remove the cellular constituents and obtain a natural ECM with three-dimensional anatomical structure and vascular system. In 2008, Ott et al first proposed perfusion-decellularization, infused sodium dodecyl sulfate (SDS) and Triton X-100 into rat heart tissue [16]. The study demonstrated that the natural components of materials such as collagen, laminin and fibronectin were preserved. So we obtained the esophageal ECM (E-ECM) scaffold by perfusion-decellularization. It was different from the previous method of Intra-luminal perfusion or immersion [17–19]. They were greatly affected by tissue thickness. In this study, the reagents were transported to the tissue space around each cell through a vascular system containing the common carotid artery and its branches. To completely and uniformly removed the cellular components of each layer of tissue. And the esophageal four-layer organizational structure (mucosa, submucosa, muscularis and serosa) and the vascular system were retained. The bone marrow mesenchymal stem cells (BMSCs) were often used as seed cells in tissue engineering and regenerative medicine due to their strong proliferation and differentiation ability [20, 21]. So we selected BMSCs as seed cells, and induced BMSCs to differentiate into myocytes by 5-azacytin in vitro [22–26]. The E-ECM scaffolds reseeded with myocytes were implanted into omenta to obtain recellular esophageal ECM (RE-ECM).
2. Materials and methods
2.1. Preparation of E-ECM scaffold
Esophagi harvested from male New Zealand rabbits (12 weeks old, weight 2.0–2.2 kg) as previously shown [26]. All animal experiments are conducted in strict accordance with the Guidelines for Care and Use of Laboratory Animals and were approved by the Chengdu Medical College Animal Care and Use Committee.
Animals were anaesthetized with pentobarbital sodium (40 mg kg−1, Chronchem) by intraperitoneal injection. All steps were performed under aseptic conditions. The cervical anterior skin and muscle were made a longitudinal incision after systemic heparinization through the auricular marginal vein. Lateral sternothyroid muscles were then identified and separated. The larynx, cervical trachea, cervical esophagus, common carotid arteries, and peripheral tissues were exposed. The bilateral common carotid arteries and their branches connected to the larynx (superior thyroid arteries and superior laryngeal arteries) were retained, and the superior and inferior ends of the bilateral common carotid arteries were ligated. The cervical esophagus with trachea, larynx, and the common carotid arteries were isolated holistically, which was moved to a super-clean bench. The harvested E-ECM scaffold via perfusion-decellularized technique with one common carotid artery as previously described [16]. Briefly, one transfusion needle was inserted into the common carotid artery, which was connected to a catheter controlled by a infusion pump. Heparinized phosphate-buffered saline (PBS, Sigma) containing adenosine (Sigma) was perfused for 15 min. This was followed by 16 h perfusion of 1% SDS (Biofroxx) in deionized water, 15 min perfusion of deionized water, 30 min perfusion of 1% Triton-X100 (Biofroxx) in deionized water. The next step involved extensive perfusion with antibiotic-containing PBS (100 U ml−1 penicillin-G, 100 U ml−1 streptomycin) for 48 h to remove any residual detergent and cellular debris. The perfusion pressure of supporting artery was maintained at 105.5 mmHg. During perfusion, the entire tissue was placed on a 200-mesh cuprum grid for filtering. The E-ECM scaffolds were placed in sterilized PBS solution for further detection.
2.2. DNA quantification
The double-stranded DNA (dsDNA) was extracted from the E-ECM scaffolds using the Universal Genomic DNA Extraction Kit (CWBIO, Beijing, China), then the dsDNA was quantified using the 2xES Taq MasterMix (Dye) kit (CWBIO, Beijing, China). The DNA fragments were observed by agarose gel electrophoresis (2%, W/V). Fresh esophagi as the control group, n = 3.
2.3. Histological and immunofluorescence analysis
Histology and immunofluorescence staining were performed to analyze the structural changes before and after esophageal perfusion. The fresh esophagi and E-ECM scaffolds were fixed in 4% paraformaldehyde for 24 h, embedded in paraffin, and cut into 5 μm thick sections. Histological analysis was performed using hematoxylin and eosin (H&E) staining. Immunofluorescence staining was performed according to manufacturer's instructions. The primary antibodies used were Major Histocompatibility Complex Class II Antibody (dilution 1:300), Rabbit Anti-collagen I Antibody (dilution 1:100), Rabbit Anti-collagen III Antibody (dilution 1:100), and Rabbit Anti-CD34 Antibody (dilution 1:200). The secondary antibody was CoraLite488-conjugated Affinipure Goat Anti-Rabbit IgG (dilution 1:100). The above antibodies were purchased from Bioss (Beijing, China). Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay for the detection of apoptotic cells in the RE-ECM was performed with the In Situ Apoptosis Detection Kit (Roche Applied Science) according to the manufacturer's protocol. Finally, 40,6-diamidino-2-phenylindole (DAPI) was used for nuclear counterstaining. Cells that stained positive were counted in at least five different fields at ×400 magnification and the mean percentage of positive cells was calculated.
2.4. Scanning electron microscopy (SEM)
The morphologies of E-ECM scaffolds were observed by a Hitachi SU8100 scanning electron microscope. The samples (n = 3) were fixed with 2.5% glutaraldehyde for 24 h at room temperature, dehydrated by a graded series of ethanol (75%, 85%, 100%) for 20 min each, and then dried by lyophilization for 24 h. These samples were sputter coated with gold, and SEM images were captured. The sample porosity were evaluated from SEM images using Image Pro-Plus 6.0 software.
2.5. Vascular imaging
To enhance the visibility of E-ECM scaffold's vascular system, red ink was injected into the common carotid artery (Deli Stationery, China) to image scaffold's vascular system [27].
2.6. Biomechanical evaluation
A tensile strength test was carried out with 3 cm long E-ECM scaffolds by computing the maximum stress in the longitudinal axis (n = 3). Mechanical properties were measured using a universal testing machine under a crosshead speed of 10 mm min−1. The material remained hydrated throughout the testing procedure.
2.7. Biological compatibility
The 1 cm long annular E-ECM scaffolds were implanted into the omenta and subcutaneous tissues of the rabbits respectively (n = 3). The animals were sacrificed after 2 weeks and 6 months post-implantation of fresh esophagi and E-ECM scaffolds. And the samples were collected and immobilized in 4% paraformaldehyde, embedded in paraffin, sliced the cross section, stained with H&E, and observed under an optical microscope.
2.8. Culture and induction of BMSCs
Nine male New Zealand rabbits (4 weeks old) were anaesthetized with pentobarbital sodium (40 mg kg−1, i.p. Chronchem). Six rabbits were used as receptors, to prepare for the next step of injecting myocytes into E-ECM scaffolds and autologous implantation into the omenta, the other three rabbits were used for other tests. And the BMSCs from each rabbits were cultured separately. The harvest site of the iliac crest was disinfected. The bone marrow was extracted from the ventral iliac crest with an 18-point needle.
The BMSCs isolated and cultured as previously described [26] Heparinized BMSCs were suspended in PBS. Mononuclear cells were isolated by centrifugation using a Histopaque density gradient (Sigma). And cells were resuspended in Dulbecco's modified eagle medium (DMEM, Gibco), finally cultured in DMEM supplemented with 15% fetal bovine serum (FBS, Hyclone), 100 U ml−1 penicillin, 100 U ml−1 streptomycin (Hyclone), and 2 mol L−1 L-glutamine (Sigma). Cultures were maintained in an incubator at 37 °C with 5% carbon dioxide. Media were changed every 3 days. The recipient rabbits were treated with penicillin for 1 week and kept sterile postoperatively. At 80% confluence, primary cells were washed twice by PBS and cultured with 5-azacytidine (10 µmol L−1, Sigma) for 24 h to differentiate into myocytes. Cells were digested with 0.25% trypsin–EDTA (Sigma) and passaged at a ratio of 1:3. The induced cells from the third to fifth generations were used. Actin expression in cells was detected by immunocytochemistry with α-sarcomeric actin antibody.
2.9. Cytotoxicity assay
BMSCs cultured in E-ECM scaffold extracts were compared with cultured in DMEM (containing 10% FBS and 1% penicillin-streptomycin) without extract. The extracts were obtained by immersing 1.5 mg of the E-ECM scaffold in 150 µl of DMEM (containing 10% FBS and 1% penicillin-streptomycin) for 7 and 14 days, respectively [28]. When the density reached 70%–80% confluency, the BMSCs were digested and inoculated into 96-well plates, at a density of 5000 cells/well. After for 24 h, the Media were replaced with DMEM (containing 10% FBS and 1% penicillin-streptomycin) or E-ECM scaffold extracts. After culturing the cells for 24 h, 48 h, and 72 h, respectively, 10 µl of Cell Counting Kit-8 (CCK-8) reagent (Biosharp) was added into each well and incubated at 37 °C for 2 h. Each group had five wells and the experiment was repeated three times. The absorbance was measured at a wavelength of 450 nm with a Gen5 microplate reader (BioTek, Winooski, VT, USA).
2.10. Cell seeding efficiency and adhesion ability
The third generation of induced cells were digested and suspended in DMEM containing 15% FBS at a concentration of 90 × 106 cells ml−1. Cells were multi-point microinjected into (20 µl each) into 5 mm long annular E-ECM scaffolds (n = 3). The scaffolds were reseeded with myocytes and then cultured in an incubator at 37 °C with 5% CO2, the remaining cells in the media were collected and quantified. And the growth of the cells on scaffolds was observed by a Hitachi SU8100 scanning electron microscope after 24 h of culture. Cells seeding efficiency at 24 h was calculated based on the formula: cell seeding efficiency (%) = (total cell number − remaining cell number)/total cell number × 100% [29].
2.11. Recellularization of E-ECM scaffold
The BMSCs from each recipient rabbit were cultured separately, the third generation of induced cells were collected and suspended in DMEM containing 15% FBS at a concentration of 90 × 106 cells ml−1. Cells were multi-point microinjected into 5 mm long annular E-ECM scaffolds (20 µl each). The scaffolds reseeded with cells were cultured in an incubator at 37 °C with 5% CO2 for 24 h, and then implanted into the omenta for growth (n = 3). After 1 week and 2 weeks, recipient rabbits were sacrificed respectively and RE-ECM were obtained for H&E staining and immunohistochemistry analysis.
2.12. Immunocytochemistry and immunohistochemistry analysis
Paraffin sections were dewaxed three time in xylenes for 10 min each. Rehydrated with a series of decreasing alcohol gradients, and rinsed in PBS. Sections were incubated in antigen retrieval solution (10 mM sodium citrate, 0.05% Tween 20, pH 6.0), heated, and naturally cooled to room temperature, rinsed three times in PBS. And then incubated in blocking solution for 1 h. Sections were exposed to primary antibody to α-sarcomeric actin antibody (1:300 dilution, mouse monoclonal antibody, skeletal muscle Ab-2, Thermo, USA) for 1 h at 37 °C. After rinsing, sections were incubated with the secondary antibody (1:100 dilution, goat antimouse IgG; China). Followed by the incubation with avidin/horseradish peroxidase for 30 min. Then reacted with the diaminobenzidine chromogenic substrate at room temperature for 5 min. Followed by a counterstain with hematoxylin. Cell slides were permeabilized with 0.2% Triton X-100 for 10 min at room temperature, and then treated as described above.
2.13. Statistical analysis
All quantitative data are expressed as means ± standard deviation. After confirming that the data conforms to the normal distribution, statistical analyses included one-way analysis of variance with a Tukey post-hoc test, or unpaired t test. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software (IBM SPSS version 26).
3. Results
3.1. Fabrication and characterization of E-ECM scaffold
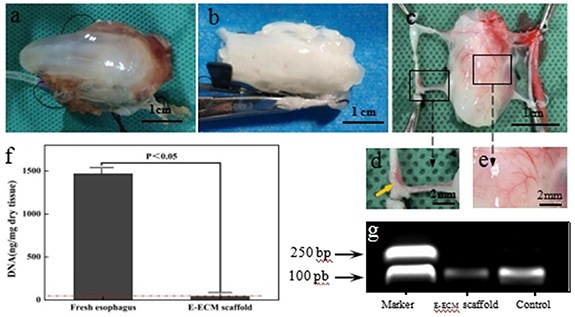
As macroscopically observed, the fresh esophagus was flesh-colored. The tissue became translucent and swollen during perfusion (figure 1(a)). After perfusion, the tissue collapsed, was white with loose structure (figure 1(b)). The fresh esophagus contained 1468 ± 103 ng dsDNA mg−1 dry tissue, which greater than 41 ± 7 ng dsDNA mg−1 dry tissue found in E-ECM scaffold (figure 1(f), p < 0.05). Approximately 94.3% of the DNA was removed after perfusion. After electrophoresis, no DNA fragments in E-ECM scaffold were presented more than 200 base pairs, while a range of DNA fragments were observed in fresh esophagus (figure 1(g)). Vascular imaging study showed that the common carotid arteries and arteriole networks of the esophagus (figures 1(c)–(e)), the ink from the right common carotid artery flowed to the left (figure 1(d)). And the vascular system remained intact in the E-ECM scaffold (figures 1(c)–(e)).
Figure 1. Characterization of esophageal extracellular matrix (E-ECM) scaffold. The tissue became translucent and swollen during perfusion (a). After perfusion, the tissue collapsed, was white with loose structure (b). Vascular imaging study showed that the red ink oozed from the end of the vessels, and the arterioles of the esophagus can be clearly seen, the vascular system remained intact in the E-ECM scaffold (c)–(e). Yellowed arrow in d indicated that the ink from the right common carotid artery flowed to the left. The fresh esophagus contained 1468 ± 103 ng dsDNA mg dry tissue, which greater than 41 ± 7 ng dsDNA mg dry tissue found in E-ECM scaffold, p < 0.05 (f). After electrophoresis, no DNA fragments in E-ECM scaffold were presented more than 200 base pairs, while a range of DNA fragments were observed in fresh esophagus (g).
Download figure:
Standard image High-resolution image3.2. Histological and immunofluorescence analysis
We recorded images with a biomicroscope equipped with a Leica microsystem (DM4000B, Leica, Germany). The H&E staining showed that the fresh esophagus was composed of four layers: mucosa, submucosa, muscularis and serosa (figure 2(a)). The organizational structure of the E-ECM scaffold were clearly visible, and no residual nuclei or cells were present (figure 2(d)). Immunofluorescence analysis showed that the immunogenicity of E-ECM scaffold were completely and uniformly removed compared with fresh esophagus (figures 2(e) and (f)). There was almost no residual membranal and nuclear component. However, we observed that collagen I and collagen III remained in the E-ECM scaffold (figures 2(i) and (j)).
Figure 2. Histology and immunofluorescence staining were performed to analyze the structural changes before and after esophageal perfusion. The H&E staining showed that the fresh esophagus was composed of four layers: mucosa, submucosa, muscularis and serosa (a). The organizational structure of the E-ECM scaffold were clearly visible, and no residual nuclei or cells were present (d). Immunofluorescence analysis (b), (c), (e), (f) showed the presence or absence of MHC Class II (green) and nuclei (blue). The collagen I and collagen III were present in the fresh esophagus (g), (h) and E-ECM scaffold (i), (j).
Download figure:
Standard image High-resolution image3.3. SEM
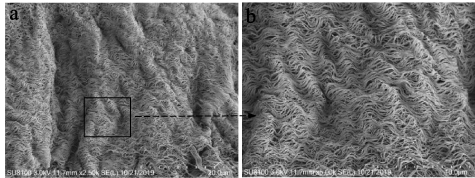
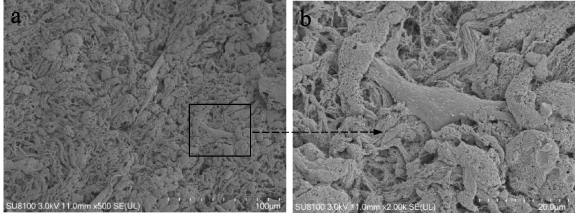
The microstructure of the samples were observed by a scanning electron microscope. In the E-ECM scaffold constructed by perfusion-decellularization, the fiber components and fiber orientation were retained, while the cells in the tissue were completely and uniformly removed to provide space for the cells to regenerate (figures 3(a) and (b)). The average porosity of the E-ECM scaffold was 61.92 ± 3.87%.
Figure 3. The microstructure of the E-ECM scaffold was observed by a scanning electron microscope. The E-ECM scaffold constructed by perfusion-decellularization, the fiber components and fiber orientation were retained, while the cells in the tissue were completely and uniformly removed to provide space for the cells to regenerate (a), (b).
Download figure:
Standard image High-resolution image3.4. Biomechanical properties
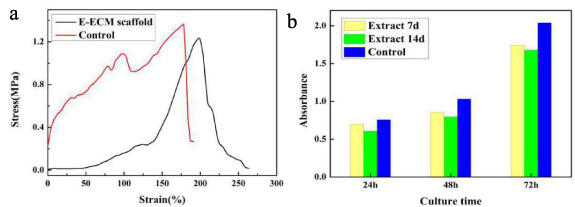
The maximal elongation percentage of the fresh esophagus was 178.18 ± 39.71% at a ultimate tensile strength of 1.36 ± 0.52 MPa. The maximal elongation percentage of the E-ECM scaffold was 198.35 ± 43.06% at a ultimate tensile strength of 1.23 ± 0.65 MPa. Based on these values, there was no significant difference in tensile strength between E-ECM scaffold and fresh esophagus (figure 4(a), p > 0.05).
Figure 4. Biomechanical evaluation and CCK-8 assay. (a) There was no significant difference in tensile strength between E-ECM scaffold and fresh esophagus, p > 0.05. (b) The data from the CCK-8 assay after culturing BMSCs for 24 h, 48 h and 72 h of incubation. The number of BMSCs gradually increased in extracts which obtained by the E-ECM scaffold immersion for 7 and 14 days, there was no significant difference compared with the control group (p > 0.05). The yellow bars represented extracts obtained by the E-ECM scaffold immersion for 7 days. The green bars represented extracts obtained by the E-ECM scaffold immersion for 14 days. The blue bars were control group.
Download figure:
Standard image High-resolution image3.5. Biocompatibility
In the fresh esophagus group, after implanting subcutaneously, the skin around the wound was obviously red and swollen. Two weeks later, H&E staining suggested severe rejection. A large number of inflammatory cells infiltrated into the fresh esophagus, mainly in the muscularis, and the myocytes were necrotic (figures 5(a) and (b)). After implanting into omentum, the wound gradually healed. One animals' wound opened, but the abdominal cavity closed. Two week later, the abdominal cavity was opened and severe congestion and edema of the omentum were observed. H&E staining revealed that inflammatory cell infiltration in fresh esophagus too, mainly in the muscularis, accompanied by myocytes necrosis (figures 5(c) and (d)).
Figure 5. The esophagus were implanted into subcutaneous and omentum, and the biocompatibility was observed by H&E staining. After the fresh esophagus was implanted subcutaneously for 2 weeks, a large number of inflammatory cells infiltrated into the fresh esophagus, mainly in the muscularis, and the myocytes were necrotic (a), (b). After greater omentum implantation for 2 weeks, inflammatory cells infiltration in the fresh esophagus too, mainly in the muscularis accompanied by myocytes necrosis (c), (d).
Download figure:
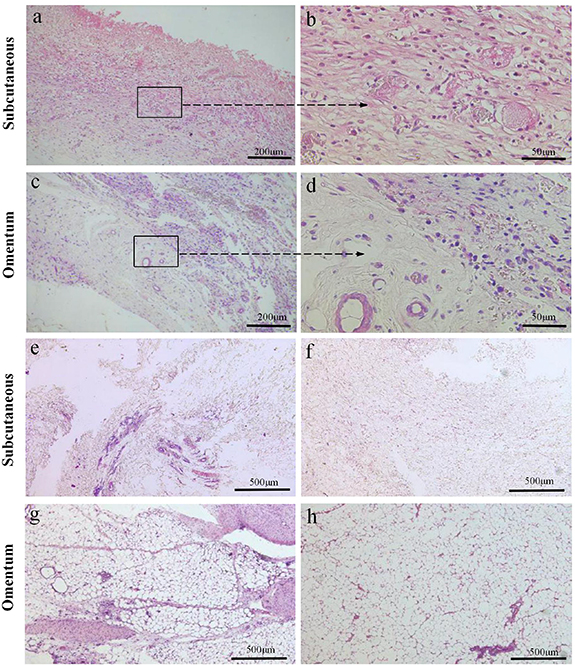
Standard image High-resolution imageIn the E-ECM scaffold group, the wound healed well after operation. Gross observation showed that after the subcutaneous implantation for 2 weeks of E-ECM scaffold, the surrounding tissue wrapt it. Six months later, scaffold disappeared. H&E staining showed that the organizational structure of E-ECM scaffold were unclear after 2 weeks of subcutaneous implantation. And fresh blood vessels appeared and inflammatory cells exuded (figures 6(a) and (b)). After 6 months, there were no residual scaffold components, subcutaneous fibrous connective tissue proliferation (figure 6(e)). After E-ECM scaffold implanted into the omentum for 2 weeks, it remained annular and surrounded by the greater omentum. After 6 months, scaffold disappeared and the connective tissue of greater omentum proliferated. H&E staining showed that after implantation for 2 weeks, the organizational structure was not discernible. At the same time, there was obvious inflammation in the scaffold (figures 6(c) and (d)). Six months later, scaffold was completely degraded, and the connective tissue hyperplasia in the greater omentum was obvious (figure 6(g)).
Figure 6. H&E staining was performed to evaluate the biocompatibility of the E-ECM scaffolds. The organizational structure of E-ECM scaffold were unclear after 2 weeks of subcutaneous implantation. Fresh blood vessels appeared and inflammatory cells exuded (a), (b). After E-ECM scaffold implanted into the omentum 2 weeks, the organizational structure was not discernible, fresh blood vessels also appeared, and inflammatory cells oozed out (c), (d). The E-ECM scaffold was completely degraded after the E-ECM scaffold was implanted subcutaneously for 6 months, there were no residual scaffold components, subcutaneous fibrous connective tissue proliferated (e). Normal subcutaneous tissue (f). After the E-ECM scaffold implantation of greater omentum for 6 months, scaffold was completely degraded, fibrous connective tissue of greater omentum proliferated distinctly (g). Normal greater omentum (h).
Download figure:
Standard image High-resolution image3.6. Characterization of BMSCs and the induced cells
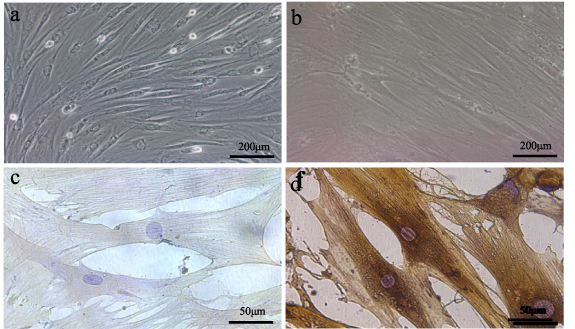
The primary BMSCs were fully adherent after 72 h, and the cells were short spindle, polygon, and irregular. About 2 weeks later, the first passage was performed at 80% cell confluency. The third generation BMSCs displayed fusiform morphology, and the cells were arranged in a spiral shape (figure 7(a)). And the third generation of induced cells were long fusiform, polar and similar to the myocytes (figure 7(b)). Immunocytochemistry analysis showed that the uninduced cells did not express α-sarcomeric actin (figure 7(c)), and the third generation cells successfully differentiated into myocytes, expressed α-sarcomeric actin (figure 7(d)).
Figure 7. The characterization of BMSCs and the induced cells. The third generation BMSCs displayed fusiform morphology, and the cells were arranged in a spiral shape (a). And the third generation of induced cells were long fusiform, polar and similar to the myocytes (b). Immunocytochemistry analysis showed that the uninduced cells did not express α-sarcomeric actin (c), and the third generation cells successfully differentiated into myocytes, expressed α-sarcomeric actin (d).
Download figure:
Standard image High-resolution image3.7. Cytotoxicity assay
After culture for 24 h, 48 h and 72 h of incubation, the number of BMSCs gradually increased in extracts which obtained by the E-ECM scaffold immersion for 7 and 14 days, there was no significant difference compared with the control group (figure 4(b), p > 0.05). These results suggested that no cytotoxicity was observed in the prepared scaffolds.
3.8. Cell seeding efficiency and adhesion ability
The E-ECM scaffold of reseeded cells was pale pink. The seeding efficiency of the cells was 54 ± 1.76%, indicating that the myocytes could adhere to E-ECM scaffold. SEM images showed that after 24 h of culture in vitro, myocytes could effectively adhere to and proliferate on E-ECM scaffold (figures 8(a) and (b)). They were further confirmed that E-ECM scaffold had good biocompatibility and no cytotoxicity.
Figure 8. SEM images showed that after 24 h of culture in vitro, myocytes could effectively adhere to and proliferate on E-ECM scaffold (a), (b).
Download figure:
Standard image High-resolution image3.9. Recellularization of E-ECM scaffold
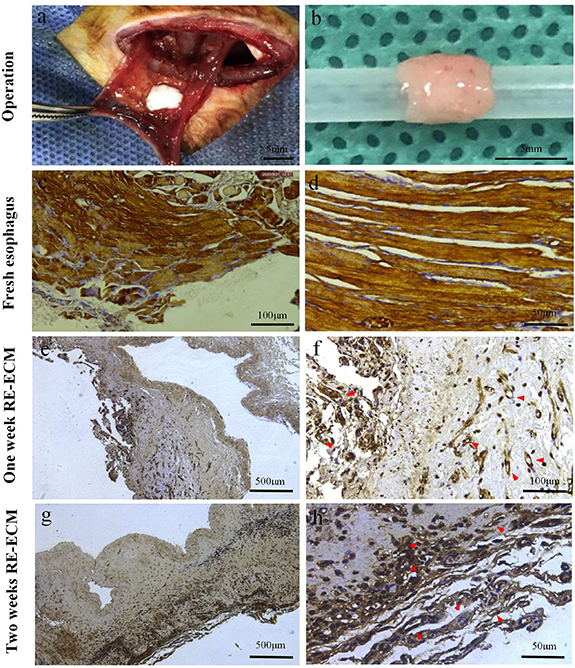
After 2 weeks of growth in the greater omenta, the RE-ECM retained its original shape, with a smooth, light salmon surface and an unblocked lumen (figure 9(b)). Immunohistochemistry analysis revealed that the hierarchies of RE-ECM were preserved, and the cells in RE-ECM were positive for α-sarcomeric actin expression, and confirmed the growth of muscle-like tissue and fresh blood vessels into RE-ECM, mainly clustered in the injection area and the muscularis of the E-ECM scaffold. In the first week, myocytes in the muscularis were arranged and regenerated in one direction, and new blood vessels could be seen (figures 9(e) and (f)). In the second week, the myocytes regenerated linearly and continuously and migrated to the deep, and the tissue vascularization was obvious. (figures 9(g) and (h)). The results showed that the BMSCs could differentiate into myocytes and continued to proliferate in vivo, the scaffold provided favorable conditions for the growth of myocytes, but their appearance was slightly different from the nature skeletal muscle (figures 9(c) and (d)). However, the E-ECM scaffold without recellularization grew in the greater omentum for 2 weeks, the organizational structure were not discernible. At the same time, there was obvious inflammation in the scaffold and no myocytes growth (figures 6(c) and (d)).
Figure 9. Recellularization of E-ECM scaffold. The operation of implanting the recellular esophageal extracellular matrix (RE-ECM) into the omentum (a). After 2 weeks of growth in the greater omenta, the RE-ECM retained its original shape, with a smooth, light salmon surface and an unblocked lumen (b). Micrographs showed immunocytochemistry staining for α-sarcomeric actin (d)–(j). Natural skeletal muscle of fresh esophagus (c), (d). In the first week, myocytes in the muscularis were arranged and regenerated in one direction, and new blood vessels could be seen (e), (f). In the second week, the myocytes regenerated linearly and continuously and migrated to the deep, and the tissue vascularization was obvious (g), (h). Red arrows in h and j indicated the new blood vessels.
Download figure:
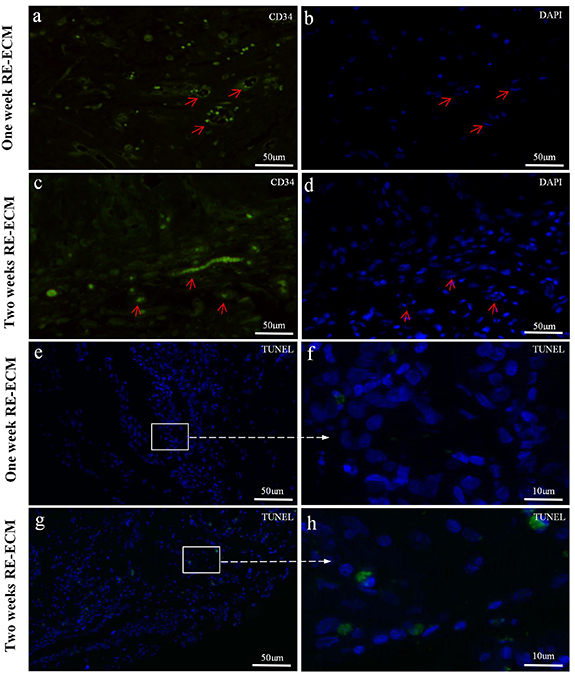
Standard image High-resolution imageAnd immunofluorescence analysis showed the presence of CD34-positive in 1 week (figures 10(a) and (b)) and 2 weeks RE-ECM (figures 10 (c) and (d)). The red arrows represented neovascularization, the endothelial nuclei were long fusiform. CD34 was expressed by vascular endothelial cells, demonstrating fresh angiogenesis in RE-ECM. In TUNEL assay, apoptotic nuclei were green stained and normal nuclei were blue stained in the 1 week (figures 10(e) and (f)) and 2 weeks RE-ECM (figures 10(g) and (h)) in vivo. The cell survival rates at 1 week and 2 weeks were 98.5 ± 3.0% and 96.4 ± 4.6%, respectively. It was demonstrated that myocytes maintained the ability for proliferation and differentiation for at least 2 weeks, and the cell activity was satisfactory in the RE-ECM.
Figure 10. Immunofluorescence analysis showed the presence of CD34 in 1 week (a), (b) and 2 week RE-ECM (c), (d) in vivo, with the red arrow indicating neovascularization, and long fusiform endothelial nuclei. In TUNEL assay, apoptotic nuclei were green stained and normal nuclei were blue stained in the 1 week (e), (f) and 2 week RE-ECM (g), (h) in vivo.
Download figure:
Standard image High-resolution image4. Discussion
In this study, the E-ECM scaffold was successfully constructed by perfusion-decellularized technique through the vascular system for the first time. We used the E-ECM scaffold with the original organizational structure and vascular system to promote the cells regeneration and tissue vascularization, obtained the RE-ECM. RE-ECM was allogeneic, which could use for repairing esophageal defect and avoided the immune effect of artificial materials and the second damage. It could preserve the organ functions to the maximum extent, reduce the occurrence of complications, and improve the postoperative life quality of patients.
The E-ECM scaffold prepared in this study had the following four characteristics: (a) retained the original four-layer organizational structure and vascular system, (b) excellent biocompatibility, (c) there was no significant difference in mechanical properties between fresh esophagus and E-ECM scaffold, and (d) In the RE-ECM scaffold, the myocytes regenerated linearly and continuously with satisfactory cell survival, myocytes migrated to the deep, and the tissue vascularization was obvious.
In animal models and preclinical studies, lamellar non-full-layer E-ECM in esophageal repair have achieved satisfactory results and can reduce the occurrence of postoperative stenosis [30]. In contrast, full-thickness reconstruction after esophagectomy remains a challenge. Recellular matrix induction of esophageal full-thickness regeneration and restoration of its function is currently the most promising method [31]. We obtained the E-ECM scaffold via perfusion-decellularization. Both H&E staining and immunofluorescence substantiated the absence of cells and residual nuclei. We could observe that the esophageal four-layer organizational structure and the vascular system were retained in E-ECM scaffold. The vascular system provides the necessary nutrition and a place for material exchange. Vascular imaging study showed that the small arteries of the esophagus were connected to each other to form a network, the ink from the right common carotid artery flowed to the left, which proved that the vessels inside the E-ECM scaffold were intact and the left and right arteries were connected. This structure was different from the layered vascular tissue produced by 3D bioprinting, which had remained a long-standing technical challenge for 3D bio-printing [32].
ECM proteins are among the most conserved proteins [31]. The immunogenicity of tissues is mainly related to cells, cell membrane antigens can cause hyperacute rejection after organ transplantation, remnants of the nuclei or other cellular components can lead to macrophage activation and induce metastasis, which are characteristics of the host inflammatory response [33–35]. Therefore, the primary condition for tissue transplantation is complete decellularization of the donor tissue. In present study, the biocompatibility and immunogenicity of the materials were directly determined by the degree of the tissue decellularation. The fresh esophagus contained 1468 ± 103 ng dsDNA mg dry tissue, which greater than 41 ± 7 ng dsDNA mg dry tissue found in E-ECM scaffold, approximately 94.3% of the DNA was removed, and no DNA fragments greater than 200 base pairs after perfusion-decellularization, confirmed by electrophoresis. Meeting valid decellularized criteria: having less than 50 ng dsDNA mg weight dry tissue, less than 200 base pairs in size in all residual DNA fragments, and a lack of visible nuclei when stained with DAPI or hemotoxylin [36]. However, we observed that collagen I and collagen III remained in the E-ECM scaffold. SEM again confirmed that the cells of the esophagus were completely and uniformly removed after perfusion through the vessel. And the average porosity of the E-ECM scaffold was 61.92 ± 3.87%. The E-ECM scaffolds were gradually degraded and disappeared after implantation for 6 months, were consistent with result published by Gilbert and Record et al [37, 38], confirmed the excellent biocompatibility of the scaffold. CCK-8 assay data showed that the cells proliferated in the E-ECM scaffold extract, there were no significant difference compared with the control group, which proved that the prepared E-ECM scaffold had no cytotoxicity.
The seeding efficiency of the cells was 54 ± 1.76%, and the E-ECM scaffold provided the cells with a suitable microenvironment for adhesion and growth. SEM showed that myocytes could effectively adhere and proliferate on E-ECM after reseeding for 24 h. After recellularizing of E-ECM scaffolds, immunohistochemistry analysis showed that the myocytes regenerated linearly and continuously and migrated to the deep, and the tissue vascularization was obvious. The cell survival rates at 1 week and 2 weeks were 98.5 ± 3.0% and 96.4 ± 4.6%, respectively. It was demonstrated that myocytes maintained the ability for proliferation and differentiation for at least 2 weeks, and the cell activity was satisfactory in the RE-ECM. This linear continuous growth trend of myocytes is more consistent with the physiological structure of the natural esophagus. Compared with the E-ECM scaffold without recellularization, which grew in the omentum for 2 weeks, inflammation was obvious in the scaffold and no myocytes were formed. And RE-ECM was positive for CD34, which was expressed by vascular endothelial cells, demonstrating fresh angiogenesis in RE-ECM again. And the germination of neovascularization might be related to the retained vascular system within the E-ECM scaffold [39]. In addition, well mechanical properties could withstand the pressure exerted by food masses and retained the swallowing function of organ. Especially fine tensile strength played an important role in reducing anastomotic leakage. Mechanical properties test results confirmed that there was no significant difference in the maximum tensile strength between E-ECM scaffold and fresh esophagus. Although the shape of the toe region of the stress–strain curve was quite different between fresh esophagus and E-ECM scaffold, this did not define the scaffold mechanical performance was not good, it was just the result of decellularization. E-ECM scaffolds became soft and ductile because the removal of cellular components and the formation of place allowing cell regeneration. The result was that a large strain occurred at a small tension and a rapid rose in stress near the maximum elongation until fractured.
There were also some problems in our experiment. The esophagus has four-layer organizational structure include mucosa, submucosa, muscularis and serosa. Myocytes are the main cells, and endothelial cells and fiber cells are also its cell components. Esophageal reconstruction requires multicellular involvement to make the tissue closer to the natural physiological structure. In the future research, we will establish animal models for reconstruction of the esophagus based on this consideration.
5. Conclusion
In this study, E-ECM scaffold was successfully constructed by perfusion-decellularized technique through the vascular system for the first time. The cells of the esophagi were completely and uniformly removed after perfusion. E-ECM scaffolds retained the original four-layer organizational structure and vascular system with excellent biocompatibility. And the E-ECM scaffolds had no significant difference in mechanical properties comparing with fresh esophagi. Myocytes regenerated linearly and continuously and migrated to the deep, and the tissue vascularization was obvious. In the RE-ECM, myocytes maintained the ability of proliferation and differentiation for at least 2 weeks with satisfactory cell survival rate. This linear continuous growth trend of myocytes is more consistent with the physiological structure of the natural esophagus. In summary, the tissue-engineered esophagus based on perfusion-decellularized technique and mesenchymal stem cells has great potential in esophageal repair. It is proposed as a promising alternative for reconstruction of esophageal defects in the future.
Acknowledgments
We acknowledge our funding sources for the generous support from Project of Education Department of Sichuan Province (No. 16ZB0274), and Project of Science and Technology Department of Sichuan Province (No. 19ZDYF0944).
Data availability statement
All data that support the findings of this study are included within the article.
Conflict of interest
The authors declare that they have no competing interests.