Abstract
Akermanite (Aker) has been widely used for bone regeneration through regulating osteogenesis of bone marrow-derived mesenchymal stem cells (BMSCs). Previously, we developed an injectable Aker/sodium alginate (Aker/SA) hydrogel to facilitate bone regeneration. However, the effect of this injectable hydrogel on the in vivo response, particularly the inflammatory response, has not been fully understood. Here, to elucidate the response following the implantable of Aker/SA hydrogel, we investigated the interaction among Aker/SA hydrogel, inflammatory cells and cells involved in bone regeneration (BMSCs). Specifically, we cultured macrophages (RAW 264.7 cell line) with the extract liquid of Aker/SA and assessed their phenotypic changes. Subsequently, BMSCs (2 × 105 cells per 24 well) were cultured with different conditioned media including that of Aker/SA hydrogel-activated macrophages to investigate their effect on cell migration. Finally, Aker/SA hydrogel was injected subcutaneously (1 × 106 cells ml−1) in rat to verify its effect in vivo. The in vitro results indicated that Aker/SA hydrogel activated macrophages towards M2 phenotype and stimulated macrophages to express anti-inflammatory factors. In addition, the conditioned medium collected from Aker-activated macrophages could accelerate the migration of BMSCs in 24 h. Consistent with the in vitro results, when the Aker/SA hydrogel was injected subcutaneously, more M2 macrophages could be observed than when the SA solution was injected after 7 d. Besides, when BMSCs were delivered via subcutaneous injection, more BMSCs were recruited by the Aker/SA hydrogel than the SA solution. All these results suggest that the Aker/SA hydrogel can modulate the immune environment at the implantation site and subsequently recruit BMSCs, which can be one of the mechanisms through which the Aker/SA hydrogel accelerates new bone formation.
Export citation and abstract BibTeX RIS
1. Introduction
A variety of natural biomaterials is characterized by good biocompatibility and can accelerate tissue regeneration. Among these, it has been shown that silicate based biomaterials such as bioactive glass and akermanite (Ca2MgSi2O7, Aker) can promote bone regeneration [1, 2]. In particular, Aker has received much attention because of its controllable degradation rate and favorable mechanical properties [3]. Previous studies have shown that bone marrow-derived mesenchymal stem cells (BMSCs) as well as osteoblasts exhibited enhanced proliferation and osteogenesis when cultured with Aker [3]. Our previous study also demonstrated the osteogenic ability of Aker both in vitro and in vivo [3]. In that study, sodium alginate (SA) was combined with Aker and glutamic acid (Glu) to form an injectable hydrogel for the regeneration of bone defects. The extracts of Aker/SA hydrogel could enhance the migration of BMSCs and stimulate the osteogenic differentiation of BMSCs. However, the in vivo response following the implantable of Aker/SA hydrogel has not been fully elucidated yet.
Implantation of a biomaterial into human body is known to induce a host reaction which can influence the outcome of integration and performance of the implant [4]. It is increasingly being recognized that the host-biomaterial interaction can play important roles in tissue remodeling and regeneration [5]. For example, biomaterials can regulate macrophage polarization to present a proinflammatory (M1) or anti-inflammatory (M2) phenotype [6], which in turn can alter the cytokines and chemokines secreted by the macrophages. The modulation of macrophages by biomaterials has been shown to be critical for successful tissue regeneration [7].
As one of the most important inflammatory cells, macrophages can modulate a series of biological reactions by secreting growth factors and inflammatory cytokines [8]. They are classically activated to display a M1 phenotype by well-known proinflammatory signals, such as IFN-γ and LPS, and produce proinflammatory cytokines such as IL-1β and IL-6 [8, 9]. Alternatively, they can be activated by molecular cues such as IL-4 and IL-13 to present a M2 phenotype and secret cytokines such as IL-10 [8, 10]. M1 macrophages have microbicidal activity while M2 macrophages have been shown to be responsible for eliciting an immunomodulatory response, matrix deposition, tissue repair and reconstruction [11, 12]. This suggests macrophage polarization could be one of the critical steps in biomaterial-mediated tissue regeneration.
In this study, we aim to investigate the interaction among Aker/SA hydrogel, inflammatory cells and cells involved in bone regeneration (BMSCs). Our hypothesis is that inflammatory cells, in particular macrophages, are involved in the response of BMSCs following the implantation of Aker/SA hydrogel.
To prove our hypothesis, the effects of Aker/SA hydrogel on the behaviors of macrophages, especially their polarization, were first evaluated, followed by an analysis on the cytokines, proteases and chemokines secreted by the macrophages. Then, the effects of the Aker/SA hydrogel-activated macrophages on the migration of BMSCs were assessed. Finally, animal experiments were performed to confirm the influence of Aker/SA hydrogel on the polarization of macrophages as well as on the recruitment of BMSCs in vivo.
2. Materials and methods
2.1. Preparation and characterization of Aker/SA hydrogel and its extracts
SA from brown algae (medium viscosity; Sigma) was dissolved in deionized water to produce a 2% (w/v) solution. Aker powders with an average diameter of 20 µm were provided by the Shanghai Institute of Ceramics, Chinese Academy of Science. Prior to experiment, the SA solution was sterilized with a 0.22 µm filter (Millipore) and stored at 4 °C while the Aker powders and Glutamate (Glu Sigma) powders were sterilized by ultraviolet light. The injectable Aker/SA hydrogel was produced according to the procedures described in figure 1(A). Briefly, 5 ml of the SA solution was first loaded into an injector which was connected to a t-branch pipe, and 0.1 g Aker powders and 0.05 g Glu powders were added into the injector. The SA solution, Aker powders and Glu powders were then mixed in the t-branch pipe and the complete gelation occurred in 5 min at room temperature to produce a hydrogel containing 2% w/v of Aker. Aker/SA hydrogel extracts were prepared according to the procedures reported in a previous study [3]. Briefly, 5 ml Aker/SA hydrogel was soaked in 20 ml serum-free Dulbecco's Modified Eagle Medium (DMEM, Gibco) at 37 °C with 5% CO2. 24 h later, the supernatant was collected and sterilized with a 0.22 µm filter. The obtained medium was labeled as Aker/SA hydrogel extracts and stored at 4 °C for further use.
Figure 1. (A) The formation of the Aker/SA injectable hydrogel. (B) The procedures of obtaining conditioned media. (C) The protocol of using Transwell to evaluate the migration of BMSCs. (D) The schematic of the in vivo experiment.
Download figure:
Standard image High-resolution imageTo measure the pH in the aqueous environment, ions released behavior and the mass loss of the Aker/SA hydrogel, 1 ml crosslinked hydrogel was soaked in 5 ml PBS solution and 0.02 g pure Aker powders was also soaked in 5 ml PBS solution for measuring the pH values using a pH meter. The extract liquid was collected at designated time points and the ions concentration was measured by inductively coupled plasma atomic emission spectroscopy (Optima 3000DV, Perkin Elmer, USA). At each time point, the weight of the Aker/SA hydrogel was measured. Three independent experiments were carried out. The images of Aker/SA hydrogel at day 0 and day 7 were taken. Finally, SEM images were also taken after the hydrogel was frozen-dried (Hitachi S-4800, Japan).
2.2. Cell isolation and culture
RAW 264.7 cells (a murine-derived macrophage cell line, provided by the cell bank of Chinese Academy of Sciences Typical Culture Preservation Committee) were cultured in DMEM with 10% (v/v) fetal bovine serum (FBS, Sigma) and 1% (v/v) penicillin–streptomycin (P/S). BMSCs from Cyagen Co. Ltd (Guangzhou, China) were cultured with total mesenchymal stem cell culture medium (Cyagen, China). The culture medium was changed every 48 h and only passages 2–7 of the BMSCs were used in this study. Both cell types were cultured in a humidified 5% CO2 incubator at 37 °C.
2.3. Quantitative real-time polymerase chain reaction (Q-RT-PCR)
To analyze the mRNA expressions of RAW, RAW cells were seeded at the density of 2 × 106 cells per well in a six-well plate. After 12 h, the medium was replaced by the Aker/SA hydrogel extracts. After being cultured for 3 d, the RAW cells were washed with PBS twice, and the RNA was collected by the E.Z.N.A Total RNA kit (OMEGA, Biotek) according to the manufacturer's instructions. cDNA was synthesized by using the ReverTra Ace-α kit (Toyobo Co. Ltd, Japan). cDNA was diluted with sterilized deionized water and primers of induced nitric oxide synthase (iNOS) (forward sequence: 5'-CTC AGC CCA ACA ATA CAA GA-3', reverse sequence: 5'-GTG GAC GGG TCG ATG TCA C-3'), transforming growth factor-β (TGF-β) (forward sequence: 5'-TGA GAA GTT CCC AAA TGG CCT C-3', reverse sequence: 5'-CTA CAG GCT TGT CAC TCG AAT TTT G-3'), tumor necrosis factor-alpha (TNF-α) (forward sequence: 5'-TGA GAA GTT CCC AAA TGG CCT C-3', reverse sequence: 5'-CTA CAG GCT TGT CAC TCG AAT TTT G-3') and vascular endothelial growth factor (VEGF) (forward sequence: 5'-TGC GGA TCA AAC CTC ACC A-3', reverse sequence: 5'-CAG GGA TTT TTC TTG TCT TGC T-3'), CD 68 (forward sequence: 5'-TGT CTG ATC TTG CTA GGA CCG-3', reverse sequence: 5'-GAG AGT AAC GGC CTT TTT GTG A-3'), CD 163 (forward sequence: 5'-TCC ACA CGT CCA GAA CAG TC-3', reverse sequence: 5'-CCT TGG AAA CAG AGA CAG GC-3'), ARG (forward sequence: 5'-CTC CAA GCC AAA GTC CTT AGA G', reverse sequence: 5'-AGG AGC TGT CAT TAG GGA CAT C-3'), urokinase plasminogen activator (uPA) (forward sequence: 5'-CAC GCA AGG GGA GAT GAA-3', reverse sequence: 5'-ACA GCA TTT TGG TGA CTT-3') and matrix metalloproteinase-2 (MMP-2) (forward sequence: 5'-CAC CTA CAC CAA GAA CTT CCG TCT G-3', reverse sequence: 5'-GTG CCA AGG TCA ATG TCA GGA GAG-3'), were mixed with SYBR-Green at the final concentration of 400 nM. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward sequence: 5'-TGC ACC ACC AAC TGC TTA G-3', reverse sequence; 5'-GGA TGC AGG GAT GAT GTT C-3') was used as the housekeep gene. The Q-RT-PCR analysis was performed using a 7900 Real-time PCR system (Applied Biosystems). The results were analyzed by the ΔΔCt method using the SDS 2.4 software and the data was normalized to the expression of GAPDH and quantified relative to the gene expressions of control samples which were cultured with normal medium.
2.4. Flow cytometry of RAW cells
Expression of macrophage markers, including iNOS (marker for M1 macrophages) and arginase (ARG) (marker for M2 macrophages), in RAW cells were detected by flow cytometry to evaluate the phenotypic changes of RAW cells in response to Aker/SA hydrogel extracts. The RAW cells were seeded at the density of 3 × 106 cells per well in six-well plate and cultured with normal medium. Then, after 12 h, the medium was switched to Aker/SA hydrogel extracts with 10% FBS and 1% P/S and the cells were further cultured for 3 d. Then, the cells were scraped off and washed with PBS twice before they were thoroughly resuspended in 250 µl fixation and permeabilization solution (the Fixation/Permeabilization Kit, BD) per tube for 20 min at 4 °C following manufacturer's instructions. After that, the cells were washed twice with the buffer (the Fixation/Permeabilization Kit, BD) and then they were stained with iNOS (at the dilution of 1:100, H2316, Santa Cruz Biotech) and ARG (at the dilution of 1:200, IC5868F, RD) separately. The cells were analyzed by a flow cytometer (FACS, AriaII, BD). For validation, three independent experiments were performed.
2.5. Indirect contact co-culture of RAW cells and BMSCs
Indirect contact co-culture was used to investigate the effects of the Aker/SA hydrogel-activated RAW cells on migration of BMSCs. The conditioned medium of RAW cells in contact with Aker/SA hydrogel extracts were used to culture BMSCs. Briefly, RAW cells were seeded at the density of 3 × 106 cells per well at the six-well plate and cultured with normal culture medium (DMEM with 10% FBS and 1% P/S). After 24 h, the culture medium was switched to the Aker/SA hydrogel extracts with 10% FBS and 1% P/S. The RAW cells were then cultured with the Aker/SA extracts for 48 h before the supernatant was collected and stored at 4 °C as conditioned medium. As figure 1(B) showed, the medium collected from the RAW cells cultured with the Aker/SA hydrogel extracts were mixed with DMEM with 1% P/S (serum-free) and labeled as Aker (RAW). The Aker/SA hydrogel extracts mixed with the conditioned medium collected from the RAW cells cultured with normal culture medium for 48 h were labeled as Aker + RAW. The conditioned medium collected from the RAW cells cultured with normal culture medium for 48 h and mixed with DMEM with 1% P/S (serum-free) was labeled as RAW. The Aker/SA hydrogel extracts were labeled as Aker and the serum-free DMEM were labeled as control. All the conditioned media used in this study were centrifuged at 1000 rpm for 3 min and the supernatants were used. The composition of the conditioned media is shown in table 1.
Table 1. The composition of different conditioned media.
| Conditioned media | Composition |
|---|---|
| Control | Normal culture medium |
| RAW | Conditioned macrophage medium (after normal cell culture) |
| Aker | Normal culture medium mixed with extract liquid of Aker/SA hydrogel |
| Aker + RAW | Mixture of conditioned media of RAW (after normal cell culture) and extract liquid of Aker/SA hydrogel |
| Aker(RAW) | Mixture of conditioned medium of RAW cultured with extract liquid of Aker/SA hydrogel and normal culture medium |
2.6. Evaluation of in vitro migration of BMSCs
To evaluate the effects of the Aker/SA hydrogel and the activated RAW cells on the migration of the BMSCs, the following experiments were performed. The BMSCs were cultured with either the Aker/SA hydrogel extracts (Aker), the culture medium of RAW cells (RAW), the Aker/SA extracts mixed with conditioned medium RAW cells cultured with serum-free medium (Aker + RAW), the conditioned medium collected from the RAW cells cultured with the Aker/SA hydrogel extracts and mixed with serum-free medium (Aker(RAW)), or the normal DMEM medium (control). After 48 h, the expression of CXCR4 of the BMSCs was detected by flow cytometry and the expression of genes relevant to cell migration was detected by Q-RT-PCR. The cells were digested with trypsin, washed with PBS twice and resuspended in the PBS solution. The single-cell suspensions were then incubated with anti-CXCR4 antibody (1:1000, Abcam, UK, ab1670) for 30 min at 4 °C. After that, the cells were incubated with the donkey anti-goat IgG (1:1000, Abcam, UK, ab150133) as the secondary antibody for 30 min at 37 °C. Then, they were washed with PBS for three times. The cells were analyzed by flow cytometry as mentioned above.
Next, Transwell plates of 24 well chambers containing 8 µm pores (3422, corning, USA) were used to detect the migratory ability of BMSCs cultured with different media as shown in figure 1(C). BMSCs were seeded at the density of 2 × 105 per Transwell with normal medium. After 6 h, the medium was removed. Serum-free medium was added on the upper chamber and different conditioned media, i.e. Aker (RAW), Aker + RAW, RAW, Aker, were added to the lower chambers. BMSCs cultured with serum-free DMEM in upper and lower chambers were used as the control group. After 24 h, cells were fixed with paraformaldehyde (4% v/v) before they were stained with 0.1% crystal violet. Cells failed to traverse across the membrane were removed.
2.7. Animal studies
All animal studies have been approved by the Animal Care and Experimental Committee of Sixth People's Hospital Affiliated to the Shanghai Jiao Tong University School of Medicine. The approval number is 2017-0116. To further investigate the effects of Aker/SA hydrogel on the phenotypic changes of macrophages as well as the migration of BMSCs, in vivo studies were carried out. The BMSCs from rats transduced with GFP gene (GFP-BMSCs) were obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd As many as six Sprague–Dawley (SD) rats were utilized in each group in the in vivo study and the animals were obtained from the Sixth People's Hospital Animal Centre (Shanghai, China). 1 ml of SA solution was mixed with 0.02 g Aker and 0.005 g Glu to form the injectable hydrogel according to the method described earlier. BMSCs were digested with trypsin/EDTA and resuspended in saline at the density of 1 × 106 ml−1. For each rat, three positions, two of which were equidistant from the third position in the middle, were indicated at each side of the rat's back for injection. As shown in figure 1(D), 1 ml of Aker/SA hydrogels, 1 ml normal saline containing GFP-BMSCs and 1 ml SA solution were implanted at the back of rats through subcutaneous injection, respectively. The distance between two adjacent injection positions was 3 cm. The saline mixed with BMSCs were injected in the midline of the other two positions so that the injected cells were roughly equidistant from different materials.
All the tissues at the midpoints between GFP-BMSCs injection points and Aker/SA hydrogel injection points (marked as black cross in figure 6(A)) or at the midpoints between GFP-BMSCs injection points and SA solution injection points (marked as red cross in figure 6(A)) were obtained 7 d after the injection. After being soaked in the formalin for 24 h, the samples were embedded in paraffin and sliced into 4 µm thick sections. To determine the effects of Aker/SA hydrogels on the migration of the implanted BMSCs, a laser microscope (Leica DMI 3000B, Germany) were used to observe the peripheral area of the injected Aker/SA hydrogel and SA solution. The GFP signals from the injected BMSCs were detected at the wavelengths of 450 nm. Images of two different positions in each sample were randomly taken for all samples.
In addition, tissue samples were fixed overnight with 4% PFA, embedded in paraffin, and then sectioned into 8 µm thick sections for immunohistochemistry staining. The sections were dewaxed, hydrated as well as incubated with 0.01 M heated buffer, following by the usage of 0.3% H2O2/methanol (v/v) to inactivate endogenous peroxidase. After 1 h incubation with 5% bovine serum albumin (BSA, Sigma), the samples were incubated with the universal macrophage marker CD68 (Abcam) and M2 macrophage marker CD163 (Abcam) antibodies for 12 h at 4 °C. Then, the samples were incubated with the solutions in the DAB kit (Gene Tech, Shanghai) for 60 s and rinsed for 15 min with PBS.
For histological staining, the samples were stained with hematoxylin for 40 s and rinsed for 30 min with deionized water. All the sections were finally hydrated and mounted for further image and analysis. The samples were imaged by using a CCD camera (Leica DFC 420C).
2.8. Statistical analysis
For all experiments, data were presented as the means ± standard deviation. At least three independent experiments were performed. Two-tailed analysis of variance was used in the statistical analysis. The difference was considered significant at p < 0.05. For analysis of in vitro cell migration images, migrated cells were randomly selected for imaging under microscope. Migrated cells in three randomly selected fields were counted and the number was analyzed by SPSS 10.0 software.
3. Results
3.1. Characterization of the Aker/SA hydrogel
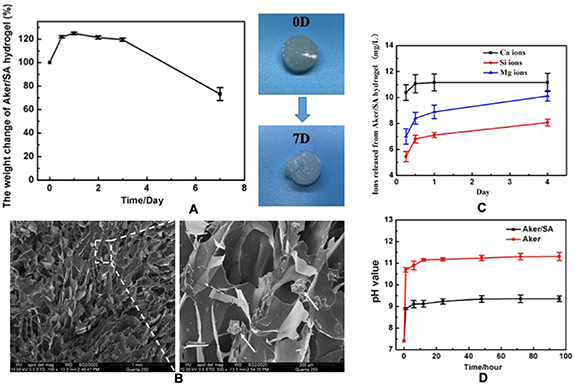
Figure 2(A) showed the weight changes in 7 d after the crosslinking of Aker/SA hydrogel. From the images, it could be noted that the weight of the Aker/SA hydrogel increased in the first 2 d, probably due to swelling, and started declining on day 2. Both the weight and images of the hydrogel taken at day 7 indicated that the Aker/SA hydrogel was stable with slow decomposition. The SEM images of the frozen dried Aker/SA hydrogel was presented in figure 2(B). A porous structure with some Aker powders could be observed, suggesting that Aker was embedded in the hydrogel. Figure 2(C) displayed the concentrations of Ca, Si, Mg ions released from Aker/SA hydrogel at different time points, showing a quick release of ions on the first day followed by a gradual release from day 1 to day 4. Our results of the pH values of the aqueous environment containing Aker/SA hydrogel or pure Aker powders demonstrated that comparing with the pure Aker powders, the combination of the Aker with the SA resulted in a lower pH value (figure 2(D)). The pH in the extract of Aker/SA hydrogel was around 9, while the pH in the extract of Aker powders was around 11.
Figure 2. (A) The percentage of Aker/SA hydrogel weight change from day 0 to day 7 and the appearance of the hydrogel at day 0 and day 7. (B) The SEM images of the frozen dried Aker/SA hydrogel. Arrow head indicates Aker powder. (C) The concentration of Ca, Si and Mg ions released from the Aker/SA hydrogel from day 0 to day 4. (D) The pH values of the extract of Aker/SA hydrogel and pure Aker powders from 0 h to 96 h.
Download figure:
Standard image High-resolution image3.2. Effect of the Aker/SA hydrogel extracts on RAW phenotype
The expression of M1 marker iNOS and M2 marker ARG of macrophages were detected by flow cytometry to assess the phenotypic change of RAW cells in response to the Aker/SA hydrogel extracts. Figure 3(A) shows that the M2 phenotype of RAW was activated by the Aker/SA hydrogel extracts as the ratio of cells expressing ARG marker in total cells (13.767 ± 1.347%) was higher than that of cells cultured with DMEM (3.667 ± 0.287%). In contrast, fewer RAW cells were activated into M1 macrophages by the Aker/SA hydrogel extracts (38.475 ± 3.947%) as compared to those cultured in control group (60.4 ± 2.411%). This is further confirmed by PCR results showing a higher expression of M2 marker, CD163 and ARG, in cells cultured with Aker/SA extracts, whereas the expression of universal macrophage marker (CD68) exhibited insignificant change (figure 3(B)).
Figure 3. (A) Flow cytometry detection of the phenotype of RAW cultured with Aker/SA hydrogel extracts and DMEM. Macrophages were stained with M1 marker iNOS and M2 marker ARG. The ratios of M2 macrophages are listed in the table. (B) The Q-RT-PCR results of the gene expression of macrophage phenotype markers and chemokines involved in the pro-inflammation and tissue regeneration. The RAW cells were cultured with Aker/SA hydrogel extracts or DMEM (n = 3; *represents p < 0.05).
Download figure:
Standard image High-resolution imageBesides, the gene expressions of the pro-inflammatory and growth factors in RAW were consistent with the results of macrophage marker expression. As shown in figure 3(B), the gene expressions of anti-inflammatory growth factors TGF-β and VEGF were increased while the expressions of pro-inflammatory cytokines iNOS and TNF-α were decreased when the RAW cells were cultured with the Aker/SA hydrogel extracts compared to those cultured with the DMEM.
3.3. Evaluation of BMSCs migration in vitro
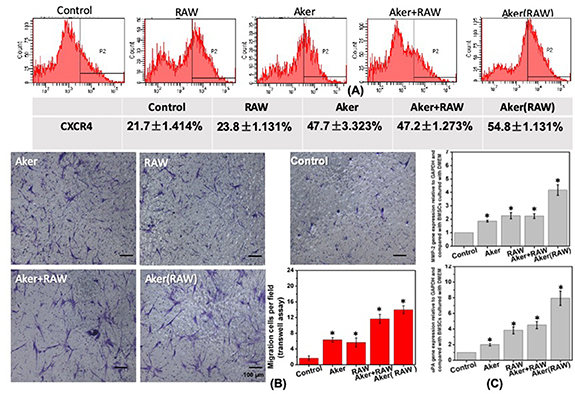
The direct effect of the Aker/SA hydrogel on the migration of BMSCs and the indirect effect of the Aker/SA hydrogel on the migration of BMSCs via modulating RAW cells were investigated. The effect of different media on the percentage of BMSCs expressing CXCR4 were determined by flow cytometry and the results were presented in figure 4(A). The percentage of CXCR4 positive BMSCs were highest when BMSCs were cultured with the Aker (RAW) medium (54.8% ± 1.131%), which suggested that the conditioned medium of the Aker/SA hydrogel-activated RAW cells (Aker (RAW)) had the strongest effect on enhancing the migration of BMSCs. When the BMSCs were cultured with extracts of Aker/SA hydrogel (Aker) and the Aker/SA hydrogel extracts mixed with the conditioned medium of RAW cells cultured with normal culture medium (Aker + RAW), the percentages of CXCR4 positive BMSCs were similar (47.7 ± 3.323%, 47.2 ± 1.273%, respectively) but higher than those in BMSCs cultured with the conditioned medium of RAW cells mixed with fresh medium (RAW) (23.8 ± 1.131%) and those in BMSCs cultured with control (21.7 ± 1.414%).
Figure 4. (A) Flow cytometry detection of the expression of CXCR4 in BMSCs cultured with conditioned media and control culture medium for 48 h. 'Control' represents BMSCs cultured with control medium; 'RAW' represents BMSCs cultured with the conditioned medium of RAW cells; 'Aker' represents BMSCs cultured with the Aker/SA hydrogel extracts; 'Aker + RAW' represents BMSCs cultured with the mixture of Aker/SA hydrogel extracts and conditioned medium of RAW cells; 'Aker(RAW)' represents BMSCs cultured with the conditioned medium of Aker/SA hydrogel-activated RAW cells. (B) The Transwell migration results of BMSCs under the influence of different conditioned media. The cells translocating through the insert membrane were stained with crystal violet. The bar chart shows the average numbers of observed cells per field. (C) The Q-RT-PCR results of the migration-related gene expression of BMSCs cultured with different conditioned media for 48 h (n = 3; *represents p < 0.05).
Download figure:
Standard image High-resolution imageFigure 4(B) shows the migration of BMSCs cultured in different media. From the images it can be observed that more BMSCs translocated through the insert membrane when they were cultured with the Aker (RAW) than in other three groups although all the conditioned media could enhance the migration of BMSCs compared to the control group. The number of migrated cells per field was further quantified. The average numbers of observed cells translocating through the membrane per field were 1.67 (control group), 6.33 (Aker group), 5.66 (RAW group), 11.7 (Aker + RAW group) and 14 (Aker (RAW) group). Based on this result, we can conclude that Aker (RAW) medium exerted the strongest stimulatory effects on BMSCs to promote their migration in vitro.
We also analyzed the expression of genes relevant to cell migration, including uPA and MMP-2, in BMSCs cultured with different media by Q-RT-PCR and the results were shown in figure 4(C). The trends of these two gene expressions matched with the trends of the expression of CXCR4 in BMSCs. The expressions of uPA and MMP-2 in BMSCs cultured with the Aker (RAW) medium were highest among all groups. In addition, there was no significant difference in the expressions of MMP-2 and uPA between the Aker + RAW group and RAW group, which were both higher than those in the Aker group and control group. Comparing between Aker and control group, the former displayed higher expressions of uPA and MMP-2 than the latter.
3.4. Evaluation of effect of Aker/SA on macrophage polarization and BMSCs migration in vivo
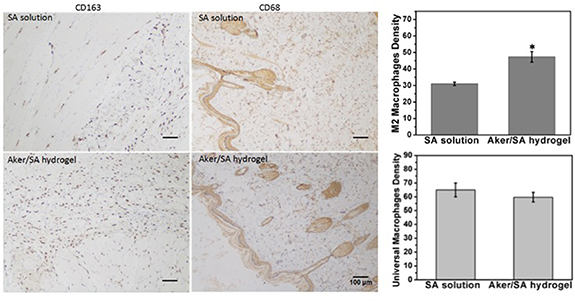
The Aker/SA hydrogel as well as the SA solution were injected subcutaneously to investigate the host inflammatory response following the implantation of different materials. The results were shown in figure 5. From the microscope images, it is noted that more M2 macrophage (CD163 positive) were present in the tissue close to the Aker/SA hydrogel injection points than in the tissue close to the SA solution injection points. Interestingly, the number of universal macrophages (CD68 positive) present was found to be similar in the two extracted tissues.
Figure 5. Universal macrophages and M2 macrophages were immunolabeled with anti-CD68 and anti-CD163 antibodies respectively and observed in tissue sections extracted at the midpoints between where BMSCs and Aker/SA hydrogel or SA solution were injected 7 d post-injection. The label shows the location of tissue collected close to either SA solution or Aker/SA hydrogel. (Right) Statistical analysis of the density of M2 macrophages and universal macrophages at day 7. The numbers of M2 macrophages and universal macrophages were quantified from three random microscopic fields (n = 3; *represents p < 0.05).
Download figure:
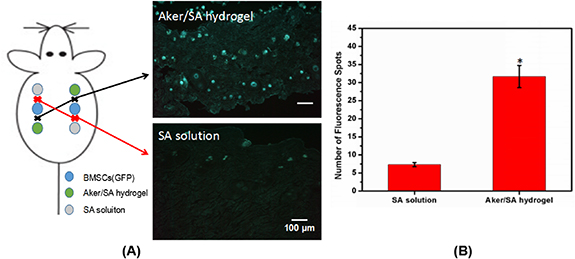
Standard image High-resolution imageIn addition, the migration of BMSCs under the effect of Aker/SA hydrogel or SA solution was examined. The fluorescent images of GFP-BMSCs in the tissues collected at the midpoints between GFP-BMSCs injection points and Aker/SA hydrogel injection points (marked as black cross) or at the midpoints between GFP-BMSCs injected regions and SA solution-injected regions (marked as red cross) were presented in figure 6(A). Our results showed that more fluorescent spots were present near the Aker/SA hydrogel injection points than near the SA-solution injection points. The quantified data was presented in figure 6(B), which further confirmed that more GFP-BMSCs migrated towards the Aker/SA hydrogel instead of the SA solution.
Figure 6. The migration of GFP-BMSCs in vivo. (A) The label 'Aker/SA hydrogel' represents fluorescent image of tissues collected at the midpoint between where Aker/SA hydrogel and GFP-BMSCs were injected. The label 'SA solution' represents fluorescent image of tissues collected at the midpoint between where SA solution and GFP-BMSCs were injected. (B) The numbers of GFP-BMSCs were quantified from three random microscopic fields (n = 3; *represents p < 0.05).
Download figure:
Standard image High-resolution image4. Discussion
The implantation of biomaterial into human body will trigger a series of host reaction and involve a variety of immune cells such as neutrophils, macrophages, and lymphocytes etc [13, 14]. For example, polymorphonuclear leukocytes can release reactive oxygen species and proteolytic enzymes which will damage not only the surface of the implanted materials but also the surrounding tissue [15]. In particular, macrophages have been proved to play an important role in not only inflammation but also tissue repair and regeneration [16].
In this work, we attempted to explore whether macrophages contribute to Aker-induced tissue regeneration. Although Aker is proven to enhance bone regeneration and studies have shown that bone regeneration is enhanced through stimulating ostenogenic differentiation of BMSCs [2, 3], few studies have focused on characterizing the immune reaction caused by the Aker and how it affects BMSCs.
Our results demonstrated that in response to Aker/SA hydrogel, the macrophage phenotype switched to M2 polarization in vivo and in vitro. Meanwhile, the expression of anti-inflammatory factors was increased. This direct effect may facilitate tissue regeneration. In addition, after macrophages were activated by Aker/SA hydrogel, the Aker/SA hydrogel-activated macrophages promoted the migration of BMSCs in vitro and recruitment of BMSCs in vivo. The recruited BMSCs are believed to participate in tissue regeneration, such as bone formation. Therefore, we speculate that the Aker/SA hydrogel may enhance tissue regeneration through modulating local microenvironment, by both directly influencing the behaviors of macrophages and indirectly activating the migration of BMSCs to the implant site.
Macrophage is an important cellular element in the immune reaction. The macrophages can be activated into the M1 and M2 phenotypes, which perform different functions including resolving the inflammation and remodeling the extracellular matrix [17, 18]. Studies have indicated that the different phenotypes of the macrophages are the result of the cross-talking of multiple signal cascades instead of a single cytokine signal [19, 20]. For example, Arcos and Vallet-Regi [21] reported that the macrophage functional polarization involving the secretion of cytokines could be affected by biomaterials such as SiO2 and polyurethane through integrin signaling. Our previous study also showed that bioglass could stimulate the macrophages towards M2 polarization and the expression of anti-inflammatory factors in macrophages can be upregulated by the extracts of bioglass [22].
In the current study, macrophages activated by Aker/SA hydrogel expressed more M2 markers and anti-inflammatory cytokine genes were up-regulated as compared to the macrophages cultured with normal medium. These results were in accordance with our previous findings [22]. In addition to in vitro study, we further designed an animal model to prove the effects of the Aker/SA hydrogel on inflammatory response and recruitment of BMSCs.
We observed more M2 macrophages in the tissue where Aker/SA hydrogel was injected than where SA solution was injected. These results further suggested that Aker/SA hydrogel could affect the phenotype of macrophage and may subsequently elicit proper inflammation response in vivo. In addition, many studies have proved that the chemokines and cytokines secreted by M2 macrophages have many different biological functions [23, 24]. The chemokines and cytokines secreted by M2 macrophages can recruit cells participating in regeneration while depletion of macrophages can impede the regeneration process [23, 25, 26]. Our results of this study proved that the Aker/SA hydrogel-activated macrophages could enhance the migration of BMSCs, as evidenced by the upregulation of CXCR4 and MMP-2 gene expression in BMSCs.
CXCR4, a specific receptor of stromal cell-derived factor-1 (SDF-1) is involved in cell migration [27, 28]. Previous study demonstrated that transplanted CXCR4-expressing BMSCs migrated towards the site with increased expression of SDF-1 [29]. Our previous study has shown that the ions released from the Aker/SA hydrogel played an important role in the motility of BMSCs by stimulating the CXCR4 expression [3]. This current study further demonstrated that the Aker/SA hydrogel-activated macrophages could further enhance the upregulation of CXCR4 expression in BMSCs which might be conducive to the recruitment of BMSCs for tissue regeneration. In addition, we found that the expressions of uPA and MMP-2, which are also involved in cell migration, were increased in BMSCs cultured with different conditioned media. In particular, the culture medium of Aker/SA hydrogel-activated macrophages was most effective in up-regulating these genes, which suggested that the Aker/SA hydrogel could enhance the migration of BMSCs through activating macrophages in vitro. In vivo experiments confirmed the results by showing the increased presence of M2 macrophages as well as the enhanced migration of GFP-BMSCs at the implant site.
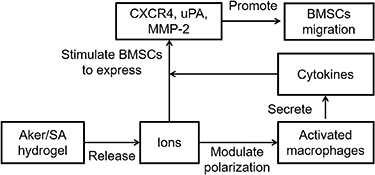
Figure 7 presents a proposed mechanism of Aker/SA-induced tissue regeneration, whereby Aker/SA, through the ions it releases, stimulate BMSCs directly and modulate the behavior of macrophages, which in turn promotes the migration of BMSCs via secretion of cytokines. Both direct and indirect effects stimulate BMSCs to express CXCR4, uPA, and MMP-2 and contribute to enhanced BMSCs migration. Previous work has proved that ions released from the silicate-based biomaterials such as bioactive glass could increase cell membrane fluidity [30]. Since bioactivities such as cell migration and differentiation are regulated by membrane fluidity, it could be one of the reasons how the Aker/SA hydrogel modulate the macrophages and BMSCs. Nevetheless, further investigation is needed to define the mechanism of action, as other variables, including pH value of the implant site, may play a role. In this study, we showed that a number of ions was released from the Aker/SA hydrogel in a few days. While it is possible to deliver the specific combination of ions directly, we speculate a sustained and higher concentration of the ions released from Aker/SA hydrogel should be a better therapeutic approach since the free ions delivered are likely to be diluted by the body fluids quickly. Moreover, ions such as Ca ions released from the hydrogel can also directly participate in the bone regeneration process [31]. Meanwhile, it is also important to recognize that the dose of Aker have influence on the host response. According to a previous study [32], a high concentration of Aker (∼100 times of the concentration used in the current study) led to macrophage apoptosis as well as lower inflammatory cytokine secretion. Our previous studies also showed that the dose of another silicate-based biomaterials, bioactive glass, affected the phenotype of RAW cells [33]. In this study, we adopted the same Aker dose reported in our previous work that demonstrated improved bone regeneration following Aker/SA hydrogel implantation [3]. This allows us to elucidate the mechanism associated with the specific hydrogel formulation.
Figure 7. The proposed mechanism of action of Aker-mediated activation of macrophages and migration of BMSCs during tissue regeneration.
Download figure:
Standard image High-resolution imageOverall, this study improved our understanding of the immune response stimulated by the presence of Aker/SA hydrogel and established a preliminary correlation between biomaterial-mediated inflammation and the activity of BMSCs. This finding will also contribute to the design of biomaterial that can actively recruit endogenous stem cells for tissue regeneration.
5. Conclusions
In the current study, we showed that Aker/SA injectable hydrogel can modulate the phenotype of macrophages and enhance the recruitment of BMSCs for potential tissue regeneration. First, we proved that the Aker/SA injectable hydrogel could affect the polarization and hence influence the secretory profile of macrophages. The Aker/SA hydrogel-activated macrophages could further enhance the migration of BMSCs in vitro and recruitment of BMSCs in vivo. Specifically, we showed that the extracts of Aker/SA hydrogel could stimulate macrophages toward M2 phenotype and the conditioned medium of Aker/SA hydrogel-activated macrophages had a strong stimulatory effect on the migration of BMSCs. When the Aker/SA hydrogel was subcutaneously injected in rats, it could stimulate macrophages to polarize toward M2 phenotype and recruit BMSCs towards the hydrogel.
Acknowledgments
This research was funded by the National Key R&D Program of China (2019YFA0111300), Research Grants Council of Hong Kong (24204819), project #BME–p5-19 of the of the Shun Hing Institute of Advanced Engineering (The Chinese University of Hong Kong), the National Natural Science Foundation of China (Grant Nos. 31771024 and 31971274), and the Interdisciplinary Program of Shanghai Jiao Tong University (Project Number: ZH2018ZDA20).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.