Abstract
Wound dressings able to deliver topically bioactive molecules represent a new generation of wound-regeneration therapies. In this article, foams based on methylcellulose cross-linked with Manuka honey were used as a platform to deliver borate bioactive glass particles doped additionally with copper. Borate bioactive glasses are of great interest in wound-healing applications due to a combination of favorable features, such as angiogenic and antibacterial properties. The multifunctional composite providing the dual effect of the bioactive glass and Manuka honey was produced by freeze-drying, and the resulting foams exhibit suitable morphology characterized by high porosity. Moreover, the performed tests showed improved wettability and mechanical performance with the addition of bioactive glass particles. Dissolution studies using simulated body fluid and cell biology tests using relevant skin cells further proved the excellent bioactivity and positive effects of the foams on cell proliferation and migration. Most interestingly, by the dual release of Manuka honey and ions from the copper-doped bioactive glass, an antibacterial effect against E. coli and S. aureus was achieved. Therefore, the multifunctional foams showed promising outcomes as potential wound dressings for the treatment of infected wounds.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Skin, the largest organ covering the entire body, has several pivotal functions including body temperature regulation, synthesis of vitamin D3, immunological surveillance, and prevention of water loss [1, 2]. The primary function of the skin is to protect the underlying bones, muscles, ligaments, and internal organs. Skin is also the organ most exposed to injury, scratches, and burns by external mechanical, physical, chemical, and biological agents [1, 3]. Moreover, illnesses like diabetes can lead to impairment in the functionality of the skin [1]. In case of any damage to the skin, a wound-healing process starts directly to restore the skin function. To guarantee an effective wound-healing process, several set conditions as temperature and oxygenation, as well as high availability of minerals, vitamins, and trace elements, are necessary at the wound site [1]. These conditions can be negatively influenced by contaminations, which can lead to deterioration of growth factors, extracellular matrix, and granulation tissue [1, 3]. Besides specific diseases, contaminations are the main reason for chronic wounds, which (in contrast to acute wounds) do not heal in less than 12 weeks and commonly reoccur [4]. The goal of research on skin regeneration and wound healing is to support the human body to heal the wound in the shortest period of time with a minimum of scarring, pain, and discomfort of the patient [4].
Depending on the kind of wound, different types of wound dressings are available to support the healing process, including sponges, hydrogels, hydrocolloids, fiber mats, films, xerogels, scaffolds, gauzes, and lints [3–5]. These different wound dressing types have their advantages and disadvantages, and need to be carefully chosen for different wounds (e.g. foams for deep wounds, films for superficial wounds). In general, an ideal wound dressing needs to keep a moist environment and at the same time it should absorb wound fluids; it should also maintain the skin temperature and pH, and not provoke immune responses or have a low adherence to skin. Moreover, it should be biocompatible, semi-permeable to water and oxygen, sterilizable, and cost effective [3, 6, 7]. Foams, which can be produced by freeze-drying, fulfill some of these criteria given their thermal insulation, high porosity, and moist environment, but due to their weak mechanical properties, they are not suitable for dry wounds as they can provoke skin maceration [1, 4]. Hydrogels, which can be freeze-dried to produce foams, are additionally known to be suitable for all stages of wound healing: vascular response, inflammatory response, proliferation, and maturation phases [5]. Typical materials used to produce foams as wound healing dressings are chitosan, alginate, polylactic acid, polyvinyl alcohol, and, most commonly, polyurethane [2, 8].
Cellulose, the most abundant naturally occurring polysaccharide, could also be used as wound dressing material based on its excellent properties. In fact, it can be obtained from renewable sources; among these the most commercially exploited is wood, but a variety of other plants contain large amount of cellulose. Moreover, some bacteria, fungi, and seaweeds produce cellulose [9, 10]. Cellulose in general is biodegradable, non-toxic, non-carcinogenic, and biocompatible. Further, it has the capacity to retain moisture, absorb exudates, and accelerate granulation [9]. It is also known that cellulose is able to stimulate wound healing by fibrogenesis [7]. In this study, we used methylcellulose (MC), which is a water-soluble cellulose derivative where methyl groups substitute hydroxyl groups [10]. MC with a substitution degree between 1.7 and 2.0 has a unique behavior: with an increase in temperature, a reversible sol–gel transition can be induced by a hydrophobic interaction [11]. This thermo-sensitive behavior makes MC an interesting candidate for wound dressings made by freeze-drying of gels.
Traditionally, wound dressings have been applied to shield the wound from external contamination, based on the idea of 'covering and concealing' [4]. However, a new trend of research is focusing on wound dressings which additionally support the skin repair and regeneration processes. This support mechanism can be further enhanced by functionalizing wound dressings with different therapeutic complexes [3, 6]. In fact, lyophilized hydrogels containing therapeutic agents are attractive, since once they are applied on the wound, they turn into a gel and provide sustained release of the therapeutic agent [12]. In order to determine the release properties of such hydrogel-based wound dressing, as well as the other crucial properties (e.g. porosity, mechanical properties, swelling properties, etc) a cross-linking process is performed. MC can be crosslinked by two main methods: gamma irradiation and chemical crosslinking [13]. The most popular chemical crosslinking agent is glutaraldehyde [14]; however, glutaraldehyde is known for its potential toxicity [15].
In this study, Manuka honey (MH) was used as a phytotherapeutic agent to provide additional functions to the basic MC foams and, for the first time, its effect on MC crosslinking was investigated [16, 17]. Bees produce MH from the nectar of the Leptospermum scoparium tree, which is native in New Zealand [18]. The key factor of MH compared to other honeys is its unique Manuka factor, which reflects the amount of methylglyoxal within the honey [19]. Based on a previous study conducted by Qiu and Netravali using glyoxal to crosslink cellulose [20], it was hypothesized in this research that the methylglyoxal within MH would be able to act as a crosslinking agent for MC. Besides containing methylglyoxal, MH can contain more than 200 substances, and is composed of around 80%–85% carbohydrates, 15%–17% water, 0.1–0.4% proteins, 0.2% ash, and small quantities of minerals, enzymes, amino acids, vitamins, and organic acids [16, 21]. MH is known to be effective against various bacteria, e.g. Enterobacter aerogenes, Helicobacter pylori, Staphylococcus aureus (S. aureus), and Escherichia coli (E. coli) [19], as well as against biofilm formation [1]. This well-known antibacterial effect is attributed to several components (e.g. methylglyoxal, lysozyme, bee peptides, flavonoids, phenolic acids [1, 3, 22, 23]) and to specific properties of MH. During degradation of MH inside the human body, low levels of hydrogen peroxide are produced, which could have also an antibacterial effect [6, 18] or, in high amounts, a dangerous effect for cells and tissues [21]. Moreover, honey in general has a low pH, between 3.2 and 4.5, which could also lead to bacteria death [2, 18]. Besides the antibacterial effect, MH is also known for its inflammation modulation effects, which promote the repair of wounds and avoid the extension of inflammatory phases [18]. The already mentioned low pH is also known to be involved in the stimulation of macrophages [19] and in induction of angiogenesis [2, 18]. Honey additionally provides topical needed nutrition to the wound and stimulates wound epithelialization [3]. Due to these known beneficial properties, honey in general has a long history in the treatment of wounds [21]. Therefore, it is not surprising that some honey impregnated dressings are available in the market, e.g. MediHoney®, Activon Tulle®, Algivon®, and Actilite® [1], and clinical trials have shown honey's beneficial effects on wound healing [23].
In order to further extend the functionality of a MC–MH wound dressing, bioactive glass (BG) in particulate form can be incorporated to potentially achieve synergistic effects of MH and BG [24]. Besides extending the functionality of the composite wound dressing, the introduction of BG has another positive effect, as ions released from BG particles can act in tandem with MH. In order to have an effective antibacterial effect, a certain amount of MH is necessary. In the ideal combination of BG and MH, the effect of each component should potentiate the effect of the other substance, and, if this is achieved, a reduced amount of BG and MH will be needed to achieve the same effect compared to using just MH or BG [24]. Indeed, due to production limitations, it is possible that the introduced amount of MH is not enough to successfully kill bacteria, and thus the addition of another antibacterial agent becomes necessary. Since the discovery of BG 50 years ago, BGs have been considered for orthopedic and bone tissue engineering applications [25, 26]. However, increasing research efforts are focusing on the use of BG in contact with soft tissues and for wound-healing applications [27–29]. In both soft and hard tissue engineering, the biodegradability of BGs results in the release of biologically active ions and in the formation of a surface CaP-rich layer, which make them highly interesting for regenerative applications [28, 30]. The release of biologically active ions leads to especially favorable effects of BG, e.g. antibacterial effects and stimulation of angiogenesis [31–34]. Based on these favorable effects, BGs offer great potential for wound-healing applications, especially in chronic wound management where angiogenic and antibacterial properties are needed. BGs can be divided into three different groups based on their glass network former: silicate, phosphate, and borate BGs [35]. Compared to silicate BGs, borate BGs have some important advantages which are relevant for wound-healing applications [36]. Borate BGs offer a high degradation rate mainly due to their lower chemical durability, and thus the ability of boron to stimulate angiogenesis can be exploited [37]. Moreover, borate BGs were found to accelerate hemostasis and cell proliferation in wound-healing approaches [29, 38, 39]. Therefore, a few years ago, a borate BG-based wound dressing (MirragenTM, ETS Wound Care LLS, USA) was approved by the US Food and Drug Administration. This product shows promising results in enabling the healing of old, non-healing wounds [29]. BGs additionally offer the advantage of being easily modified to extend their functionality. By introducing therapeutically active ions into the BG network, e.g. Cu, Ga, Ag, Zn, etc, the response of the human body can be more precisely tailored [31, 40, 41]. For instance, copper is known to have antibacterial effects and to stimulate angiogenesis; both properties have been already successfully demonstrated in vitro and in vivo using Cu-doped borate (and borosilicate) BGs [38, 42, 43].
In this work, we present the development of foams based on MC crosslinked with MH and additionally loaded with borate BG particles (with and without copper) by freeze drying. An investigation of the potential of the composite foams as multifunctional wound dressing is presented. The morphology and chemical structure of the produced foams were characterized and their dissolution kinetics and mineralization behavior were investigated. Moreover, the cytotoxicity and ability to improve wound closure was examined in cellular studies using keratinocyte-like cells and fibroblasts. Finally, the antibacterial effect of the composite foams was evaluated in contact with wound-healing relevant gram-positive bacteria S. aureus and gram-negative bacteria E.coli [1, 3, 6].
2. Materials and methods
2.1. Scaffold production
To fabricate the foams, 2.5 wt.% MC (viscosity 4000 cP, Sigma Aldrich) was dissolved in distilled water at 40°C. After cooling the solution to room temperature, 2.5 wt.% MH was added to the solution, which was kept under stirring for at least 15 min. For composite scaffolds, 0.75 wt.% B3 BG (composition in wt.%: 5.5 Na2O, 11.1 K2O, 4.6 MgO, 18.5 CaO, 56.6 B2O3, and 3.7 P2O5) or 0.75 wt.% B3C BG (composition in wt.%: 5.5 Na2O, 11.1 K2O, 4.6 MgO, 15.5 CaO, 56.6 B2O3,3.7 P2O5, and 3.0 CuO) in particulate form was added to achieve a BG content of 15 wt.%. The BGs were produced according to a previous report [44], then crushed using a Jaw Crusher (Retsch, Haan, Germany) and milled using a zirconia planetary mill (Retsch, Haan, Germany) to a fine powder with an average particle size of 5–20 µm. The produced MC-based solutions were then frozen at −20 °C and subsequently moved to the freeze dryer (Alpha 2–4 LSC plus, Christ, Germany). Cylindrical samples of 12 mm height and 9 mm diameter were obtained.
2.2. Characterization
We performed SEM analysis (Auriga 0750, Zeiss, Oberkochen, Germany) to investigate the morphology of the freeze-dried composite foams. Pore sizes were calculated based on SEM images using ImageJ (NIH, Bethesda, MD, USA) based on three foams of each kind of sample [45]. By using energy-dispersive spectroscopy (Oxford Instruments, Abingdon, UK), the presence of BG particles inside the MC–MH foams was assessed. The composite foams were additionally characterized using Fourier transform infrared spectroscopy (FTIR, IRAffinity-1S, Shimadzu Corp). FTIR spectra were collected with a resolution of 4 cm−1, 40 scans, in the range of 4000 to 400 cm−1. In order to evaluate the mechanical properties, compression tests using a universal testing machine (Instron 5960, Germany) were performed, using ten samples of each composition at the speed of 5 mm min−1 and a load cell of 100 N. Moreover, the wettability of the foams was assessed on five samples of each material group by contact angle measurement (DSA 30, Krüss, Germany) using a 3 µl water drop. The porosity of the produced freeze-dried foams was calculated based on the following formula: porosity = 1–(ρfoam/ρmaterial), where ρfoam and ρmaterial are the densities of the fabricated foams and used material, respectively. The mean density of the foam (ρfoam) was calculated by dividing the mass by the volume of ten different foams of each kind. The density of the material (ρmaterial) was measured based on the Archimedes' method (employing an analytical balance BM-252, A&D) using films made by drying the different solutions made for freeze drying in a petri dish at room temperature (10 pieces of 1 cm2 were used).
2.3. Acellular bioactivity test
In order to assess the acellular bioactivity of the fabricated freeze-dried foams, samples were immersed in simulated body fluid (SBF) according to the protocol of Kokubo et al [46] for 3, 7, and 14 d. After the different immersion times, the pH was recorded and the samples were collected for further characterization using FTIR and SEM. Moreover, the phase compositions of the freeze-dried foams were additionally characterized by powder x-ray diffraction (XRD) analysis using a diffractometer (Miniflex 600 HR, Rigaku, Japan). Data were collected over a 2θ range from 20° to 60° with a step size of 0.02°. To evaluate the release of MH during immersion in SBF, the collected dissolution medium was analyzed using a UV–Vis spectrophotometer (Specord 40, Analytik Jena, Germany) at 300 nm based on a calibration curve (R2 = 0.9989).
2.4. In vitro cell test
Prior to cell culture tests, freeze-dried foams (cut in pieces of 9 mm diameter and 2 mm height) were disinfected for 1 h under UV light. To better understand the effects of the different samples on cell response, samples were immersed in cell culture medium (CCM) composed of Dulbecco's modified eagle medium containing 10% fetal bovine serum and 1% antibiotic (penicillin and streptomycin) in the same ratio as used in the performed cell culture test and incubated in the same atmosphere as used for cell culture experiments. After 24 h incubation time, the concentration of ionic dissolution products (IDPs) was measured by optical emission spectroscopy with inductively coupled plasma (ICP-OES; Vista MPX, Varian). Prior to the ICP–OES analysis, the samples were acidified by nitric acid to pH = 2. A series of four calibration solutions was prepared to obtain a linear correlation between intensity of ions and concentration. The reference standards certified for ICP techniques were diluted to prepare the stock calibration solutions. In order to deal with non-spectral interferences, the internal standardization technique with scandium was used. Precision of the analysis for all required ions, expressed as RSD%, was below 5%. The average values including standard deviations from three replicates for each dissolved ion are reported.
In order to evaluate the possible cytotoxic effect of the fabricated foams, an indirect cell test using mouse embryotic fibroblast (MEF) cells was performed. MEF cells were cultured in a humidified atmosphere of 95% air and 5% CO2 at 37°C in the just-described CCM. To prepare cell cultures, 100 000 MEF cells were seeded in 1 ml CCM in 24-well plates. Samples, placed in TC-inserts (Sarstedt, Germany), were immersed in 1 ml CCM without making contact with the MEF cells. Cells in indirect contact to samples were incubated for 24 h in the same atmosphere as described. Cells without contact to samples were taken as positive control and cells incubated in CCM containing additionally 6% of Dimethyl sulfoxide were taken as negative control. Four replicates of each sample type were used. After 24 h of incubation, the viability of the cultivated MEF cells was evaluated by using a WST-8 assay (Sigma-Aldrich) [47] and cell morphology was assessed by using rhodamine phalloidin and DAPI based on an established protocol [48].
Moreover, the freeze-dried foams were tested in a direct cell test using human dermal fibroblasts (hDFs). The cells were grown, harvested, and counted as just described. 50 000 cells were seeded directly by drop-seeding using 50 µl CCM drop on each sample, incubated for 15 min and then filled with 1 ml CCM. Samples with hDFs on top were incubated for 24 h and 7 d under described conditions. After 24 h and 7 d, the cell viability was assessed as already described and the morphology was evaluated by SEM analysis after fixing the samples as described elsewhere [48, 49]. Foams were transferred to a new well plate prior to measuring the cell viability in order not to take into account cells attached to the bottom of the well-plate.
Additionally, based on an established protocol, an in vitro scratch test was performed using human keratinocyte-like HaCaT cells. CCM containing IDPs was prepared as described before. HaCaT cells were seeded at 500 000 cells per ml per well in a 24-well plate for 24 h under the same conditions as described before. After 24 h, by using a 200 µl pipette tip, a scratch was created on the grown HaCaT cell monolayer. After washing with PBS, cells were kept in conditioned CCM for 24 h. Additionally, pure CCM was used as positive control and CCM containing 6% dimethyl sulfoxide (Sigma Aldrich, Germany) as negative control. During this period of 24 h, the cell migration (closure of wound) was observed after 2.5, 5, 8, and 24 h using a light microscope (Primo Vert, Carl Zeiss). The test was performed in triplicate and the mean width of the scratch was calculated using ImageJ software [45].
2.5. Antibacterial test
The antibacterial effect of the fabricated samples was evaluated against the gram-positive bacteria S. aureus and the gram-negative bacteria E. coli. Bacteria strains were incubated in lysogeny broth medium at 37°C for 24 h under static conditions. Then, optical density (OD) (600 nm, Thermo ScientificTM GENESYS 30TM, Germany) of the bacteria medium was arranged to reach 0.015, according to turbidity measurement of bacterial cultures. 10 µl of arranged bacteria suspension was placed in a tube containing 1 ml of the previously described CCM and freeze-dried samples prepared as described for the cell culture experiments. After 24 h of incubation, the OD was recorded. Tubes containing pure CCM were taken as positive control and MH (same amount as incorporated in the foams) as control for the honey-containing samples. All samples were performed in triplicate. Relative bacterial viability (%) was then calculated by the following equation:
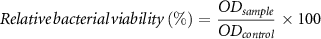
3. Results
3.1. Crosslinking of MC with MH
In figure 1, the FTIR spectrum of MH shows the typical bands previously reported in the literature [50]. In fact, OH vibrations are detectable as a broad band centered around 3250 cm−1 (OH stretching) and at 1645 cm−1 (bending deformation). The characteristic FTIR bands related to the presence of sugar (main component of the honey, usually in the range of 60%–75%) and other organic compounds (i.e. organic acids) are noticeable in the range 1500–750 cm−1 [51]. Usually, in the studies reported in the literature related to the use of MH in combination with other compounds for food or biomedical applications, the typical FTIR bands related to the presence of methylglyoxal, responsible for the antibacterial properties of MH, are not reported. A possible reason could be the fact that, being a highly reactive, low molecular mass dialdehyde, the FTIR bands of this compound overlap the other main bands of honey related to sugar and organic acids. Nevertheless, the use of IR spectroscopy has been reported to be a useful tool for the identification of methylglyoxal and the related antibacterial properties of MH, as reported by Sultanbawa et al [52].
Figure 1. FTIR spectra of MH, MC, and the MC–MH foams in the range of 4000–2500 cm−1, with the zoom on the components of the OH vibrations band for MC–MH in the range 1700–700 cm−1. The indicated bands are discussed in the text.
Download figure:
Standard image High-resolution imageGlyoxal and methylglyoxal are well known for their properties related to the crosslinking of polymers and proteins [20, 53]. The main reactions behind the crosslinking process occur in relation with the hydroxyl and amino groups of the polymer or protein to be crosslinked [54]. Considering that methylglyoxal is an essential component in MH and that the mechanism of interaction between cellulose and glyoxal in acid environment has been established [55, 56], showing the promotion of the crosslinking reaction through hemiacetalization and acetalization processes [57], the authors anticipated that a similar interaction would occur between MH and MC during the preparation of the present scaffolds.
For what concerns the FTIR spectrum of MC, the main bands related to hydroxyl groups stretching vibrations are detectable at 3440 cm−1, while the C–H stretching vibrations are represented by the peaks between 2925 and 2830 cm−1. Other two characteristic peaks of MC are the shoulder at 1150 cm−1 due to the C–O stretching from oxygen bridge and the C–O–C stretching mode of the glucosidic unit at 1050 cm−1. Moreover, the vibration of OCH3 groups is identified by the peak centered at 940 cm−1.
The crosslinking reaction is suggested to occur among the hydroxyl groups of MC and the methylglyoxal component in honey. Considering the amount of hydroxyl groups available for the reaction and the amount of methylglyoxal with respect to the total amount of both components, a low degree of crosslinking is expected. The main modifications reported in the spectrum of MC–MH, which could be attributed to the crosslinking reaction, are depicted in figure 1. In the OH vibration region, higher wavenumber values are assigned to the vibrations of 'free' hydroxyl groups, centered around 3575 cm−1, and this band is not detectable in the zoom view of figure 1, for the MC–MH spectrum. Moreover, it is possible to observe three components in the OH bands centered at 3430, 3340, and 3228 cm−1, in which the first two bands could be ascribable to intramolecular hydrogen bonded hydroxyl groups and the latter one to the intermolecular hydrogen bonded hydroxyl groups [58]. In addition to OH bands, it is also possible to notice a shift of the bands to the area of the intermolecular H bonds of hydroxyl groups. The intensity of this band is also increased with respect to the one in MC. A similar modification is observed for the peaks centered in the range 1100–1000 cm−1, mainly related to the sugar content of the honey and the glucosidic units of the MC.
3.2. Morphology of foams
In order to verify the porous structure of the produced foams, samples were observed by SEM (figure 2). All produced samples exhibited a highly porous structure, whereas foams containing (B3/B3C) BG seem to be more dense and not homogenously porous. Additionally, higher-magnification SEM images confirmed the smooth surface of MC and MC–MH foams, whereas the addition of B3/B3C BG particles led to a rougher surface. By SEM/EDX analysis, the presence of B3/B3C BG particles in the MC–MH foams could be confirmed (figures 2(E) and (F)), and the BG particles seem to be well distributed in the MC–MH foam structure. Based on the SEM images, a pore size range of 50–200 µm was found for all foams; here, the addition of B3/B3C BG particles seems not to have a significant impact on pore size. Moreover, the produced foams possess a slightly different porosity as summarized in table 1. Also, contact angle measurements showed that the addition of MH leads to a reduction of the contact angle and therefore to a higher hydrophilicity of the produced MC-based foams. The contact angle of foams containing, additionally to MH, either B3 or B3C BG was not detectable because the foams directly absorb the water drop (table 1). It should be mentioned that the contact angle measurement performed here did not take into account the porosity and roughness of the samples, which could also have a great influence.
Figure 2. SEM images of (A) MC, (B) MC–MH, (C) MC–MH–B3, and (D) MC–MH–B3C freeze-dried foams and EDX spectra of (E) MC–MH–B3 and (F) MC–MH–B3C foams.
Download figure:
Standard image High-resolution imageTable 1. Density, porosity, mean compression strength, and contact angle of the fabricated foams.
| Foam | Density (g/cm3) | Porosity (%) | Contact angle (°) | Mean compression strength (at 40%–60% of compression) (kPa) |
|---|---|---|---|---|
| MC | 0.12 ± 0.02 | 93.4 ± 0.5 | 101 ± 7 | 17 ± 3 |
| MC–MH | 0.27 ± 0.03 | 93.6 ± 0.4 | 77 ± 9 | 13 ± 4 |
| MC–MH–B3 | 0.34 ± 0.02 | 95.2 ± 0.4 | n.d. | 43 ± 6 |
| MC–MH–B3C | 0.45 ± 0.01 | 96.8 ± 0.1 | n.d. | 40 ± 6 |
n.d. = not detectable.
In general, lyophilized hydrogels based on MC and MH can be produced by dissolving the materials in distilled water. This is a great advantage compared to other polymers, where toxic solvents are needed, which could lead to possible residuals inside the obtained samples limiting their application in the biomedical field [59, 60]. During the preparation of the MC–MH sol, no phase separation occurred and, according to SEM images and EDX measurements, the two different BG particle types could be well dispersed. The produced foams provide a high porosity of more than 90%. Additionally, all samples exhibit a suitable pore size range for migration and proliferation of cells as well as vascularization. The pore size range was found to be similar to freeze-dried hydrogels containing BG based on gelatin–hyaluronic acid [61], alginate [62], collagen–hyaluronic acid [63], and MH-containing cryogels (without BG) based on silk fibroin [19, 64] and gelatin [18]. However, compared to other studies using silk-based lyophilized foams [65] and gelatin–chitosan foams [66, 67] containing BG, which show less porosity and pore size ranges, the present MC–MH based foams containing BG appear to be more suitable for tissue-engineering applications based on their pore structure. The open structure with suitable pore size is also crucial to allow nutrient, waste, and gas exchange, cell infiltration, as well as the distribution of nutrients [68].
3.3. Mechanical characterization
The produced freeze-dried foams were further mechanically characterized by a compression test, and the mean compressive strength was calculated based on the measured compression stress corresponding to 40%–60% of compression strain, as shown in figure 3. Mechanical properties affect the performance of wound dressings. Independently, if the dressing should cover a dermal wound or should fill an internal wound, the dressing should be stable enough and of sufficient structural integrity to withstand different stresses. The mechanical properties also play a crucial role in the handling in clinical routine [69]. By the addition of MH, the compression strength (table 1) of the produced foams was (not significantly) reduced, as already reported in the literature for silk fibroin cryogels containing MH [18]. However, the introduction of B3/B3C BG particles leads clearly to strengthening of the foams, whereas no significant difference between the two different borate BGs could be found. The measured compressive strength values are comparable to reported data on lyophilized foams based on silk fibroin and gelatin [18]. Therefore, the incorporation of BG is a suitable method to compensate for the lack of mechanical properties of foams, according to a 'composite' concept [70], as mentioned in the introduction. Moreover, all foams remained intact after the compression strength test, and were even able to recover their shape to a certain point after a certain time (a few hours to days). The results thus demonstrate the ability of the composite MC–MH foams containing BG to withstand (relevant) loads without breaking—an ideal property for a successful wound dressing.
Figure 3. Exemplary result of compression test of a MC foam. The mean compressive strength was calculated based on the measured compression stress corresponding to 40%–60% (marked in gray) of compression strain.
Download figure:
Standard image High-resolution image3.4. Acellular bioactivity of fabricated foams
In order to assess the bioactivity of the produced freeze-dried foams in terms of formation of calcium phosphate of foam surfaces, samples were immersed in SBF for up to 14 d. The samples were then analyzed by SEM. According to figures 4(A) and (B), as expected, foams without BG show no formation of calcium phosphate on their surfaces. Foams containing additionally B3/B3C BG particles showed the first signs of calcium phosphate formation after 3 d in SBF (figures 4(C) and (D)). The formation of calcium phosphate increased with increasing immersion time in SBF, which could be also proven by SEM images (figures 4(E) and (F)). The dissolution and bioactive behavior of BGs is well documented [26], and it is characterized by a rapid exchange between glass network modifiers and hydrogen from the solution (here, SBF), leading to an increase of pH and the breaking of the glass network. Further, calcium ions as well as phosphate groups migrate from the BG and solution in order to form amorphous calcium phosphate, which, in some cases, crystallizes into hydroxyapatite [25, 26].
Figure 4. SEM images of (A) MC foam and (B) MC-MH foam after 14 d in SBF, showing no visible formation of calcium phosphate. SEM images of (C) MC-MH-B3 foam and (D) MC-MH-B3C foam after 3 d in SBF showing first signs of calcium phosphate formation, which is more visible for (E) MC-MH-B3 and (F) MC-MH-B3C foams after 14 d of immersion.
Download figure:
Standard image High-resolution imageTo further investigate and assess the formation of calcium phosphate on the different fabricated foams, FTIR and XRD measurements were conducted. As already observed in SEM images, MC and MC–MH foams show no visible peaks in the FTIR spectra after 14 d of immersion in SBF, which could be attributed to calcium phosphate (figure 5). In contrast, the FTIR spectra of MC–MH–B3 and MC–MH–B3C foams show peaks at 1000–1100 cm−1, which can be attributed to P–O stretching [71, 72]. Further, the peaks at 560 and 600 cm−1 correspond to P–O bending vibrations [60]. Additionally, XRD measurements were performed in order to evaluate if the detected calcium phosphate had crystallized into hydroxyapatite. XRD spectra before immersion in SBF (not shown here) showed no measurable peaks for all different fabricated foams, which was expected since the polymeric matrix, as well as the introduced BG particles, did not contain any crystalline phase. Additionally, after immersion in SBF for 14 d, no XRD peaks were found for all fabricated foams (figure 5). This result could be due to the fact that the amount of hydroxyapatite on the surface is lower than the detection limit of XRD or due to the fact that the formed calcium phosphate is poorly crystallized (or a combination of both).
Figure 5. (A) FTIR spectra obtained on different foams immersed in SBF for 14 d, (B) XRD spectra of different foams after 14 d in SBF, and (C) release of MH versus immersion time in SBF.
Download figure:
Standard image High-resolution imageThe remaining SBF solution after the different immersion times was also investigated. By pH measurements, it was possible to observe an increase of pH with dissolution of the foams. After 14 d of immersion in SBF, MC and MC–MH samples led to a minor pH increase of up to 7.7 ± 0.1, whereas MC–MH–B3 and MC–MH–B3C samples led to an increase of up to 7.8 ± 0.1 (pure SBF had a pH of 7.5 ± 0.1). Moreover, the release of MH was analysed by UV–Vis spectroscopy. Here, it is important to mention that MH consists of several different compounds, which show different UV maxima [73]. Therefore, it is possible that some compounds are faster released than others, which could not be analyzed here. Moreover, it is also possible that some of the released compounds already degraded and therefore the real amount of release is higher than the measured amount. Nevertheless, as shown in figure 5(C), it could be confirmed that MH is released from the different fabricated foams containing MH. The addition of B3/B3C BG particles seems to favor the release of MH. This could be due to the increased surface area by the release of BG particles from the foams and the presence of interfaces between the particles and the matrix. More interestingly, the released amounts seem to not (significantly) increase over immersion time, which is a sign that MH and MC are well connected in the present freeze dried foams.
Although it is commonly agreed that the ability to form hydroxyapatite on a surface is a marker of the bioactivity potential of BGs (usually in connection with bone tissue applications), in wound healing applications, the role of hydroxyapatite in the wound-healing process is still an open question [29]. The formation of hydroxyapatite can lead to soft tissue calcifications, which should be avoided [31, 74]. On the other hand, hydroxyapatite has been shown to attract macrophages in a rabbit model [75], and it is also well known that a stable bonding can occur between BG and soft tissues [28]. Therefore, the fact that the produced composite foams did not show any crystalline phase after immersion in SBF should not be considered necessarily a disadvantage. On the other hand, the formation of calcium phosphate on foam surfaces shows that ions could be released from the BG particles, proving the previously mentioned 'second bioactivity mechanism' of BGs: the release of biologically active ions. Consequently, further in vitro cell tests as well as ICP measurements were performed to verify the bioactivity of the BGs and the suitability of the composite foams to be used as wound dressings. These results confirmed the formation of a CaP layer on the composite scaffolds, demonstrating that the BG preserves its bioactivity even if embedded in the polymeric matrix. Further studies are needed to assess and quantify the crystallinity of the deposited calcium phosphate.
3.5. In vitro bioactivity in contact with fibroblasts
ICP measurements verified that ions (namely B, Ca, Mg, P, K, and Na) could be released from the BGs used in this study. Since the amount of Na and K is already high in the CCM, leading to an inaccurate measurement, figure 6 shows only the released amounts of B, Ca, Cu, Mg, and P. No significant differences between the reference (pure CCM) and CCM containing dissolution products of MC and MC–MH could be found. In the case of MC–MH–B3 and MC–MH–B3C, significantly higher amounts of B, Ca, Mg, and P could be measured. Moreover, the only measurable difference between CCM containing IDPs of MC–MH–B3 and MC–MH–B3C is the release of copper from the Cu-doped B3 glass inside the MC–MH foam.
Figure 6. Ion concentration of CCM containing IDPs of the different MC-based foams measured by ICP–OES. All samples were measured in triplicate. One-way ANOVA statistical analysis denotes significant differences compared to the reference (*p < 0.05). No remarkable difference between the MC–MH foams containing B3 or B3C BGs could be found.
Download figure:
Standard image High-resolution imageAs a preliminary cytotoxic study, an indirect test using MEF cells was conducted (figure 7). According to figure 7(A), the morphology of MEF cells cultured in the presence of dissolution products of MC and MC–MH foams was similar to that of the positive control, showing a dense layer of cells. The addition of B3 particles seems to not negatively influence MEF cells; however, the cell morphology seems to be different. Interestingly, according to the measured cell viability, MC and MC–MH foams led to an increase of cell viability compared to the control, whereas no significant difference could be found for B3-containing foams (figure 7(B)). On the contrary, foams containing Cu-doped BG led to a reduced number of cells according to fluorescence images and the measured cell viability. Nevertheless measurements on both samples showed better results compared to the negative control. In contrast to this preliminary test using MEF cells, the direct cell test using hDF cells showed that the addition of B3 BG particles led to a significant increase of cell viability after 1 d; however, after 7 d, no significant differences could be found (figure 8(A)). Moreover, the addition of Cu-doped BG seems not to have a negative impact on the direct cell test: no differences between foams with and without B3C BG could be found after 1 and 7 d. SEM images of hDF cells cultured on the fabricated foams for 7 d are shown in figure 8(B). The morphology is typical for hDF cells, growing in a dense layer on top of the porous foams [76–78], which corresponds well to the results obtained by cell viability measurements.
Figure 7. Results of indirect cell culture test for 24 h incubation using MEF cells: (A) fluorescence images of actin filaments (red) and cell nuclei (blue) for the freeze-dried MC based foams, (B) relative viability of MEF cells cultured with IDPs of MC-based foams and controls (positive control was set to 100%). By one-way ANOVA statistical analysis, significant differences (*p < 0.05) could be found for all samples, except as denoted by #.
Download figure:
Standard image High-resolution imageFigure 8. Results of direct cell culture test using hDF cells. (A) Viability of hDF cells cultured directly in contact with different MC-based foams for 1 and 7 d. One-way ANOVA statistical analysis denotes significant differences (*p < 0.05). (B) SEM images of cells grown on different types of foams after culturing for 7 d.
Download figure:
Standard image High-resolution imageBy performing in vitro scratch tests, it is assumed that it is possible to observe cells migrating along the edge of the newly created scratch in order to establish cell–cell contact again, therefore closing the wound [79]. The test has been applied recently to phosphate BG fibers [80]. In figure 9, the results obtained by calculating the migration ratio of cells cultured in contact with dissolution products of the different foams are summarized and images of HaCaT cells directly after the scratch and after 24 h, cultured with both CCM (Control+) and 6% DMSO-CCM (Control-), are shown. A clear migration of cells on the positive control sample can be observed, whereas the addition of 6% DMSO to the CCM completely stopped the migration of HaCaT cells and even seems to have a negative impact on the cell morphology. The dissolution products of the fabricated foams did not show any negative impact on cell migration compared to the control; MH and B3 BG particles even showed a faster migration than the control (figure 9(A)).
Figure 9. Results obtained by an in vitro scratch test: (A) closure of scratch (presented by the migration ratio) versus time and (B) exemplary light microscope images of scratch controls after 0 and 24 h.
Download figure:
Standard image High-resolution imageIn summary, the performed cell biology tests showed that fabricated neat MC foams do not have any cytotoxic effect; the foams even showed a slight improvement of cell viability, proliferation, and migration. Further, by adding MH into the MC–MH foams, an increase of cell viability (shown in the indirect cell test using MEFs) could be achieved. A study performed by Sell et al [81] using hDFs, macrophages, and human pulmonary microvascular endothelial cells showed that high concentrations of MH can have a cytotoxic effect. Moreover, in the mentioned study, low concentrations of MH seem not to have any effect on the viability of all three cell types, and an in vitro scratch test using hDFs did not show any impact of MH [81]. Therefore, it seems that by combining MC and MH, a more favorable release of MH could be achieved, leading to improved cell migration. However, the effect of MH needs to be further investigated, since in the mentioned study different kinds as well as different amounts of MH were used. By introducing BG particles into the MC–MH foams, a further improvement of cell viability (shown after 1 d of direct culture of hDFs) and migration could be found, showing the great potential of B3 BG to enhance the biological performance of MC–MH foams. However, the effect of the addition of copper to B3 BG needs to be more precisely investigated. A previous study [82] showed a critical biological level of Cu2+ higher than 10 mg l−1 on the viability of 3T3 fibroblasts, which was not exceeded here. In a similar study [83], Cu released in concentrations around 10 mg l−1 did not have significant effects on endothelial cells. The uncertainty of a clear trend regarding the use of Cu for angiogenic effects indicates that more research is necessary to understand the effect of different concentrations of copper in wound-healing approaches, which needs to be investigated for different cell types and conditions.
3.6. Antibacterial test
As described in the introduction, wound healing could be negatively influenced by contaminations, which can lead to non-healing, chronic wounds. In the early phase of wound healing, a race between cells and bacteria to the surface of the wound/wound dressing occurs [1]. Therefore, besides being biocompatible, an ideal wound dressing should also provide an antibacterial effect, while not disturbing the attachment of cells and therefore the tissue-regeneration process. In order to analyze the efficiency against bacteria of MH and BG incorporated in the MC foams, antibacterial tests using E. coli and S. aureus were conducted. Both bacteria are well known for wound infections, whereas S. aureus is known to affect the initial phase of wound healing and E. coli is known for being involved in chronic wounds [1, 3, 6]. According to figure 10, MC foams did not have any impact on bacteria growth, whereas MC–MH foams showed a clear reduction of S. aureus, and even a stronger effect is observed on E. coli. The more pronounced effect of MH on E.coli has been already reported [52]. This result is in accordance with the antibacterial effect shown by MH alone. Moreover, the introduction of B3 BG particles shows a minor (not significant) effect on the growth of both kinds of bacteria. Most interestingly, the presence of B3 particles seems to disturb the antibacterial effect of MH inside the MC–MH–B3 composite foam. Moreover, the MC–MH foam containing Cu-doped B3 BG shows more pronounced antibacterial effect compared to MC–MH foams. Here, the relative bacteria viability of E.coli decreased from around 60% to almost 0% (figure 10). Therefore, it seems that the incorporation of B3C BG particles adds an extra effect for killing bacteria to the MC–MH foam and therefore increases its suitability for applications on infected and chronic wounds. Copper is a well studied antibacterial agent; for instance, copper ions have been reported to penetrate bacteria, leading to DNA degradation [84]. Based on the differences in the structure of gram-positive and gram-negative bacteria, gram-negative bacteria have a higher degree of protection and therefore copper ions are more effective against gram-positive bacteria [85, 86]. Moreover, it needs to be pointed out that both MH and antibacterial ions such as Cu offer an advantage compared to antibiotics. It is well known that in recent years, fewer new antibiotics have been developed and more resistance against available antibiotics is continuously developing [3, 24]. Unlike antibiotics, herbal extracts as MH and therapeutic ions can preserve their effectiveness, as suggested by experiments where bacteria have not developed any resistance even after continuous exposure to these compounds [87, 88].
Figure 10. Relative bacteria viability of E.coli and S. aureus in contact with the different fabricated MC-based foams. All samples were measured in triplicate. One-way ANOVA statistical analysis denotes significant differences (*p < 0.05); # symbolizes significant differences (p < 0.05) compared to MC for both kinds of bacteria.
Download figure:
Standard image High-resolution image4. Conclusion
Freeze-dried foams based on MC crosslinked with MH were successfully produced. The foams showed high porosity and improved wettability. The wettability and mechanical properties could be further improved by the addition of two different borate BGs in particulate form. The addition of BG particles further provides bioactive properties, as shown in in vitro studies using SBF. Most interestingly, the composite foams exhibited promising results in indirect and direct cell culture tests, using MEF and hDF cells, by improving cell proliferation and migration. Moreover, MH released from the foams was able to kill bacteria to a certain extent, which was further enhanced by the introduction of copper-containing BG particles. However, further studies are necessary to evaluate the dose-dependent effect of MH and more precisely the effect of copper ions released from the BG component. Nevertheless, the produced foams based on MC crosslinked with MH and further improved by the addition of BG particles showed promising results for consideration as wound dressings capable of preventing infections, and represent a versatile technology platform for a new generation of antibiotic-free wound dressings based on natural products and ion-releasing BGs.
Acknowledgments
Prof. Leena Hupa (Process Chemistry Centre, Åbo Akademi, Turku, Finland) and Åbo Akademi's Johan Gadolin Scholarship are gratefully acknowledged for a visiting fellowship for KS. The authors acknowledge partial contribution of the project of the Centre for Functional and Surface Functionalized Glass (CEGLASS), ITMS code 313011R453, funded from the European Regional Development Fund, Operational Programme Research and Innovation, where ICP-OES measurements were carried out.
Conflicts of interest
There are no conflicts to declare.










