Abstract
Wound dressings based on bacterial cellulose (BC) can form a soft and conformable protective layer that can stimulate wound healing while preventing bacteria from entering the wound. Bacteria already present in the wound can, however, thrive in the moist environment created by the BC dressing which can aggravate the healing process. Possibilities to render the BC antimicrobial without affecting the beneficial structural and mechanical properties of the material would hence be highly attractive. Here we present methods for functionalization of BC with ε-poly-L-Lysine (ε-PLL), a non-toxic biopolymer with broad-spectrum antimicrobial activity. Low molecular weight ε-PLL was cross-linked in pristine BC membranes and to carboxymethyl cellulose functionalized BC using carbodiimide chemistry. The functionalization of BC with ε-PLL inhibited growth of S. epidermidis on the membranes but did not affect the cytocompatibility to cultured human fibroblasts as compared to native BC. The functionalization had no significant effects on the nanofibrous structure and mechanical properties of the BC. The possibility to functionalize BC with ε-PLL is a promising, green and versatile approach to improve the performance of BC in wound care and other biomedical applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Bacterial cellulose (BC) is a biopolymer produced by Gluconacetobacter xylinus (more recently reclassified as Komagataeibacter xylinus) and to a certain extent by some other bacteria [1]. While having the same chemical structure as cellulose from plants, impurities like hemicellulose and lignin are not present, and the fibers are significantly thinner giving BC unique and attractive chemical and physical properties [2, 3]. BC displays high chemical stability, high tensile strength and flexibility, large water holding capacity, permeability to gases and liquids, and excellent biocompatibility [4]. BC has consequently been explored as a material in a wide range of applications, including bioelectronics [5], fuel cells [6], selective absorbents [7], and biosensors [8, 9], in addition to tissue engineering [10–12], and wound dressings [13–16].
BC has been explored in wound care applications since the 1980s due to its ability to form a soft and conformable protective layer that can stimulate the healing process [17]. In addition, the small pore sizes created by the nanofibrillar cellulose network prevent bacteria from entering the wound. On the other hand, bacteria already present in the wound can thrive in the moist environment created by the BC wound dressing. Wound infections severely aggravate wound healing and can be life threatening [18]. At the same time, the alarming problems with antibiotic resistance and the fact that most antiseptic compounds (e.g. iodine) can have a negative impact on the healing process leaves much to be desired in creating an optimal treatment for infected patients [19]. Possibilities to provide BC wound dressings with inherent antimicrobial properties would thus be extremely attractive. Several strategies to adsorb, covalently bind, or physically entrap antimicrobial compounds in BC, including antibiotics, silver nanoparticles, chitosan, and cationic antiseptics, have been developed [17]. A group of antimicrobial compounds that has been less extensively investigated in this context are antimicrobial peptides (AMPs) [20]. AMPs are an integral part of the first line of host defense against infections in a wide variety of organisms and can show broad-spectrum antimicrobial activity [21, 22]. ε-poly-L-Lysine (ε-PLL) is an AMP produced by e.g. Streptomyces albulus [23]. ε-PLL is non-toxic to humans, water soluble and biodegradable, and utilized as a food preservative [24, 25]. ε-PLL adsorbs to, and disrupts the integrity of, the bacterial cell membrane, which in addition to effectively killing the bacteria reduces the risk for development of resistance [26]. ε-PLL is thus an attractive candidate in the search for novel strategies to combat infections, especially in wound care applications and in combination with BC since a high active concentration of the antimicrobial agents then can be provide locally in the wound [27].
In order to render the BC antimicrobial and prevent rapid compound leeching, the ε-PLL must be chemically tethered to the BC nanofibers or physically trapped within the fibrous matrix. A number of strategies have been demonstrated for chemical functionalization of BC-based materials including silylation [28, 29], oxidation of the cellulose using 2,2,6,6-tetramethylpiperidine-1-oxy catalyzed reactions [30], or oxidation using plasma [31]. Such methods are very versatile and enable further chemical functionalization by a wide variety of species, including antimicrobial compounds [17], but are laborious and require use of organic solvents and most often have to be carried out under nitrogen atmosphere [32, 33]. These functionalizations also often affect both the structure of the cellulose fibers and the mechanical properties of the materials [28, 30, 31]. BC can also be prepared as a composite materials, where BC membranes or BC fiber slurries are soaked or mixed with e.g. polymers or metallic nanoparticles, that either adsorb [16, 34, 35], or are cross-linked within the membrane [11]. Although such strategies are more facile, crosslinking of functional molecules in the BC membrane may lead to loss of function, whereas adsorption-based strategies may suffer from poor long-term stability and limit the possibilities to sterilize the materials. Further development of methods for functionalization of BC with AMPs that do not influence the structural and mechanical properties of the BC or reduce the biocompatibility of the BC membrane and enable sterilization using standard methods without loss of antimicrobial activity is hence needed.
Here, we propose two strategies for efficient functionalization of BC wound dressings with ε-PLL in aqueous conditions that preserves both the natural fibrillar structure and mechanical properties of the native BC. In the first approach, ε-PLL was covalently conjugated using carbodiimide chemistry to carboxymethyl cellulose (CMC) adsorbed to the BC. CMC has previously been reported to adsorb to both BC and thin films of cellulose from plants [36, 37], and as shown here, can be utilized to provide long term stable anchoring of ε-PLL to BC that can withstand both ethanol sterilization and autoclaving. As a second approach we investigated the possibility to cross-link the ε-PLL within the BC meshwork using carbodiimide chemistry to form a stable interpenetrating network (IPN). The ε-PLL was a relatively low molecular weight molecule consisting of approximately 25–35 lysine residues (∼4–5 kDa) and previously has been reported to exhibit bactericidal properties when incorporated in a hydrogel [38]. Both the CMC and ε-PLL functionalized BC retained the same cytocompatibility as native BC. In addition, ε-PLL functionalized BC membranes exhibited contact inhibition of Staphylococcus epidermis. The proposed strategies for providing antimicrobial properties to BC are rapid, green, and versatile, and preserve the overall beneficial properties of BC, opening up new avenues for the use of BC in treatment of infected wounds.
Materials and methods
Materials
All chemicals, if not further specified, were obtained from Sigma-Aldrich (USA). BC wound dressings were supplied by S2Medical AB (Linköping, Sweden). The membranes were cut into discs of 6 mm diameter using a biopsy punch (Stiefel, UK), and were stored in 70% ethanol. ε-PLL hydrogen chloride (ε-PLL, 3–4 kD) was obtained from Handary (Belgium). Cell culture medium was supplied from Fisher Scientific (USA).
Functionalization of BC membranes
Prior to functionalization, BC membranes were equilibrated in Milli-Q water (MQ) to remove residual ethanol. BC membranes were functionalized by physical adsorption of sodium CMC (average Mw ∼ 250 000, degree of substitution 0.7) which supplies carboxyl groups for easy coupling of other molecules. The BC membranes were incubated for 1.5 h in 0.5 mg ml−1 or 1.0 mg ml−1 CMC and 50 mM sodium chloride dissolved in MQ or in 10 mM acetate buffer (Ac, pH 3.9). Before further functionalizations, membranes were rinsed in MQ. For recording of Fourier transform infrared spectroscopy (FTIR) spectra and assessing the successful adsorption of CMC and its ability to be used as an anchor molecule for further modification, human serum albumin (HSA) was coupled as a reporter molecule. For coupling of HSA 0.4 M using N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDC) and 0.1 M N-hydroxysuccinimide (NHS) 0.1 M (both dissolved in MQ) were mixed in a 1:1 ratio and incubated with the membranes for 15 min. Excess solution was rinsed off and the samples were incubated with 5 mg ml−1 HSA in 10 mM acetate buffer (pH 3.9). Membranes not activated with EDC/NHS or not containing CMC were used as controls. For testing of stability, samples were washed for 30 min in 2 M NaCl to remove electrostatically bound HSA and for 30 min in 70% ethanol to test stability to ethanol sterilization. Stability during autoclaving was tested by autoclaving HSA coupled BC-CMC for 20 min at 121 °C. Immobilization of peptides on BC was carried out using a lysine-rich coiled coil peptide (KV) previously designed and synthesized by Aronsson et al [39]. For functionalization of membranes with ε-PLL, BC and BC-CMC were incubated for 1 h with 5 mg ml–1 ε-PLL. Subsequently membranes were rinsed with MQ and incubated with EDC/NHS solution prepared as described above and incubated for 1 h and finally rinsed in MQ. As a reporter molecule for the detection of bound ε-PLL as well as free amines methyl red (MR) was coupled to the membranes using EDC/NHS chemistry. 1 mg ml−1 MR was dissolved in ethanol and mixed 1:1 with EDC/NHS solution prepared as described above and incubated for 15 min. The activated MR solution was then added to ε-PLL functionalized membranes and incubated for 1 h. Excess dye was removed by washing the functionalized BC membranes in MQ for 2 d.
FTIR-attenuated total reflection (FTIR-ATR) measurements of HSA and peptide functionalized BC membranes
FTIR spectra of BC and BC-CMC (MQ and Ac) membranes before and after immobilization of HSA were recorded using a Bruker Vertex 70 instrument in an ATR setup. Measurements were done with a resolution of 4 cm−1 and 16 sampling steps per spectrum. Spectra were recorded from 600 to 4500 cm−1. The resulting data was analyzed by normalizing resulting spectra to maximum of the highest peak of the BC signal. The mean values of the amide I and II band intensities at approximately 1655 cm−1 and 1540 cm−1, respectively, were used for further statistical analysis.
MR functionalized membranes
For the analysis of primary amines available for conjugation, BC, BC-ε-PLL and BC-CMC-ε-PLL membranes were reacted with MR as a reporter dye as described above and were measured in the pH range 4.5–2.0. Spectra were recorded using a fiber optics setup using an Ocean Optics QE65 Pro spectrophotometer with an Ocean Optics halogen HL-2000-FHSA light source in the wavelength range 350–1000 nm. The absorbance at 460 nm at pH 4.5 was used as an indicator of the amount of bound MR. The pH responsiveness was observed by assessing absorption difference between pH 3.9 and 2.0 at 550 nm as well as the ratio of 460–550 nm.
X-ray photoelectron spectroscopy (XPS) and sample preparation
XPS was used to investigate the elemental composition of BC, ε-PLL and CMC-ε-PLL functionalized BC. Samples for XPS were prepared by washing BC, BC-PLL and BC-CMC-PLL membranes for 24 h after functionalization and subsequent air drying overnight and attaching them to silicon wafers using copper tape. For each membrane the XPS spectra were acquired from two individual samples at two different spots using an Axis Ultra DLD spectrometer (Kratos Analytical) employing monochromatic AlKα radiation (hν = 1486.6 eV) with the x-ray anode operating at 150 W. The base pressure in the analysis chamber of the instrument during spectra acquisition was better than 1.1 × 10−9 Torr (1.5 × 10−7 Pa). All spectra were collected at normal emission angle from the area of 0.3 × 0.7 mm2. Survey scans were acquired using Epass = 160 eV with energy steps of 0.5 eV and narrow scans of O1s, N1s and C1s were acquired using Epass = 20 eV with energy steps of 0.1 eV. The low-energy electron flood gun was used for charge compensation. XPS spectra were aligned against the C–C/C–H component of the C1s peak set at 285 eV. Data evaluation was carried out using CasaXPS software (version 2.3.17).
Scanning electron microscopy (SEM), atomic force microscopy (AFM) and sample preparation
Samples for SEM analysis were chemically dried using ethanol in a series of 10 min incubations with increasing concentrations (30%, 50%, 70%, 95%) and finally two 15 min incubations in 99.5% ethanol. The ethanol was subsequently exchanged by hexamethyldisilazan (30%, 75% and 100%), with 10 min incubation at each concentration. Samples were then left to air dry overnight and subsequently sputter coated by platinum (Leica EM SCD 500) prior to imaging. The pore and fiber structure of BC, BC-CMC, BC-ε-PLL and BC-CMC-ε-PLL were observed using a SEM (Gemini LEO1550) at 5 kV acceleration voltage. Samples for AFM were dehydrated and dried as described above for SEM. Samples were then analyzed using a Veeco Instruments Dimension 3100 SPM AFM in tapping mode. The data was analyzed using WSxM 4.0 Beta 8.2 software [40].
Rheology of BC and functionalized BC membranes
Oscillatory rheology measurements were acquired with a Discovery HR-2 hybrid rheometer (TA instruments) using a 8 mm parallel plate geometry at 20 °C. BC, BC-CMC and BC-ε-PLL membranes were cut to be slightly larger than the geometry. To adjust for variability in membrane thickness the gap height was adjusted to give an axial force in the range 0.02–0.03 N. Frequency sweep measurements were done in the range 0.1–100 rad s−1 with a fixed strain of 0.1%. All measurements were done in triplicates.
Cell culture
Primary human fibroblasts, previously isolated from dermis by Palm et al [41] were seeded into 6 well plates at an initial concentration of 8000 cells/well and cultured in Dulbecco's modified Eagle medium high glucose, supplemented with 10% fetal bovine serum without addition of antibiotics, at 37 °C and 5% CO2. Cells were allowed to attach to the culture plastic overnight and sequentially covered with BC, BC-CMC, BC-ε-PLL or BC-CMC-ε-PLL. All materials were sterilized using 70% ethanol and cut to cover the whole well. Cells were observed over the course of 13 d using an Olympus CKX41 light microscope.
Antimicrobial assay
Single colonies of Escherichia coli MG1655 and Staphylococcus epidermidis were inoculated into 5 ml Luria-Bertani broth and incubated at 37 °C overnight on shaker set at 200 rpm. Bacterial cultures (100 μl) were spread onto blood agar plates and BC, BC-CMC, BC- ε-PLL or BC-CMC-ε-PLL membranes, were cut into ∼5 mm disks and placed onto the agar plates. The agar plates were incubated for 1 d at 37 °C and subsequently for 30 d at 4 °C. Bacterial growth was monitored under light microscope (Olympus SZX9) and images were captured at 200× magnification.
Statistical analysis
Data presented are shown as mean ± standard deviation. For statistical analysis paired t-test using GraphPad Prism version 6.01 was applied. Groups were seen as statistically different with an α-value <5% (p < 0.05).
Results and discussion
Adsorption and stability of CMC on BC
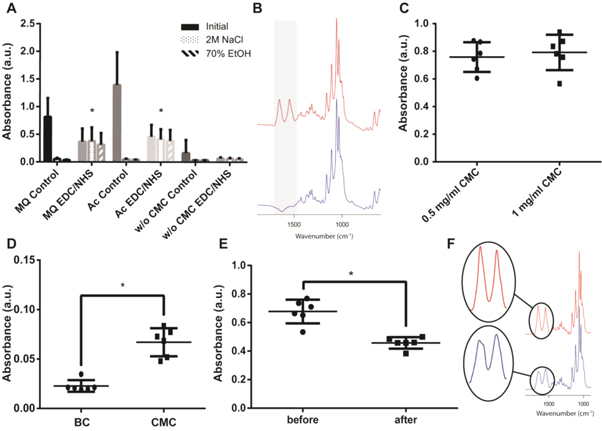
CMC was adsorbed to BC as a means to introduce functional groups to the BC to facilitate further functionalization of the material by ε-PLL using standard carbodiimide chemistry (EDC/NHS). The relative amount of adsorbed CMC and stability of CMC functionalized BC to sterilization were investigated using ATR-FTIR (figure 1). Because of the large IR absorption of BC and the overlapping vibrational modes with CMC, the extent of CMC was difficult assess directly by ATR-FTIR. The adsorption of CMC was hence first evaluated by conjugation of HSA to the CMC functionalized BC using EDC/NHS. The pronounced amide I and II bands of the protein allowed for indirect quantification of available CMC as well as the stability of the functionalization. The effect of two different conditions for CMC adsorption was tested, using either CMC dissolved in MQ water or acetate buffer (Ac) pH 3.9, both containing 50 mM NaCl to reduce electrostatic repulsion. For the immobilization of HSA, Ac was chosen as a buffer since the isoelectric point (pI) of HSA is above pH 4.0, therefore increasing the interaction with the carboxyl groups of CMC. After an initial rinse with water, non-activated BC-CMC appeared to bind more HSA than EDC/NHS activated BC-CMC (figures 1(A), S1(A) is available online at stacks.iop.org/BMM/13/025014/mmedia). On further investigation this was, however, shown to be due to electrostatic interactions, as rinsing in 2 M NaCl led to a complete loss of bound HSA in control groups, while leading to no change when covalently bound via CMC (figures 1(A), S1). The larger amount of HSA bound in the control groups prior to rinsing is most likely due to the EDC/NHS activation of the carboxyl groups in CMC that reduce the overall net charge, lowering the electrostatic attraction of HSA. BC without CMC showed very little protein binding clearly indicating that CMC adsorption to BC enabled the conjugation of HSA (figure 1(A)). The different conditions used for CMC adsorption proved to have no significant effect on the final amount of bound protein, as indicated by the intensities of the amide bands. However since adsorption in Ac showed slightly better results in significance testing than MQ in comparison to their respective control (p-value of 0.0081 and 0.0221 respectively) Ac was used in further experiments.
Figure 1. (A) Relative amounts of HSA bound after conjugation, remaining HSA after wash in 2 M NaCl and after wash in 70% ethanol. (B) FTIR spectra of native BC (blue) and HSA coupled to BC-CMC (red). (C) Relative amount of bound HSA using different CMC concentrations (after rinsing in 2 M NaCl). (D) Conjugation of coiled-coil peptides to BC-CMC. Stability to autoclaving, (E) FTIR peak intensity and (F) FTIR spectra before (red) and after (blue) autoclaving.
Download figure:
Standard image High-resolution imageTwo different concentrations of CMC (0.5 and 1 mg ml−1) were tested to investigate possible limitation in binding due to depletion of the CMC in the solution. However, increasing the concentration of CMC did not significantly increase the amount of HSA bound (figure 1(C)), indicating that CMC was present in excess. Since HSA is a rather large molecule (∼66 kD) the possibility to conjugate small peptides comparable in size to ε-PLL to BC-CMC a synthetic coiled coil polypeptide (KVSALKEKVSALKEKNSALKWKVSALKE) was used [39]. Due to the lower molecular weight of the peptide (MW ∼ 3 kD) as compared to HSA the intensities in the FTIR spectra were significantly reduced but the amide peaks were still readily visible. Significantly more (p-value of 0.0005) polypeptides were found on the BC membranes when coupled via CMC as compared to the control group (no CMC) showing almost no adsorption of peptide (figure 1(D)).
Interestingly, washing the BC-CMC in 70% ethanol for 30 min, a frequently used sterilization method for in vitro testing of materials, did not result in any loss of covalently bound HSA (figures 1(A), S1(C)). For clinical testing, however, autoclaving is often the sterilization method of choice. In contrast to sterilization by ethanol, autoclaving resulted in a significant broadening and reduction (p-value of <0.0001) in the intensity of the amide bands (figure 1(E)). The lower intensity combined with the broadening of the amide I band (1655 cm−1) and the appearance of a new peak at ∼1630 cm−1 indicates that the bound proteins denatured due to the autoclaving rather than detaching (Figure 1(F)) [42].
Functionalization of BC with ε-PLL
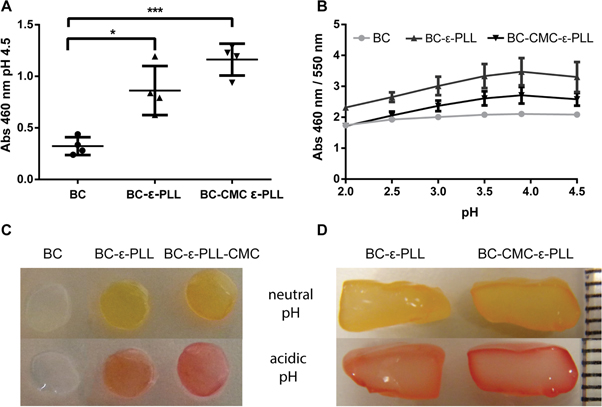
To render the BC antimicrobial, ε-PLL was coupled to BC-CMC using carbodiimide chemistry by first soaking the membranes in an aqueous solution of ε-PLL (5 mg ml–1) followed by a quick rinse in MQ water and addition of EDC/NHS. BC membranes without CMC were also treated under identical conditions to investigate the possibility to trap sufficient ε-PLL within the BC meshwork without the use of CMC. In contrast to BC functionalized by HSA and the coiled coil peptide, confirming the presence and conjugation of ε-PLL by FTIR was more difficult. This is presumably due to the amino acids in ε-PLL being linked in an ε-configuration resulting in slightly lower amide band intensities, making them difficult to observe considering the massive BC background signal. This effect was also apparent when comparing high concentration solutions of HSA and ε-PLL (100 mg ml−1), where ε-PLL reached just about 1/3 of the intensity of HSA (figure S2). Therefore an indirect method to detect ε-PLL by coupling of the pH sensitive dye MR to the bound ε-PLL using EDC/NHS was employed. Due to the strong absorbance of MR in the visible wavelength range, MR bound to the membranes could be detected using UV–vis spectroscopy and correlated to the concentration of ε-PLL (figure 2). Very little unspecific binding of MR to unfunctionalized BC could be seen, whereas functionalized materials showed a clear coloration due to the bound MR. The MR absorption peak at 460 nm at pH 4.5 was used as an indicator of the total amount of primary amines available on the membrane (figure 2(A)). ε-PLL and CMC-ε-PLL functionalized membranes showed clearly significant differences compared to native BC with respect to bound MR (p-value of 0.0419 and 0.0004 respectively). The large amount of MR on CMC-ε-PLL, as indicated by a high total absorbance, indicate efficient conjugation of ε-PLL to the BC fibers. Interestingly, a fairly high MR signal was also obtained for ε-PLL crosslinked in BC membranes without CMC, indicating that CMC was not required to retain the ε-PLL. Since ε-PLL molecules have one terminal carboxyl group in addition to the numerous primary amines, it can hence be crosslinked to some extent by EDC/NHS and possibly form an IPN or pseudo IPN within the BC membrane.
Figure 2. UV–vis absorbance of BC membranes labeled with MR and assessment of functionalization depth. (A) Absorbance at 460 nm at pH 4.5, (B) response to pH change (ratio), (C) visual appearance of the membranes at neutral (top) and acidic pH (bottom). (D) Sectioning of thick MR-modified BC clearly indicate that the functionalization depth was limited to about ∼0.4 mm (the distance between two lines on the scale represents 1 mm).
Download figure:
Standard image High-resolution imageIn addition, the MR retained its pH responsive properties after conjugation to the BC. However, the immobilized MR showed a shift in the pH range of the colorimetric response from pH 4.4–6.2 to pH 2–4, likely a result of the carboxyl group being used for conjugating the MR to the ε-PLL. Absorbance spectra of the functionalized materials were recorded at different pH-values (figure S3). When comparing the ratio of absorbance at 460/550 nm, similar pH response curves were seen for all materials except for unfunctionalized BC, which as expected, showed almost no response (figure 2(B)). The pH response of MR functionalized BC membranes, could also be seen clearly by eye, which also allowed observing that the color changes were reversible (figure 2(C)).
In addition to demonstrate the presence of ε-PLL on the membranes, the possibility to covalently anchor indicator dyes to ε-PLL functionalized BC enabled a convenient method to estimate the penetration depth of the functionalization. BC have quite small pore sizes, which restrict diffusion of macromolecules in the membranes. The effects of diffusion limitations were clearly observed on thick pieces of BC (∼3 mm) when the BC was sectioned after the MR conjugation using a sharp scalpel. The sectioning showed that MR conjugated to the thicker BC-ε-PLL and BC-CMC-ε-PLL had penetrated to a depth of approximately 0.4 mm into the materials, clearly visible as a colored red or yellow rim surrounding an uncolored core (figure 2(D)). This clearly indicates that for thin BC membranes (<1 mm), a fairly homogenous functionalization is to be expected, while thicker pieces will primarily be functionalized at the surface.
Elemental characterization of ε-PLL functionalized membranes
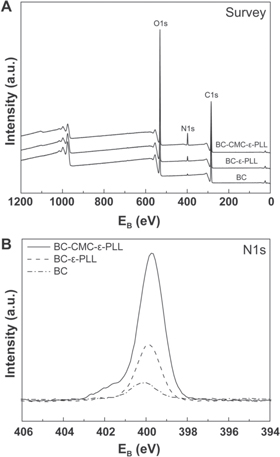
To further confirm the presence and relative amount of ε-PLL in the BC membranes, XPS was used to determine the elemental compositions of native BC, BC-ε-PLL and BC-CMC-ε-PLL membranes. Survey spectra (figure 3(A)) and narrow spectra of the major constituents (carbon, nitrogen and oxygen) were recorded. As pure cellulose would, except for impurities, not contain any nitrogen, any increase in nitrogen content could thus be attributed to bound ε-PLL. As expected, native BC did only contain a small amount of nitrogen. ε-PLL functionalized membranes showed a significant increase in nitrogen content, which could be increased even further by CMC functionalization before the crosslinking of ε-PLL (figure 3, table 1). This observation also correlates with the data obtained by indirect detection of ε-PLL using MR conjugation indicating that a larger amount of ε-PLL is found in the CMC functionalized BC membranes.
Figure 3. XPS survey spectra (A) and high-resolution N1s core level spectra (B) for BC-ε-PLL-CMC, BC-ε-PLL and BC.
Download figure:
Standard image High-resolution imageTable 1. XPS elemental composition of BC, BC-ε-PLL and BC-CMC-ε-PLL.
| XPS elemental composition (atomic %) | |||
|---|---|---|---|
| C1s | O1s | N1s | |
| BC | 62.2 | 37.2 | 0.6 |
| BC-ε-PLL | 62.8 | 35.1 | 2.2 |
| BC-CMC-ε-PLL | 65.2 | 28.4 | 6.4 |
Morphological characterization of functionalized BC
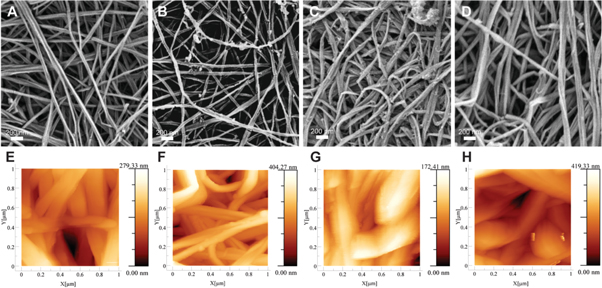
To observe possible changes in the nanofibrous structure of the BC membranes after functionalization, the native BC as well as CMC, ε-PLL and CMC-ε-PLL functionalized BC membranes were investigated by SEM and AFM. The fibers in native BC showed a thickness of approximately 50–100 nm in SEM (figure 4(A)). Both individual fibrils and bundles of fibers could be seen. The meshwork structure of the nanofibers creates pores with sizes smaller than 200 nm. The small pores prevent bacteria from migrating through the membrane, which is beneficial in wound care applications. The presence of pores is, however, critical to enable fluid and gas exchange through the membranes. The fiber thickness seemed to be unchanged after functionalization and no additional material blocking the pores could be observed, indicating that the proposed functionalization primarily form thin layers surrounding the individual fibers (figures 4(B)–(D)).
Figure 4. SEM and AFM of BC (A), (E), BC-CMC (B), (F), BC-ε-PLL (C), (G) and BC-CMC-ε-PLL (D), (H).
Download figure:
Standard image High-resolution imageAFM measurements were carried to further characterize the surface of CMC, ε-PLL and CMC-ε-PLL functionalized samples in a less dehydrated state (figures 4(E)–(H)). Overall the fiber diameter appeared substantially larger than in the SEM micrographs, which is likely a combined result of tip deconvolution effects and the sample being imaged under ambient conditions and thus being slightly hydrated. Both SEM and AFM indicate that membranes functionalized with ε-PLL appeared to have a slight increase in fiber thickness, while CMC functionalized fibers show slightly smaller in fiber diameters, as compared to unfunctionalized BC. These small variations in fiber thickness and absence of material blocking the pores in the membranes clearly indicate that the functionalization leads to formation of a thin layer of crosslinked ε-PLL surrounding the BC fibers, possibly combined with a dilute continuous IPN of crosslinked ε-PLL.
Rheology of BC and functionalized BC membranes
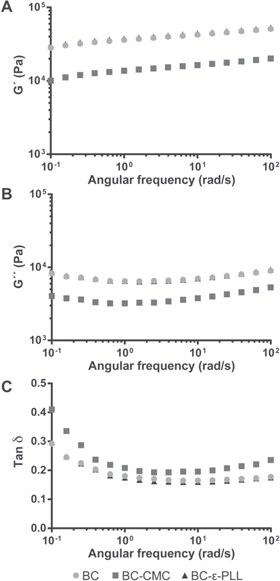
Covalent strategies for functionalization of BC tend to significantly influence the mechanical properties of the material, which in turn can have a negative influence on the properties of BC in wound dressing [28, 30, 31]. To assess the influence of the functionalization on the viscoelastic properties of hydrated BC, a rheological analysis of BC, BC-CMC and BC-ε-PLL membranes was carried out. Storage (G') and loss (G'') modulus, and therefore also their ratio (the loss tangent), were very similar for BC and BC-ε-PLL membranes (figure 5). Interestingly, BC-CMC showed lower values in both moduli and higher loss tangent compared to the other membranes. A higher loss tangent indicates a more liquid-like material, suggesting an increase in the water content of the membranes due to the CMC adsorption. This could be explained by additional swelling and better water storage capability caused by an influx of counter ions attracted by the CMC layer. Overall storage and loss moduli were linear and remained within the same range for all frequencies indicating Newtonian behavior.
Figure 5. Mean storage modulus (G') (A), loss modulus (G'') (B) and loss tangent (Tan δ) (C) of BC, BC-CMC and BC-ε-PLL membranes in an angular frequency range of 0.1–100 rad s−1 (n = 3).
Download figure:
Standard image High-resolution imageCytocompatibility of BC, BC-CMC, BC-ε-PLL and BC-CMC-ε-PLL
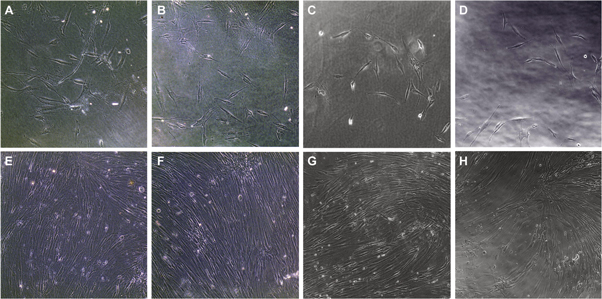
In order to investigate the effect of the functionalization of the BC on the cytocompatibility of the membranes, human dermal fibroblasts (HDF) were cultured in direct contact with BC, BC-CMC, BC-ε-PLL or BC-CMC-ε-PLL (figure 6). HDF cells were used due to their important effects on wound healing [33]. The membranes were placed on top of the cultured cells in order to simulate the covering of the wound and to investigate the membrane permeability to gases and nutrients. Due to the optical transparency of BC membranes, the cells could be observed without having to remove the membranes. Cells cultured in contact with BC and functionalized BC showed a nearly confluent state and a typical elongated morphology with alignment of the cells to each other after 13 d of culture (figures 6(E)–(H)). No differences in in cell morphology and proliferation rate were seen when cultured under native BC as compared to functionalized BC membranes. It is important to note that none of the membranes stimulated an increase in the proliferation rate in fibroblasts, as an exaggerated proliferation in vivo could contribute to scar formation.
Figure 6. Human dermal fibroblast cultures cultured in contact with BC (A), (E), BC-CMC (B), (F), BC-ε-PLL (C), (G) and BC-CMC-ε-PLL (D), (H) membrane, directly after application of the membranes (A)–(D) and after 13 d of culture (E)–(H).
Download figure:
Standard image High-resolution imageAntimicrobial effect of ε-PLL functionalized BC
In order to investigate the antimicrobial activity of the functionalized BC when in direct contact with bacteria, membranes were incubated at 37 °C on blood agar plates seeded with Escherichia coli MG1655 and Staphylococcus epidermidis. After 24 h incubation, BC and BC-CMC membranes were covered by bacteria, whereas a significant reduction in growth of S. epidermis was seen on, and in the immediate vicinity of both the BC-ε-PLL and BC-CMC-ε-PLL membranes (figures 7, S4). The effect was even more pronounced after storage of the agar plates at 4 °C for 30 d, strongly indicating that the antimicrobial activity of both the BC-ε-PLL and the BC-CMC-ε-PLL was retained for an extended time period. Negligible antimicrobial effect was, however, seen for E. coli. This is likely a result of the higher concentrations of ε-PLL required to kill E. coli as observed in suspension, or a difference in the actual mechanism of membrane distribution in Gram positive and negative bacteria, which warrants further studies.
Figure 7. Antimicrobial effect of BC functionalization with ε-PLL on S. epidermidis. Bacteria cultures after 1 d at 37 °C (A)–(D) and subsequent culture for 30 d at 4 °C (E)–(H) on BC (A), (E), BC-CMC (B), (F), BC-ε-PLL (C), (G) and BC-CMC-ε-PLL (D), (H).
Download figure:
Standard image High-resolution imageConclusions
In conclusion, this work describes new methods for rendering BC wound dressings antimicrobial using the AMP ε-PLL and very benign chemistry, i.e. aqueous media and ambient conditions. Two strategies were evaluated; using carbodiimide chemistry to cross-link the ε-PLL within the BC mesh or by conjugating the ε-PLL to CMC that was first physisorbed to the BC. CMC was shown to efficiently adsorb to the BC fibers and exhibited stability to high ionic strength and could also be stored and washed in 70% ethanol and autoclaved without any significant changes to the degree of functionalization. Successful functionalization of the BC with ε-PLL was confirmed by conjugation of MR to the primary amine groups in ε-PLL, also showing a penetration depth profile of the ε-PLL of about 0.4 mm into the nanocellulose meshwork. Elemental analysis using XPS measurements verified the presence of immobilized ε-PLL by showing a substantial increase in nitrogen content. Additionally, no significant alterations in the BC nanofibrous structure and pore size were observed after functionalization, suggesting small effects on gas and nutrition exchange through the membrane, while retaining a barrier function against larger particles (e.g. bacteria). The viscoelastic assessment of BC membranes showed no changes when functionalized with ε-PLL, but suggested an increase in water retention capacity in CMC functionalized membranes. Membranes functionalized by CMC and ε-PLL were shown to retain the cytocompatibility of native BC. Importantly, ε-PLL functionalized BC exhibited efficient contact inhibition of S. epidermidis. In addition to the simple and green functionalization strategy, the possibility to render BC antimicrobial without affecting the structural and mechanical properties of the material is of large interest for fabrication of dressings for prevention and treatment of infected wounds.
Acknowledgments
The authors would like to thank Jörgen Bengtsson for technical support. The authors would like to thank Linköping University and the Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (Faculty Grant SFO-Mat-LiU No. 2009 00971), the Carl Trygger Foundation, and the Knowledge Foundation for financial support.