Abstract
We previously developed a biomaterial scaffold that could effectively provide seed cells to a lesion cavity resulting from traumatic brain injury. However, we subsequently found that few transplanted human umbilical cord mesenchymal stem cells (hUC-MSCs) are able to migrate from the scaffold to the lesion boundary. Stromal derived-cell factor-1α and its receptor chemokine (C-X-C motif) receptor (CXCR)4 are chemotactic factors that control cell migration and stem cell recruitment to target areas. Given the low expression level of CXCR4 on the hUC-MSC membrane, lentiviral vectors were used to generate hUC-MSCs stably expressing CXCR4 fused to green fluorescent protein (GFP) (hUC-MSCsCXCR4/GFP). We constructed a scaffold in which recombinant human brain-derived neurotrophic factor (BDNF) was linked to chitosan scaffolds with the crosslinking agent genipin (CGB scaffold). The scaffold containing hUC-MSCsCXCR4/GFP was transplanted into the lesion cavity of a rat brain, providing exogenous hUC-MSCs to both lesion boundary and cavity. These results demonstrate a novel strategy for inducing tissue regeneration after traumatic brain injury.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Abbreviation
| hUC-MSCs | human umbilical cord mesenchymal stem cells |
| SDF-1α | Stromal derived-cell factor-1α |
| CXCR4 | chemokine receptor 4 |
| BDNF | brain derived neurotropic factor |
| CGB scaffolds | chitosan scaffolds linking brain derived neurotropic factor, using genipin |
Introduction
Traumatic brain injury (TBI) often results in mortality or long-term disabilities such as dysphasia or cognitive deficits [1]. The primary destruction and secondary injuries such as ischemia and inflammation caused by TBI may cause extensive tissue loss of cerebral parenchyma which results in cavities formation [2]. To date, there are no effective clinical treatments for repairing cerebral parenchyma following TBI [3]. Recently, stem/progenitor cell transplantation has shown great promise for this purpose [4]. However, nerve repair remains a challenge, and new treatment methods are required to minimize neuronal death and provide exogenous seed cells to replenish lost neurons and promote the recovery of neurological function after TBI [5].
Recent advances in stem cell research have included the use of human umbilical cord mesenchymal stem cells (hUC-MSCs) in disease models [6–10]. Owing to their immunosuppressive capacity and ability to differentiate into various cell types [11, 12], hUC-MSCs are a major candidate for cell-based therapies, particularly in regenerative medicine. Our previous work demonstrated that chitosan-based scaffolds can provide a suitable microenvironment for hUC-MSC attachment and proliferation [5] and may therefore serve as a basis for TBI treatment.
Secondary injury resulting from trauma, including delayed release of inflammatory/biochemical mediators and ischaemia, can cause continuous neuronal damage at the lesion cavity edge. Cell migration is a critical aspect of stem cell recruitment to lesioned areas; however, there are currently no effective methods to induce the migration of transplanted seed cells to lesion sites so as to replace cells lost through injury. Stromal cell-derived factor (SDF)-1α and its receptor chemokine (C-X-C motif) receptor (CXCR)4 are important chemotactic factors for stem cells [13]. Together they enhance proliferation, promote chain migration and transmigration, and activate intracellular pathways modulating neural stem cells [14]; SDF-1α has been shown to be critical for MSC migration [15], and high levels of SDF-1α/CXCR4 binding at injury sites help to retain mobilised CXCR4-positive cells [16]. However, only a small proportion (~1%) of cultured MSCs express CXCR4 [17–22].
To overcome this limitation and to determine whether SDF-1α/CXCR4 can induce the migration of transplanted hUC-MSCs, in this study we transfected hUC-MSCs with a lentiviral vector encoding CXCR4 and examined the effects of CXCR4 overexpression on cell migration in vitro and in vivo. hUC-MSCsCXCR4/GFP were induced to differentiate into neurons in the presence of recombinant human brain-derived neurotrophic factor (BDNF) released from cellular scaffolds. Our findings provide a potential strategy for the therapeutic application of SDF-1α/CXCR4 to promote the migration of transplanted hUC-MSCs to the site of brain injury following TBI.
Materials and methods
Stable expression of CXCR4 in hUC-MSCs
hUC-MSCs were purchased from the stem cell bank at the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in 75 cm2 flasks in 10 ml Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1, v/v) containing 10% fetal bovine serum (FBS; Gibco/Life Technologies, Carlsbad, CA, USA) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The medium was replaced every 3 d. When cultures reached 80% confluence, cells were resuspended with 0.25% Trypsin Express (Gibco/Life Technologies) and reseeded in 75 cm2 flasks at a density of 3 × 105 cells/flask. Third-passage hUC-MSCs were transfected with pLV-null-enhanced green fluorescent protein (eGFP) and pLV-CXCR4-eGFP lentiviral vectors. Blasticidin (5 μg ml−1) was used to screen stable hUC-MSCCXCR4/GFP and hUC-MSCGFP lines. Transfection efficiency was confirmed by fluorescence microscopy [21].
Quantitative real-time (qRT-)PCR and western blot analysis
CXCR4 and GFP expressions levels in hUC-MSCs, hUC-MSCsGFP, and hUC-MSCsCXCR4/GFP were evaluated by qRT-PCR and western blotting. The following forward and reverse primer sequences were used: CXCR4, 5'-AGT CTG GAC CGC TAC CTG G-3' and 5'-ATG GTG AGC AAG GGC GAG GAG-3' and GFP, 5'-GCA AAG ATG AAG TCG GGA ATA-3' and 5'-TCA AAG ATC TAC CAT GTA CAG CTC GT-3'. Total RNA was isolated using RNAqueous-4PCR Total RNA Isolation kit (AM1914; Invitrogen, Carlsbad, CA, USA) and qRT-PCR was carried out in triplicate using FastStart Universal SYBR Green Master (Roche, Indianapolis, IN, USA). Each 20 μl reaction contained 9 μl SYBR Green I mix, 5 μl cDNA, 2 μl each forward and reverse primers, and 2 μl RNase-free H2O. The mixture was incubated at 95 °C for 10 min, followed by 42 cycles of 95 °C for 15 s and 60 °C for 60 min. The specificity of the PCR product was confirmed by melting curve analysis (obtained between 60 °C and 95 °C) and 2% agarose gel electrophoresis. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and western blotting were performed according to standard protocols. Briefly, cells were lysed in lysis buffer containing 15 mM Tris–HCl (pH 7.5), 120 mM NaCl, 25 mM KCl, 1 mM EDTA, 0.5% Triton X-100 (Sigma, St. Louis, MO, USA), and 100 × Halt Protease Inhibitor Single-Use cocktail (Thermo Scientific, Waltham, MA, USA). Whole cells lysates (50 μg per group) were separated using 4%–12% Novex Bis-Tris SDS-acrylamide gels (Invitrogen), then electro-transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), which were washed three times in phosphate-buffered saline with 0.05% Tween-20 (PBST) followed by overnight incubation at 4 °C with rabbit antibodies against HIF-1a (ab2185) and glyceraldehyde 3-phosphate dehydrogenase (ab181602; all from Abcam, Cambridge, MA, USA). After three washes with PBST, membranes were incubated with secondary antibody conjugated with horseradish peroxidase in 0.5% milk for 2 h at room temperature. Immunoreactivity was detected using the ECL Western Blotting Substrate (ID 32109; Pierce, Rockford, IL, USA). Signal intensity was quantified with a Quantity One analysis system (Bio-Rad).
Detection of cell surface marker expression
To confirm the identity of transfected hUC-MSCsCXCR4/GFP, surface antigen expression was evaluated by flow cytometry using phycoerythrin-conjugated antibodies against cluster of differentiation CD34, CD73, and CD105; fluorescein isothiocyanate-conjugated antibodies against CD45, CD90, and CD14; and an antibody against human leukocyte antigen HLA-DR (all from BD Pharmingen. San Jose, CA, USA). After incubation with the antibodies, cells were centrifuged and washed twice with PBS before they were sorted with a BD FACscan instrument (BD Pharmingen).
Cell proliferation assay
The viability of hUC-MSCsCXCR4/GFP was measured with Cell Counting Kit (CCK)-8 (Dojindo, Kumamoto, Japan). Cells transfected with pLV-CXCR4-eGFP or pLV-null-eGFP—i.e. hUC-MSCsCXCR4/GFP and hUC-MSCsGFP, respectively—served as positive and negative controls. Untransfected hUC-MSCs were also used as controls. Cells were seeded in 96-well plates at a density of 1 × 104 cells in 100 μl DMEM/F12 medium (Gibco/Life Technologies) supplemented with 10% FBS and incubated at 37 °C in 5% CO2/95% air. After 0, 12, 24, 36, 48 and 72 h, 10 μl CCK-8 solution were added to each well and the cells were incubated for 30 min at 37 °C; absorbance was measured at 450 nm on a Synergy 2 multimode microplate reader (BioTek, Winooski, VT, USA).
Fabrication of chitosan scaffolds with genipin-immobilized BDNF (CGB scaffolds)
Scaffolds were constructed as previously described [4]. Crosslinked scaffold solution was generated by adding 9 mg genipin (Yuanyebio, Shanghai, China) and 2 mg recombinant human BDNF (PeproTech, Rocky Hill, NJ, USA) to 10 ml acetic acid (1%) containing 0.2 g chitosan. The solution was stored in a freeze dryer (Thermo Scientific) at −56 °C for 24 h after vigorous mixing; the scaffolds were then immersed in 0.1 mol/l NaOH to neutralize the remaining acetic acid. After overnight drying at room temperature, scaffolds were repeatedly rinsed with ultra-pure water and sterilized by ultraviolet radiation for 1 h.
Adhesion and activity assays
hUC-MSCsCXCR4/GFP (1 × 104) were cultured in medium containing 1% penicillin, 1% streptomycin (Solarbio, Beijing, China), and 10% FBS and added to CGB scaffolds in 24-well plates. Samples were incubated at 37 °C in 5% CO2/95% air. Scaffolds with adherent hUC-MSCsCXCR4/GFP were examined on day 1 by scanning electron microscopy. In briefly, the CGB scaffolds with or without hUC-MSCsCXCR4/GFP were fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) for 30 min, washed in ice-cold PBS, blocked in O.C.T. and processed in a cryostat (Leica CM1950, Germany). Standard 25 mm thick sections were cut, then affixed to poly-lysine coated glass slides, then store in drying case before scanning. The images of scanning electron microscope (SEM) were randomly collected of each group (N = 3). Cell viability in the CGB and control groups (cells incubated with or without CGB scaffolds, respectively) was assessed with the CCK-8 assay. After adding 10 μl CCK-solution to each well for 1 h at 37 °C, absorbance was measured at 450 nm on a microplate reader. Cell viability was calculated using the equation: viability (%) = (absorbance of experimental group—absorbance of control group)/absorbance of control group × 100%.
Transwell migration assay
The role of the SDF-1α/CXCR4 signaling axis in hUC-MSC migration was assessed with the transwell assay using 8 μm inserts (BD Pharmingen). A total of 1 × 104 cells were resuspended in 100 μl serum-free DMEM/F12 medium and loaded into the upper wells while the lower chambers were filled with 500 μl complete medium (DMEM/F12 supplemented with 10% FBS). For migration assays, SDF-1α was added to the lower chamber at a concentration of 50, 75, or 100 ng ml−1; another group was also treated with AMD3100 blocking reagent. After incubation in a humidified chamber of 5% CO2/95% air at 37 °C for 24 h, cells were fixed with 500 μl methanol for 15 min. For the migration assay, the inner surface of the upper chamber was wiped with cotton swabs to remove non-migrating cells; the chambers were then washed with 500 μl PBS and stained with 500 μl haematoxylin for 1 min at room temperature. After another wash with 500 μl PBS, transwell membranes were removed and placed on slides and stained cells in five random microscopic fields at 40 × magnification were counted using Image J software (National Institutes of Health, Bethesda, MD, USA).
Differentiation of hUC-MSCsCXCR4/GFP
hUC-MSCsCXCR4/GFP at 1.5 × 104 single-cell suspension in 1 ml media with DMEM/F12 (1:1, v/v) was add to 24-well plate. After 6 h, when the cells attached to the plate, the media was replaced by 1 ml neuronal differentiation medium, which contains DMEM/F12 and added with 50 ng ml−1 bFGF (13256029; Gibco, USA), 250 ng ml−1 SHH (PMC8033; Gibco, USA), 100 ng ml−1 FGF-8 (PHG0184; Gibco, USA), 1 × N2 supplement (17502048; Gibco, USA), 50 μg ml−1 Vit-C. After cultured for 9 d, CGB scaffolds were appended, placed in apical chambers of transwell inserts (CGB scaffold group); the group without the scaffold served as a control. After culturing for 3 d, cells in the lower chamber were removed and processed for immunocytochemistry. Cells were fixed with 4% paraformaldehyde and permeabilised with 0.1% Triton X-100 (Sigma) for 10 min. After blocking nonspecific binding in 10% goat serum for 30 min at RT, cells were incubated overnight at 4 °C with a mouse antibody against microtubule-associated protein (MAP)-2 (1:300, ab11267; Abcam) and a rabbit antibody against neuronal nuclei (NeuN) (1:500, ab177487; Abcam). Cells were then labelled with iFluor 633 goat anti-mouse IgG (1:300, 16478; AAT Bioquest, Sunnyvale, CA, USA) and Alexa Fluor 594 goat anti-rabbit IgG (1:300, ab150080; Abcam) and counterstained with 4,6-diamidino-2-phenylindole (DAPI; Lonza, Basel, Switzerland).
Animal experiments
Animal experiments were approved by the Nantong University Animal Experimentation Committee and were carried out in accordance with their ethical guidelines, which were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A TBI model was generated as follows. Male Sprague-Dawley rats (12 weeks old; 380–450 g) were anesthetised with zoletil 50 (55 mg kg−1 body weight). A piece of skull over the left frontal cortex was removed by drilling. A dural incision was made to expose the forebrain, and defects with a diameter and thickness of 2 mm were created with a scalpel in the cortex at 2 mm to the right and left of bregma. After hemostasis, CGB scaffolds containing hUC-MSCsCXCR4/GFP or hUC-MSCsGFP were implanted into the injury sites. For control animals, saline was instead injected into the site (N = 6). After 2 weeks and 6 weeks, brains were harvested after transcardial perfusion with 250 ml saline and 250 ml 4% paraformaldehyde in 0.1 M PB and postfixed with 4% paraformaldehyde (Sigma) in 0.1 M PB (pH 7.4) for 2 h. After dehydration in 20% and 30% sucrose solutions, brains were sectioned and embedded in optimum cutting temperature compound (Surgipath, Richmond, IL, USA) and sectioned on a cryostat (CM1950; Leica, Wetzlar, Germany). The 10 μm thick sections were collected on poly-L-lysine-coated glass slides and stained for hematoxylin-eosin (HE) stain and immunocytochemical analysis. In brief, to identify differentiating neurons derived from hUC-MSC, sections of 6 weeks were incubated overnight at 4 °C with a mouse antibody against microtubule-associated protein (MAP)-2 (1:300, ab11267; Abcam), after blocking nonspecific binding in 10% goat serum for 30 min at RT, then labelled with iFluor 594 goat anti-mouse IgG (1:300, 16468; AAT Bioquest, Sunnyvale, CA, USA). Nuclei were counterstained with DAPI. Sections were mounted with mounting medium and analysed by fluorescence microscopy (Axiostar scope A1, Carl Zeiss, Jena, Germany).
Statistical analysis
All data are presented as mean ± standard deviation and were analysed with SPSS 10.0 software (SPSS Inc., Chicago, IL, USA). Differences between groups were evaluated by one-way analysis of variance (ANOVA) and were considered significant at P < 0.05 (designated as * in figures).
Results
CXCR4 is stably overexpressed in hUC-MSCsCXCR4/GFP
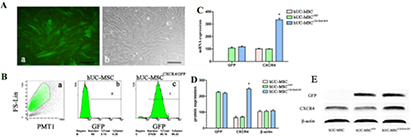
The efficiency of CXCR4 transfection was evaluated by qRT-PCR and western blotting. Protein localisation was determined by confocal microscopy (figure 1(A)), while flow cytometry was used to quantify transfection efficiency (figure 1(B)). CXCR4 expression was higher in hUC-MSCsCXCR4/GFP than in control cells (hUC-MSCsGFP), as determined by qRT-PCR (figure 1(C)) and western blotting (figures 1(D) and (E)). These results indicate that transfected hUC-MSCsCXCR4/GFP stably express CXCR4.
Figure 1. CXCR4 overexpression in human umbilical cord mesenchymal stem cells (hUC-MSCs). (A) hUC-MSCs expressing GFP. (B) The fraction of CXCR4/GFP + cells was 96.43%, as determined by flow cytometry. (C). CXCR4 mRNA expression levels in MSCs was determined by qRT-PCR (*P < 0.05). (D) and (E) CXCR4 protein expression was determined by Western blotting and β actin was used as a loading control. CXCR4 levels were upregulated in MSCsCXCR4/GFP as compared to control cells, as depicted by histograms (*P < 0.05 versus MSC controls).
Download figure:
Standard image High-resolution imagehUC-MSCsCXCR4/GFP express stromal and MSC markers
Flow cytometry was used to identify immunophenotype of hUC-MSCsCXCR4/GFP. From the results, we can confirm that hUC-MSCsCXCR4/GFP expressed the stromal/MSC markers CD73, CD105, and CD90 but not the haematopoietic or endothelial markers CD14, CD34, CD45, CD79a, or HLA-DR (figure 2). These results demonstrate that the immunophenotype of hUC-MSCsCXCR4/GFP was unaffected by transfection.
Figure 2. Flow cytometric analysis of human umbilical cord mesenchymal stem cells stably expressing CXCR4 fused to GFP (hUC-MSCsCXCR4/GFP). Cells expressed the stromal/MSC markers CD73, CD105, and CD90, but not the haematopoietic and endothelial markers CD14, CD34, CD45, CD79a, or HLA-DR.
Download figure:
Standard image High-resolution imagehUC-MSCsCXCR4/GFP are compatible with CGB scaffolds
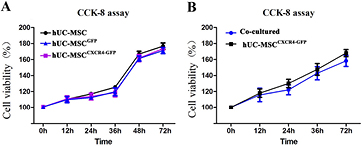
There was no difference in viability between transfected cells and negative controls (P > 0.05) (figure 3(A)), suggesting that the transfection was only mildly cytotoxic to hUC-MSCsCXCR4/GFP, which were available in large numbers for the repair of lesion cavities after TBI. The CCK-8 assay also showed no differences in viability between hUC-MSCsCXCR4/GFP grown in the presence or absence of a CGB scaffold (P > 0.05) (figure 3(B)). The scaffolds were only mildly cytotoxic to hUC-MSCsCXCR4/GFP, indicating excellent biocompatibility. Scanning electron micrographs revealed a smooth scaffold surface with adherent and proliferating hUC-MSCsCXCR4/GFP (figure 4), suggesting that the scaffold can serve as a carrier for hUC-MSCCXCR4/GFP.
Figure 3. Human umbilical cord mesenchymal stem cells stably expressing CXCR4 fused to green fluorescent protein (hUC-MSCCXCR/GFP) viability assays. (A) Viability was similar between hUC-MSCsCXCR4/GFP, hUC-MSCsGFP, and control hUC-MSCs, as determined by the CCK-8 assay (P > 0.05). (B) hUC-MSCCXCR4/GFP viability was similar in the presence and in the absence of the CGB scaffold (P > 0.05).
Download figure:
Standard image High-resolution imageFigure 4. Cells overexpressing CXCR4 cultured on CGB scaffolds. (A) and (B) Scanning electron micrographs of the morphology and surface topology of the porous CGB scaffold (A); the scaffold surface was smooth and had complex 3D structures to which hUC-MSCsCXCR4/GFP adhered (red arrows) (B). Scale bars: 500 μm.
Download figure:
Standard image High-resolution imageCXCR4 overexpression stimulates hUC-MSCCXCR4/GFP migration
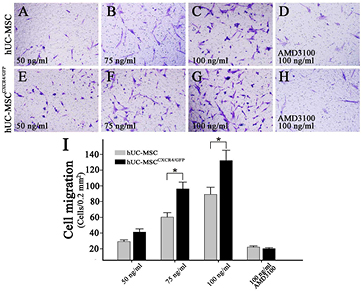
The migration of hUC-MSCs and hUC-MSCsCXCR4/GFP in response to 50, 75, and 100 ng ml−1 SDF-1α was examined by the transwell assay. The number of migrating cells was 25 ± 2.1, 56 ± 5.7, 85 ± 9.1, (figures 5(A)–(C)) respectively, for hUC-MSCs cells and 37 ± 4.1, 92 ± 8.6, 128 ± 13.1, respectively, for hUC-MSCsCXCR4/GFP (figures 5(E)–(G)) in a random microscopic field at 40 × magnification (0.2 mm2); the number was higher for cells overexpressing CXCR4 than for control cells at each concentration of SDF-1α. In addition, hUC-MSCCXCR4/GFP migration at 100 ng ml−1 SDF-1α was abrogated by treatment with AMD3100 (figures 5(D)–(H)), a specific inhibitor of the SDF-1α/CXCR4 axis. The effect of CXCR4 on hUC-MSC migration was concentration-dependent (figure 5(I)). These results indicate that CXCR4 overexpression stimulates hUC-MSC migration in the presence of SDF-1α.
Figure 5. Determination of hUC-MSC migration with the transwell assay. (A)–(H) hUC-MSCs and hUC-MSCsCXCR4/GFP showed concentration-dependent migration in response to SDF-1α ((A)–(C) and (E)–(G)). SDF-1α-dependent hUC-MSCGFP and hUC-MSCCXCR4/GFP migration was blocked by AMD3100 ((C) versus (D) and (G) versus (H)). (I) CXCR4 overexpression enhanced hUC-MSC migration (hUC-MSCCXCR4/GFP versus hUC-MSCGFP, *P < 0.05).
Download figure:
Standard image High-resolution imageCGB scaffolds induce hUC-MSCsCXCR4/GFP differentiation
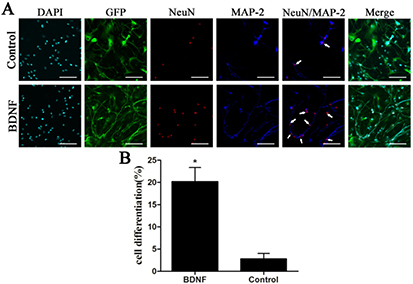
hUC-MSCsCXCR4/GFP were cultured in neuronal differentiation medium with or without BDNF for 12 d and examined by immunocytochemistry to detect MAP-2 and NeuN double-positive neurons. The percentage of neurons was 20.5% ± 2.40% in BDNF-induced cells as compared to 4.3% ± 1.18% in the control group (P < 0.05; figure 6). These results indicate that BDNF released from CGB scaffolds enhances the differentiation of hUC-MSC into neurons.
Figure 6. (A) Neurons were identified by MAP-2 (blue) and NeuN (red) immunoreactivity and neurons are indicated by an arrow. (B) The percentage of neurons out of the total number of DAPI-positive cells was 20.5% ± 2.40% and 4.3% ± 1.18% in the presence and absence of BDNF, respectively. The difference between the two groups is significant (*P < 0.05, (B)) Scale bars: 100 μm.
Download figure:
Standard image High-resolution imageMigrating hUC-MSCsCXCR4/GFP contribute to tissue regeneration in vivo
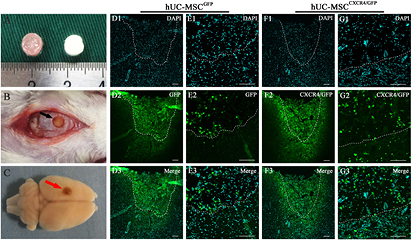
Rat brains were harvested 14 d after transplantation of CGB scaffolds containing hUC-MSCsCXCR4/GFP, hUC-MSCsGFP (figures 7(A)–(C)), or saline. Transplanted hUC-MSCsCXCR4/GFP and hUC-MSCsGFP were identified by green fluorescence and all cells were stained with DAPI (figures 7(D) and (F)). In the lesion boundary zone, the number of GFP-positive cells was higher in the hUC-MSCCXCR4/GFP/CGB scaffold as compared to the hUC-MSCGFP/CGB scaffold group (figures 7(E) and (G)), indicating that overexpression of CXCR4 promotes the migration of transplanted hUC-MSCs from the scaffold to the lesion. Haematoxylin and eosin staining revealed that the scaffold filled the wound cavity 2 weeks after transplantation (figure 8(A1)), in contrast to the severe tissue loss and distortion of the cerebral cortex tissue observed in the saline control group (figure 8(A2)). The wound size was decreased by implantation of the CGB scaffold and newly formed extracellular matrix was present, indicating that the CGB scaffold containing hUC-MSCsCXCR4/GFP was compatible with brain tissue. Also, the immunofluorescence was done to indentify nerve regeneration 6 weeks after transplantation of CGB scaffolds containing hUC-MSCs CXCR4/GFP and containing hUC-MSCsCXCR4/GFP. The double stains of GFP and MAP-2 indicating neurons differentiated from hUC-MSCs. In the transplantation area, the number of GFP and MAP-2 positive cells in the hUC-MSCCXCR4/GFP/CGB scaffold was same with the hUC-MSCGFP/CGB scaffold group. However, in the lesion boundary zone, the number of GFP and MAP-2 positive cells was higher in the hUC-MSCCXCR4/GFP/CGB scaffold as compared to the hUC-MSCGFP/CGB scaffold group (figures 8(E) and (I) white arrow). This result indicated transplantation of hUC-MSCCXCR4/GFP/CGB scaffold not only benefit to the regeneration of the lesion site but also the lesion boundary zone of TBI.
Figure 7. (A) CGB scaffold containing hUC-MSCs. (B) and (C) CGB scaffolds containing hUC-MSCsGFP or hUC-MSCsCXCR4/GFP were transplanted into lesion cavities resulting from TBI in rats (B) and brains were harvested 14 d after transplantation (C). Black and red arrows indicate a transplanted scaffold in the injury cavity. (D)–(G) Migration of GFP-positive cells from hUC-MSCsGFP/CGB scaffolds (D) and (E) and hUC-MSCsCXCR4/GFP/CGB scaffolds (F) and (G) in the lesion cavity (broken lines) 14 d after transplantation. The number of GFP-positive cells at the lesion boundary zone was higher in the hUC-MSCCXCR4/GFP/CGB scaffold (G1–G3) as compared to the hUC-MSCGFP/CGB scaffold group (E1–E3). Scale bars: 100 μm.
Download figure:
Standard image High-resolution imageFigure 8. (A) Gross morphological examination of the brain lesions by haematoxylin and eosin staining after transplantation of CGB scaffolds containing hUC-MSCsCXCR4/GFP (A1) or saline (A2). Broken black lines show the wound margin between native and regenerated brain tissue. (B)–(I) The coronary sections with immunohistochemical analysis of the injured rat brain tissues at 6 weeks post-transplantation of hUC-MSCGFP/CGB scaffold and hUC-MSCCXCR4/GFP/CGB scaffold. (E) and (I) The selected sites showing the number of GFP and MAP-2 double-positive cells was higher in the hUC-MSCCXCR4/GFP/CGB scaffold as compared to the hUC-MSCGFP/CGB scaffold group. Ectogenic neurons are indicated by an arrow and endogenic neurons are indicated by an arrow head. The white dash line presents the contour of damaged cavities. Scale bars: 100 μm.
Download figure:
Standard image High-resolution imageDiscussion
There are few effective treatments for TBI [1], which is associated with the loss of neurons and a high mortality rate. Cell-based therapies have shown promise as a potential therapeutic strategy; biocompatible scaffolds can provide 3D support and influence cell properties, thereby improving their survival [23, 24]. hUC-MSCs have many advantages—including their abundance and low immunogenicity—for the treatment of TBI.
In our previous work, we designed a mold based on the size of lesion cavities in a TBI model and used it to construct a chitosan scaffold for hUC-MSCs [5]. The chemokine receptor CXCR4 along with its ligand SDF-1α are important chemotactic factors for stem cells that determine their retention and/or migration [13, 17]. Implanted MSCs have been shown to migrate to injury sites in ischemic brain [25], infarcted myocardium [26], and bone fractures [27]; the local concentration of SDF-1α is upregulated after tissue injury, and its interaction with CXCR4 is critical for this process. Several studies have reported that MSCs lost CXCR4 expression after prolonged culturing or repeated subculturing [18–21]. We therefore hypothesized that enhancing CXCR4 expression could improve the therapeutic efficacy of transplanted MSCs by stimulating their migration to sites of injury.
We demonstrated that CXCR4 overexpression did not affect hUC-MSC surface marker expression or differentiation potential, but enhanced their migration in response to SDF-1α both in vitro and in vivo. We also showed that hUC-MSC overexpressing CXCR4 adhered to and proliferated on the scaffolds. Moreover, BDNF released from CGB scaffolds induced the differentiation of CXCR4-overexpressing hUC-MSCs into neurons, which contributed to the regeneration of brain tissue at the lesion boundary, also with the potential benefit for proliferation and differentiation of neural stem cell.
In conclusion, the present results indicate that hUC-MSCs overexpressing CXCR4 migrate in response to SDF-1α from CGB scaffolds to the site of brain injury. The transplanted CGB scaffold with hUC-MSCsCXCR4/GFP encapsulated can match the lsssion cavity of injured brain caused by TBI which was shown by HE staining. It was evidenced that the BDNF released from CGB scaffolds induced the differentiation of these hUC-MSCs into neurons. Also, in vivo, the encapsulated hUC-MSCsCXCR4/GFP can not only differentiated into neurons in the scaffold site but also in the lesion site border to replenish the apoptosis of neurons. These findings suggest that introducing hUC-MSCsCXCR4/GFP to the injury site via CGB scaffolds may be a potential therapeutic strategy for the treatment of TBI.
Acknowledgments
The authors thank the Surgical Comprehensive Laboratory at the Affiliated Hospital of Nantong University for technical assistance and equipment support. This work was supported by the National Nature Science Foundation of China (grant no. 81271368) and the Natural Science Foundation of Jiangsu Province (grant no. BK2012654).