Abstract
Objective. Schwann cells (SCs) transplanted in damaged nervous tissue promote axon growth, which may support the recovery of function lost after injury. However, SC transplant-mediated axon growth is often limited and lacks direction. Approach. We have developed a zinc oxide (ZnO) containing fibrous scaffold consisting of aligned fibers of polycaprolactone (PCL) with embedded ZnO nanoparticles as a biodegradable, bifunctional scaffold for promoting and guiding axon growth. This scaffold has bifunctional properties wherein zinc is released providing bioactivity and ZnO has well-known piezoelectric properties where piezoelectric materials generate electrical activity in response to minute deformations. In this study, SC growth, SC-mediated axon extension, and the presence of myelin basic protein (MBP), as an indicator of myelination, were evaluated on the scaffolds containing varying concentrations of ZnO in vitro. SCs and dorsal root ganglion (DRG) neurons were cultured, either alone or in co-culture, on the scaffolds. Main results. Findings demonstrated that scaffolds with 1 wt.% ZnO promoted the greatest SC growth and SC-mediated axon extension. The presence of brain-derived neurotrophic factor (BDNF) was also determined. BDNF increased in co-cultures for all scaffolds as compared to SCs or DRGs cultured alone on all scaffolds. For co-cultures, cells on scaffolds with low levels of ZnO (0.5 wt.% ZnO) had the highest amount of BDNF as compared to cells on higher ZnO-containing scaffolds (1 and 2 wt.%). MBP immunostaining was only detected in co-cultures on PCL control scaffolds (without ZnO). Significance. The results of this study demonstrate the potential of the ZnO-containing scaffolds for SC-mediated axon growth and its potential for use in nervous tissue repair.
Export citation and abstract BibTeX RIS
1. Introduction
An injury to central or peripheral nervous tissue severs axons resulting in loss of function. Endogenous growth of severed axons may contribute to the recovery of function; however, such a growth response is limited by the injury environment, especially in the central nervous system [1, 2]. Current therapies to promote axon growth and functional recovery after nervous tissue injury have limited efficacy [3], which fuels the search for more effective approaches to promote and guide axon growth after nervous tissue damage.
Pre-clinical studies demonstrated the potential of a Schwann cell (SC) transplant to promote axon growth in damaged nervous tissue, which often is associated with functional recovery [4, 5]. The clinical feasibility of SC transplantation for nervous tissue repair was shown in phase I clinical trials in patients with spinal cord injury [6] and peripheral nerve injury [7]. However, the preclinical studies have revealed that SC transplant-mediated axon growth in damaged nervous tissue is limited and that the axons often fail to grow in a preferred longitudinal direction thereby potentially limiting their chances to become functionally integrated.
To address these limitations of SC transplants, tissue engineering approaches using SCs in combination with scaffolds containing aligned fibers have gained considerable interest [8, 9]. Aligned fibers can further enhance axon growth [10] and provide longitudinal directionality to growing axons [9]. In addition, these fibrous scaffolds can incorporate molecules/factors, such as neurotrophins, for release [11] to further accelerate axon growth in combination with SCs. Zinc (Zn) has an important role in regulating the expression of neurotrophins, specifically brain-derived neurotrophic factor (BDNF) [12–14], which can mediate axon growth. Zinc also is an important element for neuronal development and axon growth, associated with the activation of a number of downstream pathways [15], and providing neuroprotection [16]. To date, the effect of Zn-containing scaffolds on axon growth in combination with SCs has not been evaluated.
The present study examined the use of scaffolds consisting of aligned fibers, composed of polycaprolactone (PCL) and ZnO, in combination with SCs for promoting axon growth. PCL was chosen because of its biocompatibility and relatively slow degradation rate [17] allowing for the dissolution/release of Zn. In addition, ZnO may be attractive because of its well-characterized piezoelectric properties, generating electrical activity in response to mechanical deformation [18]. Electric fields are known to support axon growth [19, 20] through, at least in part, increasing neuronal regeneration-associated gene expression [21].
In this in vitro study, we cultured SCs and dorsal root ganglion (DRG) neurons, alone and in combination, on the ZnO-containing scaffolds and evaluated SC growth, SC-mediated axon growth, the presence of myelin basic protein (MBP), and the presence of BDNF. Our findings demonstrated that the ZnO-containing scaffold facilitated significantly greater SC-mediated axon growth when compared with PCL alone (without ZnO). Also, the ZnO-containing scaffold affected the presence of MBP and BDNF in co-cultures. Our results indicate that ZnO-containing PCL scaffolds may hold promise for SC transplant-mediated axon growth for repair and recovery after nervous tissue damage.
2. Materials and methods
2.1. Fabrication of electrospun scaffolds
Aligned fibers containing ZnO were prepared by electrospinning. The electrospinning solution consisted of PCL (80 kDa, Sigma-Aldrich, St. Louis, MO) dissolved into methylene chloride (Thermo Fisher Scientific, Wantham, MA) at 10% wt/wt and ZnO nanoparticles (100 nm, Sigma-Aldrich, MO), which were added to the solution at 0, 0.5, 1, and 2% wt/wt. The ZnO concentrations were selected based on previous work using ZnO-containing scaffolds for other applications [22] and since neural cell attachment did not occur at concentrations above 2%. Solution was stirred overnight, followed by 20 min sonication in an iced water bath and 1 min probe sonication (Branson Digital Sonifier, VWR, Radnor, PA). The solution was transferred to a syringe and electrospun at ambient conditions (21 °C–22 °C 16%–56% humidity) using an 18-gauge needle, ejection rate of 2 ml h−1, 12–22 kV of applied voltage. Aligned fibers were collected on a rotating mandrel at a distance of 20–30 cm from the needle to the mandrel and a rotation speed of 3000 rpm. All scaffolds were air dried for one week to remove the residual solvent.
2.2. Fiber morphology, diameter, and alignment
Fiber morphology, dimensions and alignment were determined by scanning electron microscopy (SEM). Scaffolds were sputter coated with gold-palladium and viewed by SEM (Jeol, JSM-7900F, Tokyo, Japan) using an accelerating voltage of 1–1.5 KV and a working distance of 10 mm. Fiber diameter (10 measurements per image at 3 locations) and fiber alignment (10 measurements per image at 2 locations) were determined using Image J (National Institutes of Health, Bethesda, MD). For fiber alignment, a line was drawn perpendicular to a fiber in the SEM image and angles between fibers, chosen at random, and the line were measured. The percentage of fiber alignment was calculated according to a previously published protocol [8]. Briefly, the percentage of alignment was calculated using the following equation:
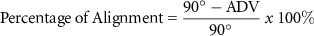
where the absolute deviation value (ADV) is the absolute value of the measured angle from 90. Analysis was performed using ImageJ software.
2.3. ZnO loading and Zinc release
To verify the amount of ZnO loaded into the scaffold, samples were subjected to thermogravimetric analysis (TGA; TA Q50, New Castle, DE). Operating conditions consisted of a heating rate of 50 °C min−1 up to 500 °C in nitrogen at 20 ml min−1. The remaining weight was a measure of the ZnO content in the scaffold. N of 3 per group was used. Cumulative zinc release was measured for each scaffold according to previously reported protocols [22]. Briefly, the scaffolds were immersed in phosphate buffer saline (PBS) for 4 weeks at 37 °C. The PBS was collected and the amount of Zn was determined at days 4, 7, 14, 21, and 28 by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7900, Agilent Technologies, Santa Clara, CA). N = 2 per group per time point.
2.4. Mechanical testing
Tensile testing was performed using an Instron model 3342 (Instron, Norwood, MA). N = 7 samples per group were used. Scaffolds were cut into strips measuring 5 mm width × 15 mm length × 0.3 mm thick. Samples were hydrated by immersion in PBS overnight prior to mechanical testing. Samples were tested in tension at a deformation rate of 5 mm min−1 until failure. Stress/strain curves were generated. The Young modulus, yield strength, ultimate stress, and ultimate strain were determined from the stress/strain curves. The Young modulus was determined by measuring the slope of the linear region of the stress/strain curve. Yield strength was the stress at which the material began to plastically deform (where the linear portion ceased). Ultimate stress was the stress at failure and ultimate strain was the strain at failure.
2.5. SC growth and morphology
SCs were cultured based on previously published protocols [8]. GFP transfected SCs (GFP-SCs) were obtained from the University of Miami. Briefly, purified SC cultures were obtained from sural/sciatic nerves of adult female Fischer rats, following previously published protocols [23]. Rats were purchased from Envigo Inc. (Frederick, MD), housed at the University of Miami, and in accordance with an animal protocol approved by the University of Miami Institutional Animal Care and Use Committee (IACUC). The resulting cultures were purified to greater than 95% [24]. At passage 2 and at approximately 50% confluence, SCs were transduced with a lentiviral vector encoding enhanced GFP at a multiplicity of infection of 30. The transduction efficiency was greater than 90%. GFP-SCs were expanded in SC medium consisting of Dulbecco's Modified Eagle Medium (DMEM) (Thermo Fisher Scientific) containing 2 μM forskolin (Sigma-Aldrich, Inc., Watham, MA), 2.5 nM heregulin (Genentech, Inc., San Francisco, CA), 2% bovine pituitary extract (Alfa Aesar, Tewksbury, MA), 10% fetal bovine serum (GE Healthcare Life Sciences, Marlborough, MA),), and 0.1% gentamicin (Thermo Fisher Scientific) until 80% confluency prior to seeding onto scaffolds. PCL only and ZnO-containing PCL scaffolds, 6 mm in diameter and 0.4 mm thick, were inserted into 96-well polypropylene plates and were sterilized by 100% ethanol for 20 min followed by rinsing with PBS and pre-treatment with the SC medium overnight. GFP-SCs were seeded at 3.5 × 104 cells cm−2 on the scaffolds and cultured for 14 d. Cell number was determined by the PicoGreen Assay (Thermo Fisher Scientific) on days 1, 4, 7, and 14 according to previously reported protocols [8]. Briefly, samples were harvested, rinsed and cells were lysed using papain in cysteine buffer at a concentration of 27.5 mg ml−1. dsDNA was labeled using the PicoGreen reagent and the fluorescent intensity for each sample was measured at 480 nm excitation and 520 nm emission using a fluorescent plate reader (FLX800, BioTek Instrument, Inc., Winooski, VT). Cell number was determined by using a standard curve relating fluorescence intensity to known cell numbers. N = 6 per group per time point. Cell morphology was observed on the scaffolds. Samples were fixed in 4% paraformaldehyde at each time point and viewed using a confocal microscope (C1si, Nikon, Tokyo, Japan).
2.6. DRG axon growth on scaffolds
Rat dorsal root ganglia (DRGs) were cultured on the scaffolds as intact DRG explants, purified DRGs, or purified DRGs co-cultured with SCs, following previously reported methods [8]. In accordance with an approved animal protocol by the Rutgers University IACUC, DRGs were isolated from embryonic Sprague Dawley rats (Charles River, MA, USA) at 16 day gestation (E16) as published [25]. Scaffolds were sterilized as stated above and pre-treated with neurobasal (NB) media overnight. NB media consisted of neurobasal media (Thermo Fisher Scientific) supplemented with 2% of B27 supplement (Thermo Fisher Scientific), 1% of penicillin/streptomycin (Thermo Fisher), 1 mM glutamax (Thermo Fisher Scientific), 0.08% glucose (Thermo Fisher Scientific), and 50 ng ml−1 of 7S NGF (Thermo Fisher Scientific). Intact DRG explants were placed directly on scaffolds and cultured in NB media at 37 °C, 5% CO2. For purified DRGs, intact DRG explants were cultured in NB media supplemented with mitotic inhibitors (10 mM 5-Fluoro-2ʹ-deoxyuridine, Sigma-Aldrich and 10 mM Uridine, Sigma Aldrich) for 3 d to prevent SC and fibroblast proliferation, according to a previously reported method [26]. DRGs were then axotomized in order to simulate SCI by severing axons by carefully scraping around the DRG with forceps, which was adapted from a previous protocol [8]. Great care was taken to not damage the ganglion and to ensure that all axons were severed. Then, the DRGs were placed on the scaffolds and cultured in NB media. For the purified DRGs co-cultured with SCs group, GFP-SCs were seeded onto the scaffolds at 2 × 106 cells cm−2 and cultured in SC media for one day. Purified and axotomized DRGs were placed on the SC-laden scaffolds and cultured in co-culture media (NB-C) containing 0.4% glucose, 1% penicillin/streptomycin, 1 mM glutamax, 50 ng ml−1 of 7S NGF, and 10% FBS in minimum essential media (MEM, Thermo Fisher Scientific). All groups were cultured for 3 d. Samples were fixed with 4% paraformaldehyde, rinsed with PBS and permeabilized with 0.1% Triton X-100 in blocking solution (10% goat serum in PBS) for 60 min. After rinsing with PBS, polyclonal rabbit anti-rat neurofilament 200 primary antibody (1:1000, Sigma-Aldrich, Inc.) in blocking solution was added to each sample and kept overnight at 4 °C. Samples were then rinsed with PBS followed by adding goat anti-rabbit Alexa Fluor 594 secondary antibody (1:500, Thermo Fisher Scientific, Inc.) in blocking solution and incubated for 1 h at room temperature. Samples were rinsed with PBS and imaged using confocal microscopy. Images were merged using ImageJ to view the entire length of the axons. Axon growth was quantified by measuring the 10 longest axons on each side of the DRG. N = 3 samples per group were used for each study.
2.7. BDNF presence
BDNF presence in the media was measured using an Invitrogen™ BDNF Rat ELISA Kit (Thermo Fisher Scientific), following manufacturer's protocol. Cell culture media was collected on day 3 for SCs alone, axotomized DRGs, and SCs co-cultured with DRGs groups. N = 4 per group was used. Absorbance was measured at 450 nm using a Microplate Reader (Emax, Molecular Devices, San Jose, CA)).
2.8. MBP presence in co-cultures
MBP presence was determined in co-cultures using dissociated DRGs and SCs according to previously established protocols [27]. Briefly, intact DRG explants were isolated as described above and placed in 0.25% trypsin/EDTA for 15–20 min. The DRG was then placed in NB media, centrifuged and then resuspended in media containing 10% FBS, 0.05 mg ml−1 gentamycin (Thermo Fisher Scientific), 1 mM glutamax, 0.08% glucose, and 50 ng ml−1 of 2.5S NGF (Bio-Rad Laboratories, Inc., Hercules, CA) in MEM. Dissociated DRGs were seeded onto scaffolds, which were sterilized and pre-treated in NB media as described above, cultured in NB media, and the following day, the media was replaced with NB media supplemented with mitotic inhibitors. After 3 d of purification, SCs were seeded onto each scaffold at 221 500 cells cm−2 in MEM containing 0.08% glucose, 0.05 mg ml−1 gentamycin, 1 mM glutamax, 10% FBS and 50 ng ml−1 of 2.5S NGF. After 1 week, 50 μg ml−1 ascorbic acid (Sigma Aldrich) was added to the media to induce myelination. Cultures were maintained in media containing 50 μg ml−1 ascorbic acid with routine media changes for 2 weeks before fixing with 4% paraformaldehyde. Scaffolds were then kept in 100% methanol for 20 min at −20 °C and then placed in a blocking solution containing 2% BSA, 0.3% triton X-100, 5% donkey serum (Thermo Fisher Scientific), and 5% goat serum in PBS for 1 h at room temperature. Samples were then rinsed and incubated with rabbit anti-rat neurofilament (1:1000) and mouse anti-MBP (MBP, 1:200, Biolegend, CA, USA) in the blocking solution overnight at 4 °C. Samples were rinsed with PBS and incubated with anti-rabbit Alexa Fluor 488 (1:800) and donkey anti-mouse Alexa Fluor 594 (Thermo Fisher Scientific) in blocking solution for 1 h at room temperature. Samples were imaged for NF and MBP immunostaining using confocal microscopy. In order to ensure the presence of SCs on the scaffolds over the time period of the culture, experiments were repeated with GFP-SCs and immunostained for NF at the end of the myelination culture.
2.9. Statistics
For scaffold characterization, SC growth, axon growth, and BDNF presence experiments, one-way ANOVA was used to test statistical significance, followed by Tukey-Kramer post-hoc tests. Statistical significance was determined for p < 0.05. Results were shown as mean ± standard deviation (S.D.).
3. Results
3.1. Fiber morphology and ZnO loading characterization
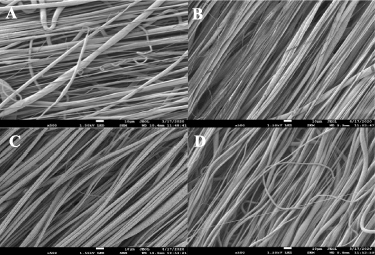
All scaffolds had uniform fiber morphology, as shown in the SEM images (figure 1). The fiber diameter was approximately 3–4 μm for all scaffolds. Statistical differences in fiber diameter were detected between 1% ZnO and 0.5% ZnO groups. The % alignment for all groups was found to be above 90% (table 1). By TGA analysis, the amount of ZnO loaded into the scaffold post-processing was determined to be 0.692% for 0.5% ZnO, 0.913% for 1% ZnO, and 2.26% for 2% ZnO groups.
Figure 1. SEM images of each of the groups is shown. PCL containing (A) 0% ZnO, (B) 0.5% ZnO, (C) 1% ZnO, and (D) 2% ZnO. Scale = 10 μm. Magnification = 500×.
Download figure:
Standard image High-resolution imageTable 1. The fiber diameter and percent alignment for ZnO-containing scaffolds.
| Group | Fiber diameter (μm) | %Alignment |
|---|---|---|
| 0% ZnO | 2.73 ± 2.05 | 94.99 ± 3.98 |
| 0.5% ZnO | 2.63 ± 0.95 | 97.94 ± 1.27 |
| 1% ZnO | 3.66 ± 0.85 | 97.12 ± 2.78 |
| 2% ZnO | 2.88 ± 1.87 | 92.34 ± 13.68 |
a p < 0.05 compared to 0.5% ZnO. Values are Mean ± S.D., n = 20 per group for alignment and n = 30 per group for fiber diameter.
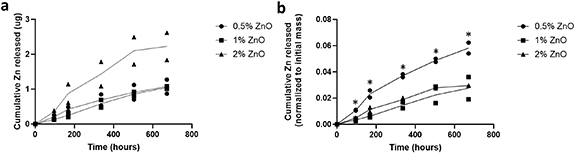
3.2. Zinc release
The release of Zn over time was examined using ICP-MS. In figure 2(a), the amount of Zn released for all of the groups yielded no significant differences. As shown in figure 2(b), there was a significant difference in normalized Zn release for the 0.5% ZnO group compared to all other groups. Less than 6% (i.e. 0.06 mass of Zn released normalized to initial mass of Zn in the scaffold) Zn was released from the scaffold for all groups.
Figure 2. Zn release for all groups over time. Cumulative Zn release (a) and cumulative Zn release normalized to the initial mass of Zn (b). n = 2 per group per time point. *p < 0.05 compared to all other groups.
Download figure:
Standard image High-resolution image3.3. Mechanical testing
As shown in table 2, all scaffolds exhibited a strain greater than 0.25. Significant differences were detected between the 0% ZnO and 2% ZnO groups for yield strength and ultimate stress. Also, significant differences were detected for ultimate stress between 1% ZnO and 2% ZnO. The Young's modulus for all groups was between 10 and 20 MPa where no significant differences were detected.
Table 2. Mechanical properties of ZnO-containing scaffolds.
| Group | Young modulus (MPa) | Yield strength (MPa) | Ultimate stress (MPa) | Ultimate strain |
|---|---|---|---|---|
| 0% ZnO | 19.13 ± 4.10 | 1.45 ± 0.39 | 2.02 ± 0.62 | 0.46 ± 0.14 |
| 0.5% ZnO | 13.39 ± 5.82 | 0.86 ± 0.45 | 1.36 ± 0.98 | 0.43 ± 0.20 |
| 1% ZnO | 18.14 ± 13.37 | 1.09 ± 0.70 | 1.84 ± 0.48 | 0.29 ± 0.09 |
| 2% ZnO | 10.71 ± 8.98 | 0.45 ± 0.28 | 0.86 ± 0.21 | 0.26 ± 0.10 |
a p < 0.05 as compared to the 0% ZnO group. b p < 0.05 as compared to 1% ZnO group. Values are Mean ± S.D., n = 7 per group.
3.4. SC growth and morphology
As shown in figure 3, the 1% ZnO group had significantly higher cell number (as normalized to day 1) as compared to 0% ZnO (PCL) at day 14. Significantly lower cell number was detected between 2% ZnO and all groups at day 14.
Figure 3. SC number normalized to day 1 for ZnO-containing groups. a p < 0.05 as compared to 0% ZnO group (PCL) at day 14, b p < 0.05 as compared to 2% ZnO group at day 14 and c p < 0.05 between 7 and 14 d. N = 6 per group per time point.
Download figure:
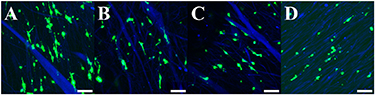
Standard image High-resolution imageBy confocal microscopy, it was observed that GFP-SCs were extended along the length of the aligned fibers for each group except for 2% ZnO where the cells were rounded at days 1 and 14 in culture (figures 4 and 5). Less blue autofluorescence was detected for the fibers at day 14 for the 1% and 2% ZnO groups (figure 5).
Figure 4. The GFP-SCs are shown on the scaffolds at day 1. Magnification = 20×. Scale = 100 μm. (A) 0% ZnO group, (B) 0.5% ZnO group, (C) 1% ZnO group, and (D) 2% ZnO group. Green = GFP, blue = fibers.
Download figure:
Standard image High-resolution imageFigure 5. The GFP-SCs are shown on the scaffolds at day 14. Blue is autofluorescence of the fibers with less intensity for 1 and 2% ZnO groups. Magnification = 40×. Scale = 50 μm.
Download figure:
Standard image High-resolution image3.5. DRG axon growth and co-culture with SCs
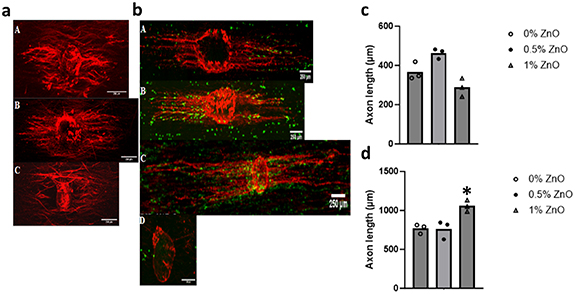
For DRG explants seeded onto scaffolds, axon growth was observed for all scaffold groups (figure S1) and no significant differences in axon length could be detected between groups (figure S1). However, differences could be detected when using axotomized DRGs and in co-culture with SCs. No significant differences in axon length were determined for axotomized DRGs at day 3 (figures 6(a) and (c)). The average axon length for the axomotized DRGs was approximately 400 μm for 0, 0.5 and 1% ZnO groups. Axotomized DRGs did not attach to the 2% ZnO group. In co-culture with SCs (figures 6(b) and (d)), axon length increased significantly for all groups (0, 0.5 and 1% ZnO groups) in comparison to groups without SCs (p < 0.05). The 1% ZnO group was significantly higher, by approximately 30%, than the 0 and 0.5% ZnO groups. DRGs in co-culture with SCs on 2% ZnO groups did not extend axons.
Figure 6. Axotomized DRG axon growth at day 3 (a) and in co-culture with SCs (b). (A) 0% ZnO, (B) 0.5% ZnO, (C) 1% ZnO, and (D) 2% ZnO groups. Note: axomtomized DRGs did not attach for the 2% ZnO group. Neurofilament = red, GFP = green, scale bar = 250 μm. Axon length (μm) for axomtomized DRGs alone (c) and in co-culture with SCs (d) at day 3. *p < 0.05 compared to all other groups. n = 3 samples per group, n = 20 axons per sample.
Download figure:
Standard image High-resolution image3.6. BDNF presence
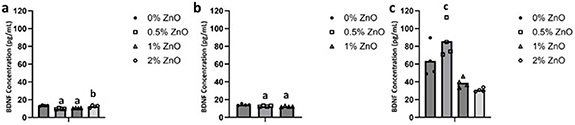
The presence of BDNF in the media was measured for SCs, axotomized DRGs and both in co-culture for 0%, 0.5% and 1% ZnO groups (figure 7). Co-cultures of SCs and axotomized DRGs had significantly higher levels of BDNF than SCs and axotomized DRGs alone. For DRGs and SCs co-cultured on scaffolds, significant differences in the amount of BDNF were detected between the 0.5% and 1% ZnO groups. For SCs alone, significant differences in the level of BDNF were detected between 0% ZnO and 0.5% and 1% ZnO groups (13.34 ± 0.57 pg ml−1, 9.82 ± 0.65 pg ml−1, and 10.58 ± 0.26 pg ml−1, respectively) and 0.5% and 2% ZnO groups (12.45 ± 1.31 pg ml−1). For axotomized DRGs alone, significantly lower BDNF levels were determined for 0.5% and 1% ZnO groups in comparison to 0% ZnO (12.56 ± 0.61 pg ml−1, 12.22 ± 0.67 pg ml−1, and 14.17 ± 0.89 pg ml−1, respectively). Since DRGs did not attach to 2% ZnO group, BDNF measurement is not shown for this group.
Figure 7. The amount of BDNF in the culture media at day 3 for (a) SCs alone, (b) axotomized DRGs alone, and (c) axotomized DRGs and SCs in co-culture on scaffolds. a p < 0.05 compared to 0%, b p < 0.05 compared to 0.5%, c p < 0.05 compared to 1% and 2% ZnO groups. n = 4 per group.
Download figure:
Standard image High-resolution image3.7. MBP presence in co-cultures
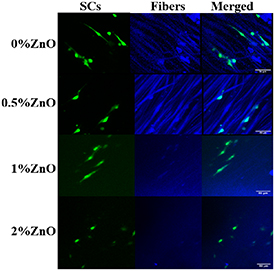
The presence of MBP was observed in co-cultures of dissociated DRG neurons with SCs on scaffold groups (0, 0.5 and 1% ZnO groups) for 4 weeks. As shown in figure 8, neurofilament-positive axons were present for all groups examined. However, MBP staining was only detected for the PCL alone (0% ZnO) group. SCs were still present for all groups as shown by the presence of GFP-SCs in co-culture with the dissociated DRG neurons using the same culture conditions.
Figure 8. (a) Dissociated DRG neurons and SCs were co-cultured for up to 4 weeks on 0% ZnO, 0.5% ZnO, and 1% ZnO scaffolds. Neurofilament (NF) = green and MBP = red, scaffold fibers = blue. (b) GFP-SCs used in the co-culture using same culture conditions as in a. NF = red, SCs = green. Scale bar = 100 μm.
Download figure:
Standard image High-resolution image4. Discussion
This study examined the effect of Zn-containing scaffolds on SC growth and SC-mediated axon growth. The Zn-containing scaffolds consisted of aligned fibers, which provides contact guidance for axon growth [28]. SCs extended and axons grew along the length of the fibers. The Zn-containing scaffolds, with low levels of ZnO, increased SC growth and SC-mediated axon growth. The addition of ZnO to the scaffolds had no effect on axon growth for DRGs alone. The amount of BDNF increased on scaffolds containing low levels of ZnO in co-cultures of SCs and DRGs, indicating that BDNF may play a role and co-culture conditions may have an effect on the presence of BDNF. The high level of ZnO, the 2% ZnO group, did not support SC growth and DRG attachment. Our results indicate that PCL fibers containing low levels of ZnO may serve as a scaffold for SCs to enhance their effect on axon growth and to provide direction to the growing axons.
SC growth as well as SC-mediated axon growth occurred on scaffolds containing 1% ZnO. However, such an effect was not observed for axotomized DRGs when cultured alone, irrespective of the ZnO content. This may have been due to the presence of SCs on the ZnO-containing scaffolds where SCs grew in number on the ZnO-containing scaffolds. SCs are known to secrete neurotrophins and may provide other molecular cues that improve axon growth [29]. Scaffolds containing 2% ZnO did not support the attachment of axotomized DRGs. Cortical neurons have been shown to attach favorably to pure ZnO substrates [30]. Interestingly, for biomaterial composites containing relatively high amounts of ZnO, astrocyte adhesion decreased [31]. The effect of ZnO content in a composite scaffold on cell adhesion may differ depending upon the neural cell type. Zn is released at low levels from the scaffold over time wherein the majority of the Zn is retained in the scaffold, which may have a favorable effect on cell growth, cell adhesion, and axon growth. In addition, high levels of soluble zinc can lead to cytotoxicity in the form of DNA damage to CNS neurons [32]. Future work may need to examine the effect of ZnO-containing scaffolds on cell survival, specifically examining cell death and apoptosis.
BDNF levels in the culture media increased when SCs and DRGs were in co-culture on the scaffolds. Significantly greater BDNF was found in co-culture for the 0.5% ZnO group as compared to the 1% and 2% ZnO groups; however, axon growth was greatest for the 1% group when compared to scaffolds without ZnO. Interestingly, the amount of Zn released for the 0.5% and 1% Zn-containing scaffolds was similar, indicating that there may be other factors that contributed to promoting axon growth. SCs secrete multiple factors that can promote axon growth including BDNF [29]. However, too much BDNF may hamper axon growth [33] and can regulate SC myelination [34]. Further studies are needed to elucidate the mechanisms and role of BDNF on axon growth in co-cultures on Zn-containing scaffolds. In addition, although the amount of Zn released from the 0.5% and 1% Zn-containing scaffolds was similar, the greatest amount of Zn released when normalized to the initial Zn present in the scaffold was for the 0.5% group. This result may indicate that there are subtle differences in the distribution of ZnO in the fiber at the lower weight%, which may also affect axon growth. In addition, the significant differences in the fiber diameters between 0.5% and 1% groups may also affect axon growth due to changes in contact guidance provided by the fibers [35].
The Zn-containing scaffolds may affect SC favorably to support axon growth but may not support myelination. MBP, as an indicator of myelination, was only detected in co-cultures on scaffolds without ZnO (PCL control). It was detected in co-cultures on ZnO-containing scaffolds. However, SC number was significantly higher for the 1% ZnO group in comparison to the control, without ZnO. This finding may suggest that the ZnO scaffolds may retain SCs in a growth-supporting state that limits maturation and myelination [36]. Additional studies are needed to demonstrate myelination occurred in the co-cultures on scaffolds without ZnO including co-localization of the MBP staining with the SCs and the formation of SC myelin sheaths. The zinc released from the scaffolds may also impact zinc transport and cause changes in intracellular zinc levels impacting a number of signaling pathways [37]. Our results are promising, demonstrating the potential of the PCL fibers containing ZnO as a favorable scaffold for SC supported repair of nervous tissue injuries.
Acknowledgments
The authors would like to thank support from the NJ Commission on Spinal Cord Research-Exploratory Grant CSCR19ERG008 and the NSF Science and Technology Center—Center for Engineering Mechanobiology (CEMB)—1548571.
Data availability statement
All the data that support the findings of this study are contained within the main manuscript or supplementary materials except for the data in the tables. The data used to create the tables is available at the following DOI https://doi.org/10.7916/awxr-br84.
Conflict of interest
The authors report no conflict of interest in this paper
Supplementary data (0.2 MB PDF)