Abstract
Objective. The application of cerebellar transcranial alternating current stimulation (tACS) is limited by the absence of commonly agreed montages and also the presence of unpleasant side effects. We aimed to find the most effective cerebellar tACS montage with minimum side effects (skin sensations and phosphenes). Approach. We first simulated cerebellar tACS with five montages (return electrode on forehead, buccinator, jaw, and neck positions, additionally focal montage with high-definition ring electrodes) to compare induced cerebellar current, then stimulated healthy participants and evaluated side effects for different montages and varying stimulation frequencies. Main results. The simulation revealed a descending order of current density in the cerebellum from forehead to buccinator, jaw, neck and ring montage respectively. Montages inducing higher current intensity in the eyeballs during the simulation resulted in stronger and broader phosphenes during tACS sessions. Strong co-stimulation of the brainstem was observed for the neck. Skin sensations did not differ between montages or frequencies. We propose the jaw montage as an optimal choice for maximizing cerebellar stimulation while minimizing unwanted side effects. Significance. These findings contribute to adopting a standard cerebellar tACS protocol. The combination of computational modelling and experimental data offers improved experimental control, safety, effectiveness, and reproducibility to all brain stimulation practices.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Transcranial alternating current stimulation (tACS) is a non-invasive brain stimulation (NIBS) technique for studying and modulating brain oscillations, where a weak electric current in form of a sinusoidal wave is delivered to the target area via two or more scalp electrodes [1, 2].
Recently, the cerebellum has become a target for many NIBS studies with promising results [3]. The cerebellum with its broad connections to various cortical and sub-cortical areas contributes to multiple motor, behavioural and cognitive functions. Using tACS on the cerebellum has opened new possibilities to decipher the complexities of cerebellar physiology [4]. Furthermore, through finely-tuned tACS, one can entrain and modulate oscillations [4–6] which allows for exploration and even manipulation of the cerebellum's functions in different networks in healthy and disease states. For instance, the cerebellum-motor cortex inhibition and motor-adaptation can be studied through cerebellar tACS [3, 4, 7], or clinical interventions such as entraining and suppressing tremor in Parkinson's disease and essential tremor are made possible by this technique [8, 9].
With increasing applications of cerebellar tACS, attention has been paid to the detrimental influence of peripheral side-effects of tACS that might interact or counteract with transcranial effects of stimulation [10, 11]. Major reported side effects of tACS are skin sensations [12–14]. Inducing a high electric field in the skin (even exceeding 4–7 V m−1) during stimulation can cause unpleasant sensations such as itching, pricking, warmth, and pain and result from direct stimulation of cutaneous nerves [12, 15]. Another possible phenomenon during cerebellar tACS is the perception of flickering lights or flashes in the visual field called phosphenes [4, 16], which were thought to be the result of visual cortex stimulation [17] but were later shown to have a retinal origin [18–20]. Both of these side effects have been reported to be montage- and frequency-dependent [10–12, 21]. A major factor determining the intensity of perceived side-effects is the placement of tACS electrodes [21, 22] that, however, also impacts the intensity of electric field density in the cerebellum [23].
Currently, there is no comprehensive investigation of skin effects and phosphenes during cerebellar tACS that takes electrode montage as well as stimulation frequency into consideration [15]. An experimental investigation for finding optimal stimulation conditions that can have potential in clinical applications would require repetitive tACS sessions with human participants exploring different stimulation parameters online. Evidently, a purely experimental approach would be challenging and limited in terms of safety and ethical concerns, methodological complexities, and importantly quantifying and interpreting the results [24]. In-silico or modelling studies however offer solutions to overcome such challenges and even provide the opportunity to precisely observe the effects of stimulation inside the brain tissue. A common protocol is to simulate multiple tACS sessions with a variety of parameters and compute the distribution of induced current inside the target region by solving the current continuity equation subjected to the appropriate boundary conditions, and considering the conductive media (i.e. the skull and brain having different tissues) [25].
Even with increasing interest and promising findings, the application of cerebellar tACS, particularly in clinical setups is limited; mainly due to the presence of unpleasant side effects and lack of standard protocols for stimulation and clinical interventions. Addressing this gap, the present study aims to find the optimum parameters for an effective cerebellar tACS with minimum side effects, by taking advantage of a combined computational and experimental approach. First, by modelling tACS with variable electrode montages we were able to quantify current density in the cerebellum. Besides, it allowed for the assessment of induced current in other brain areas that may be associated with stimulation side effects. Second, we applied in-vivo tACS with selected montages in healthy subjects in a controlled experimental setup and investigated the side effects of skin sensations and phosphenes. This in turn promotes the convenient application of cerebellar tACS by enabling researchers and clinicians to deliver successful stimulations with the least unpleasant side effects and consequently supports the establishment of optimal standardized tACS protocols.
2. Methods
2.1. Computational methods
2.1.1. Main electrode position
Simulations were carried out using a boundary element model (BEM) approach with a realistic three-shell head model comprising skin, bone and brain [26]. For the head model, we used the standard MNI152 combined with a detailed cerebellar surface model computed from the Spatially Unbiased Atlas Template of the Cerebellum and Brainstem (SUIT) [27]. Electric fields were estimated as the sum of the linear combinations between a leadfield  and the injected currents from all stimulation electrodes
and the injected currents from all stimulation electrodes  at each intracranial location
at each intracranial location  as
as
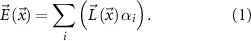
The leadfield was constructed using exact low-resolution electromagnetic tomography (eLORETA;[28]). Patch electrodes of the size 5 × 5 cm were approximated as an array of nine points, located 0, 1, 2, and 3 cm away from the inion (figure 1(A)). The inion was defined as [0 −120 −21] in MNI coordinates according to Tsuzuki and colleagues [29]. We evaluated the distribution of electric currents on the surface of the cerebellum by two-sided signed rank tests applying Bonferroni correction for multiple comparisons.
Figure 1. (A) Four patch locations for the posterior main tACS electrode. Electrode patches were approximated by five points. Relative to the inion (upper left most blue dot), the four patches were moved by 0, 1, 2 or 3 cm to the right. The return electrode on the buccinator muscle was not varied. (B) Electric fields resulting from the four montages on the whole brain (upper row) and the isolated cerebellum (lower row). (C) Median electric field strength in the ipsilateral right hemisphere (upper plot) or contralateral left hemisphere (lower plot) of the cerebellum (*** indicates p < 0.001).
Download figure:
Standard image High-resolution image2.1.2. Return electrode position
To find the optimum return electrode position i.e. the montage, full tACS sessions were simulated using SimNIBS (version 3.2.4, [30]). The same standard MNI152 head model was used and conductivities of various tissues were assigned according to Wagner and colleagues [31]. The electrodes were all rectangular-shaped in two sizes of 5 × 5 and 5 × 7 cm, modelled as NeuroConn silicone rubber electrodes (conductivity = 29.4 S m−1, thickness = (1 mm) covered with sponge (conductivity = 1 S m−1, thickness: 2.5 mm) [32]. The main electrode center (5 × 5 cm) was fixed at 2 cm lateral to the inion (optimal position based on the simulation). The return electrode (5 × 7 cm) center was positioned on the center coordinates of four of the most popular montages in literature i.e. the forehead, the ipsilateral buccinator muscle, lower jaw, and lower neck. Amplitude was set to  2 mA to simulate the real peak-to-peak amplitude of tACS [33].
2 mA to simulate the real peak-to-peak amplitude of tACS [33].
To determine the current distributions induced by the stimulation, SimNIBS first solves the Laplace equation:

where  is the electrical conductivity of specific tissue and
is the electrical conductivity of specific tissue and  is the induced electrical potential. This is modelled by setting boundary conditions. Having this solution, the electrical (E) and current (J) distributions flow through the electrodes are calculated:
is the induced electrical potential. This is modelled by setting boundary conditions. Having this solution, the electrical (E) and current (J) distributions flow through the electrodes are calculated:


Finally, corrections and linear scaling are made to ensure the current flow matches the previously set values in the electrodes [34, 35].
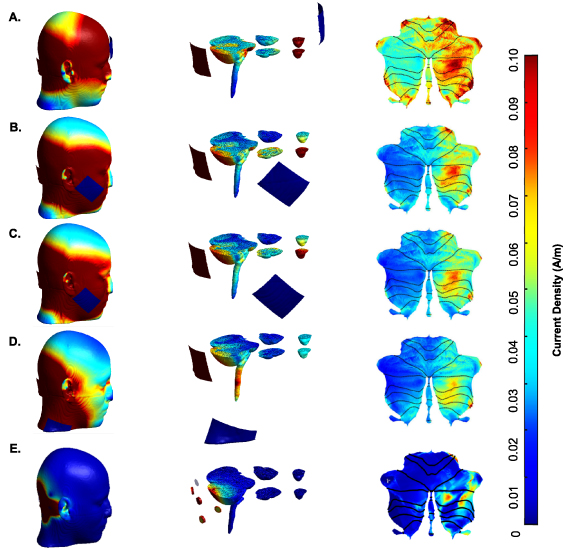
The effect of the stimulations was investigated via region of interest (ROI) analysis. Lobule-specific current densities were extracted and computed using SUIT toolbox in MATLAB, based on which cerebellum flatmaps 3 were drawn (figure 2). In addition, co-stimulation of the eyeballs (which can indicate the probability of inducing phosphenes [18]), as well as the brainstem were investigated by creating corresponding ROI masks in MRIcroGL 4 [36], applying them to the SimNIBS 3D head model outputs 5 , and finally extracting current density distributions in the two regions.
Figure 2. Simulations of different tACS montages, the intensity of stimulation can be seen on the skin, the cerebellum, and the eyeballs for different montages: (A) return electrode on the forehead, (B) buccinator muscle, (C) jaw, (D) neck, and (E) 4 × 1 ring electrode montage. Left: full scalp view, Middle: sliced view of cerebellum and eyeballs, Right: cerebellum flatmaps showing current density distribution.
Download figure:
Standard image High-resolution image2.1.3. Focal 4 × 1 electrode arrangement
A separate simulation of cerebellar tACS with a high-definition 4 × 1 ring electrode montage was performed to compare how the electric field distribution and density of cerebellar stimulation differ from using two rectangular-shaped electrodes as described in sections 2.1.1 and 2.1.2. To this end, five circular electrodes with 1.2 cm diameter were modelled and arranged according to Saturnino and colleagues [37] with the center electrode positioned 2 cm lateral to the inion and the four periphery electrodes at equal distances of 3.5 cm around it. The same rubber material and tissue and electrode conductivities of previous simulations were used. The current for the central electrode was set to +2 mA and each of the four surrounding electrodes to −0.5 mA. The analysis including extraction of current density distribution in the cerebellum and lobular ROI analysis with SUIT were performed same as previous simulations as described in section 2.1.2.
2.2. Experimental methods
2.2.1. Subjects
Seven healthy right-handed volunteers (4 male, 33 ± 7 years old) participated in this study after providing informed written consent. None had a history of neurologic or psychiatric disorders or tACS contraindications. The procedure has been approved by the local ethics committee of Hamburg and was conducted in accordance with the Declaration of Helsinki.
Participants were seated in a comfortable chair in a near-dark room, 2 meters away from a monitor (55'', Sony Group, Tokyo, Japan) displaying a black screen with a white cross in the middle in order to hold the visual focus of the participants constant throughout the experiment.
Four stimulation blocks, one for each montage, were performed consecutively for each participant in a pseudo-randomized order with ∼60 s pauses in between. Blocks consisted of three stimulation frequencies (5, 10, and 30 Hz) and one sham condition in a randomized order. Each stimulation frequency or sham stimulation was applied for three minutes. Eyes were closed for the second half of each stimulation. Participants were blinded to stimulation conditions.
During stimulation, questions were asked to assess the participants' perceived skin sensations including itching, warmth, pricking, and pain; as well as the phosphene intensity and area in the visual field (separately for open and closed eyes conditions). All intensity levels were evaluated by discrete ordinal values of 1:absent, 2:light, 3:moderate, 4:clear, and 5:strong sensation. Phosphene areas were reported by cross-hatching or drawing inside a blank rectangle representing the monitor screen and afterward quantified as ratios of the shaded part to the whole rectangle area. During stimulation, participants were also observed for breathing difficulties or autonomous dysfunctions that could be due to co-stimulation of the brainstem [38].
2.2.2. tACS
Stimulation was delivered via a battery-driven alternating current stimulator (DC Stimulator Plus, NeuroConn, Ilmenau, Germany), using standard rubber electrodes enclosed in saline-soaked sponges (0.9% NaCl) fixed and held on in place by medical elastic bondage (MaiMed GmbH, Germany). Impedance levels were kept under ⩽10 kΩ during all stimulation blocks.
The main electrode (5 × 5 cm2) was placed 2 cm lateral to the inion and the return electrode (5 × 7 cm2) on the four chosen locations (see section 2.1). Frequencies of 5 Hz, 10 Hz, and 30 Hz were applied, with 2 mA peak-to-peak amplitude, except for the sham block which comprised 30 s of ramp up and down.
2.3. Statistical analysis
All statistical analyses were carried out using Python 3 (Python Software Foundation, www.python.org) or MATLAB (R2020, The MathWorks Inc). Computational data for the main tACS electrode failed normality and was thus compared by Wilcoxon signed rank tests (Bonferroni-correction was applied). One-way ANOVA was used to investigate the effect of montage on current density distributions. The Scheirer–Ray–Hare (SRH) test was performed to study the effect of montage and frequency on skin sensations and phosphene intensity; and two-way ANOVA for the same effect on phosphene areas. When relevant, post-hoc Tukey tests were applied and corrected for multiple comparisons by the Benjamini–Hochberg method with a false discovery rate of 5% [39].
3. Results
3.1. Computational results
3.1.1. Optimal position of the main electrode
Moving the electrode to a more lateral position reduced contralateral co-stimulation continuously with the lowest levels for the montage 3 cm lateral to the inion (figure 1(C); all statistical comparisons were highly significant with p < 0.001). Electric currents in the ipsilateral hemisphere of the cerebellum were comparable between 0 and 1 cm relative to the inion, highest for 2 cm and lowest for 3 cm relative to the inion (0/1: p = 0.101, 0/2: p = 0.333, 0/3: p < 0.001, 1/2: p < 0.001, 1/3: p < 0.001, 2/3: p < 0.001).
Comparing simulated current density distributions on cerebellar surfaces shows that positioning the main electrode 2 cm lateral to the inion optimally targeted the right cerebellar hemisphere with only minor co-stimulation of the contralateral hemisphere.
3.1.2. Effect of montage on the stimulation strength in the cerebellum
One-way ANOVA revealed a significant effect of montage on the mean current density in the right hemisphere of the cerebellum (F(3,48) = 3.59, p = 0.02). Post-hoc pairwise Tukey test showed significant differences in forehead-neck (p = 0.02), but not in forehead-jaw (p = 0.05), forehead-buccinator (p = 0.11), buccinator-jaw (p = 0.98), buccinator-neck (p = 0.86) or jaw-neck (p = 0.98) couples. More detailed analysis of individual cerebellar lobule activations can be found in supplementary results (available online at stacks.iop.org/JNE/19/026060/mmedia).
3.1.3. Effect of montage on co-stimulation of the eyeballs and the brainstem
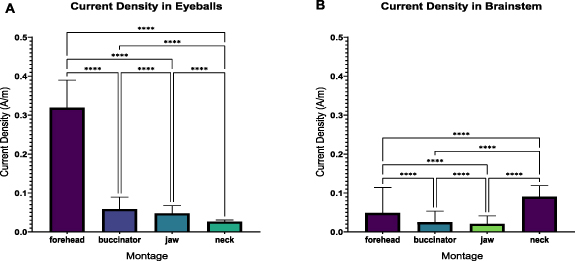
We conducted ROI analysis of both eyeballs and the brainstem using SimNIBS and MRIcroGL, supplementary table S1 shows the descriptive statistics of current density in both areas separately. One-way ANOVA was used to investigate the effect of stimulation montage on the current density distributions in the ROIs. Results revealed a significant effect both in eyeballs (F(3, 44 380) = 133 304, p < 0.0001) as well as brainstem (F(3, 95 164) = 15 530, p < 0.0001). In both cases, the post-hoc Tukey test showed a significant difference in mean current density between all pairs of montages (forehead-buccinator, forehead-jaw, forehead-neck, buccinator-jaw, buccinator-neck, jaw-neck) as can be seen in figure 3. Co-stimulation of the eyeballs was strongest in the forehead montage followed by buccinator, jaw, and neck, respectively. The brainstem was most strongly stimulated by the neck montage followed by the forehead and buccinator and least by jaw montage.
Figure 3. Comparison of mean current density in (A) eyeballs and (B) brainstem between the four tACS montages i.e. forehead, buccinator, jaw, and neck. All differences in means are statistically significant.
Download figure:
Standard image High-resolution image3.1.4. Comparing classical and 4 × 1 ring montage
The mean current density in cerebellar lobules was compared between each of the four classical montages and the 4 × 1 ring electrode arrangement using one-way ANOVA. Current distribution induced by ring montage was significantly lower than all the four montages (compared to forehead: F(1, 24) = 100, buccinator: F(1, 24) = 41, jaw: F(1, 24) = 33, neck: F(1, 24) = 49, with p < 0.0001 for all tests).
3.2. Experimental results
3.2.1. Effect of montage and frequency on skin sensations
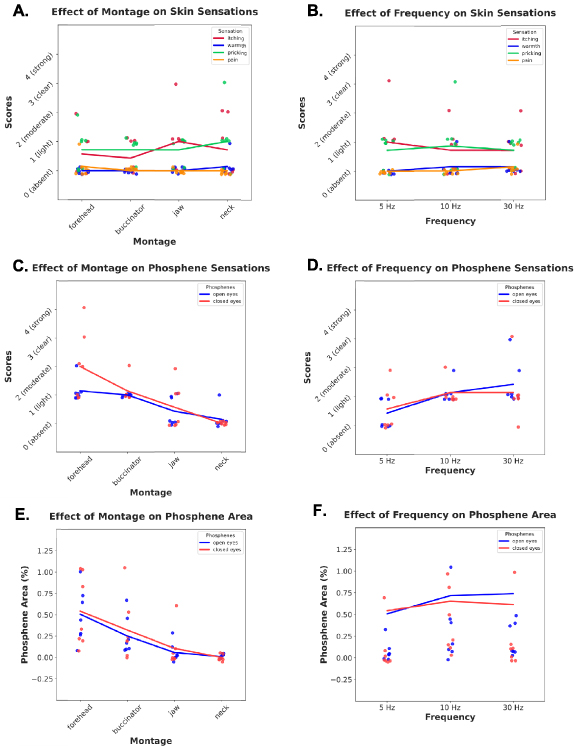
All participants finished the experiment without reporting pain, discomfort, or other serious adverse effects (supplementary table S2), e.g. breathing difficulties or palpitations. Two-way SRH revealed that neither montage, frequency, nor their interaction affected the four skin sensation intensities (figures 4(A)–(B) and supplementary table S3).
Figure 4. (A)–(D) Effects of montage (forehead, buccinator, jaw, neck) and frequency (5, 10, 30 Hz) on skin sensations and phosphene scores, as scattered individual scores based on self-reports of each subject, as well as line representations of averaged scores associated with ANOVA analyses. (E), (F) Effects of montage and frequency on percentage of phosphene area, calculated for every individual based on the area of the drawn representation of phosphenes divided by total area of the monitor screen, beside line representations of averaged areas associated with ANOVA analyses.
Download figure:
Standard image High-resolution image3.2.2. Effect of montage and frequency on phosphene intensity
In open eyes condition, phosphene intensity scores were affected by montage (H(3,72) = 8.15, p = 0.04) but not by frequency (H(2,72) = 0.93, p = 0.62), nor the interaction (H(6,72) = 0.83, p = 0.99). Post-hoc Tukey test indicated no significant pairwise differences. In closed eyes condition, phosphene scores were different across montage (H(3,72) = 52.65, p < 0.001), but not frequency (H(2,72) = 3.39, p = 0.18). Also, there was no interaction (H(6,72) = 2.74, p = 0.83). A post-hoc test showed significant pairwise differences between all montages, with the highest scores for forehead, followed by buccinator, jaw, and neck (figures 4(C)–(D)).
3.2.3. Effect of montage and frequency on phosphene areas
The area of perceived phosphenes in the visual field was affected by both montage (F(3,72) = 19.89, p < 0.001) and frequency (F(2,72) = 4.75, p = 0.01) according to two-way ANOVA, although not by the interaction (F(6,72) = 0.50, p = 0.79). Post-hoc test showed the difference between forehead and other montages (buccinator: p = 0.01, jaw: p < 0.001, neck: p < 0.001), buccinator-neck (p < 0.001), but not jaw-neck (p = 0.35). The effect of frequency was significant between 5 Hz and 10 Hz (p = 0.01), but not 5 Hz and 30 Hz (p = 0.05), or 10 Hz and 30 Hz (p = 0.82) (figure 4(E)–(F)).
Using Spearman's rank correlation test, it was found that phosphene intensity is significantly correlated with the area of perceived phosphene, both in open eyes condition (rs = 0.84, p < 0.001) and closed eye condition (rs = 0.82, p < 0.001).
4. Discussion
Our simulations confirm that electrode placement is a crucial determinant of the resulting electric field and consequently current density in the cerebellum. Our experimental data show that montage affects both the effectiveness of cerebellar tACS and the side effects, confirming previous findings in regard to the former [4, 22, 25, 40] and the latter [13, 17, 21]. Between the tested montages in our study, the forehead led to the strongest stimulation effect in the ipsilateral hemisphere of the cerebellum, whereas stimulation with high-definition ring electrodes was least effective in terms of current distribution in the cerebellum, in line with recent findings [23]. The reason for the strong stimulation with the forehead montage could be attributed to the direction of current flow vectors through the cerebellum. The current field direction i.e. the straight line from the inion to the forehead passes directly through the cerebellum and this leads to induction of higher current densities compared to montages where the current is led away from the cerebellum (for example the neck montage), in line with the study by Klaus and colleague who showed the variability of induced field direction caused by changes of electrode positioning in transcranial direct current stimulations [23]. However, the forehead montage is also associated with the strongest phosphene effect, which is in line with our simulations showing the highest current density induced in the eyeballs with this montage. The higher current density in eyeballs explains stronger phosphenes considering the retinal origin of this phenomenon [20]. As the rectangular return electrode is moved further down, cerebellar current density and phosphenes tend to decrease.
Skin sensations were low throughout all tACS sessions and unaffected by the choice of montage, which could be due to keeping the impedance low by using elastic bondage that maximizes electrode-skin contact [41]. Also, the choice of classical large sponge electrodes contributes to low skin sensations, as opposed to small multiple ring electrodes which are associated with more severe cutaneous sensations [2]. In addition, frequency alteration did not affect the skin sensations intensities, which is confirmed by some [14], but not other publications [12, 13]. The ambiguity of whether cutaneous effects of tACS are frequency-dependent remains unclear. However, Hsu and colleagues [12] provide a detailed analysis of frequency as a stimulation parameter and report a reduction in skin sensations with higher frequencies, a finding that we did not observe. Of note, their reported level of cutaneous sensations was altogether low, as was ours. Therefore, this difference could be attributed to floor effects when evaluating phosphenes or skin sensations. Another explanation for the divergent findings in regard to frequency-dependent effects is the variability of methods used in different studies, e.g. the applied montages, electrodes, and frequencies [3, 4, 7, 9].
Perception of phosphenes showed no effect of stimulation frequency. This finding contrasts with other previous studies [10, 13] which could be due to the large inter-individual variability of reports that did not allow to find potentially weak effects in a small sample. We found a significant effect of montage that favoured neck and jaw montages. In some previous studies, neck or shoulder montages have been chosen for positioning the return electrode in order to avoid phosphenes [41–43]. However, according to our data, the neck position (or below the neck) leads the current away from the cerebellum and significantly decreases current density in the target region. Notably, our simulations additionally showed that such montages are associated with unwanted co-stimulation of the brainstem. The difference between the buccinator and the jaw position is highlighted and investigated in our study since they are in fact different in terms of distance to the eyes; however, in literature, this fine difference is often neglected. There are studies in which the jaw montage has been used but the position is reported as the buccinator [44], probably since buccinator is a common term in stimulation literature. Comparing these two remaining montages, our results favour the jaw montage because it stimulates the cerebellum with a more evenly spread current density, and it is associated with a lower co-stimulation of the brainstem and retina.
Having a sample size of seven participants in the experimental part of this study brings about limitations in terms of generalizing our findings to cerebellar tACS studies, even though this is not uncommon among studies which involve cerebellar stimulation [8, 42, 45]. Also, the intrinsic limitations of the computational model such as applying a BEM and simplifying the physics of subjects' heads with one averaged head model (MNI152), and considering fixed conductivity values for various tissues, need to be taken into account. Future studies are encouraged to control for interindividual variability by creating subject-specific head models based on MRI images and investigating the montage/frequency interaction during cerebellar tACS more exhaustively and in larger cohorts.
By combining computational simulations with experimental data, we present converging evidence for qualitative and quantitative differences between common montages for the stimulation of the cerebellum. Taken together, our study proposes the jaw montage as an optimal choice for cerebellar tACS that minimizes side effects while maintaining an effective ipsilateral stimulation of the cerebellum. Due to the frequency-invariance, this protocol is applicable for a wide range of experimental conditions. Ultimately, adopting a standard protocol and combining it with pre-stimulation techniques (simulation) will improve experimental control, safety, and effectiveness of cerebellar tACS as well as it will pave the road for reproducible experimental results.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Funding
This work was funded by the EU-project euSNN (MSCA-ITN-ETN H2020-860563).
Financial disclosures
Fatemeh Sadeghihassanabadi is supported by a grant from the EU (euSNN, MSCA-ITN-ETN H2020-860 563).
Jonas Misselhorn is supported by the Deutsche Forschungsgemeinschaft (SFB 936/A3).
Christian Gerloff received funding from Deutsche Forschungsgemeinschaft, European Union, Federal Ministry of Education and Research (BMBF), German Statutory Pension Insurance Scheme (RV Nord), National Innovation Fund, Wegener Foundation, and Schilling Foundation; he received honoraria as speaker or consultant from Amgen, Boehringer Ingelheim, Daiichi Sankyo, Abbott, Prediction Biosciences, Novartis, and Bayer outside the submitted work.
Simone Zittel is supported by a grant from the Deutsche Forschungsgemeinschaft (1807/2-1) and from the EU (euSNN, MSCA-ITN-ETNH2020-860 563). She received honoraria as speaker or consultant from Merz Pharmaceuticals and BioMarin.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
- 3
Surface-based representation of the cerebellum.
- 4
- 5
MSH file which can be viewed by Gmsh software (https://gmsh.info/).