Abstract
Objective. Proprioception is the sense of one's position, orientation, and movement in space, and it is of fundamental importance for motor control. When proprioception is impaired or absent, motor execution becomes error-prone, leading to poorly coordinated movements. The kinaesthetic illusion, which creates perceptions of limb movement in humans through non-invasively applying vibrations to muscles or tendons, provides an avenue for studying and restoring the sense of joint movement (kinaesthesia). This technique, however, leaves ambiguity between proprioceptive percepts that arise from muscles versus those that arise from skin receptors. Here we propose the concept of a stimulation system to activate kinaesthesia through the untethered application of localized vibration through implanted magnets. Approach. In this proof-of-concept study, we use two simplified one-DoF systems to show the feasibility of eliciting muscle-sensory responses in an animal model across multiple frequencies, including those that activate the kinaesthetic illusion (70–115 Hz). Furthermore, we generalized the concept by developing a five-DoF prototype system capable of generating directional, frequency-selective vibrations with desired displacement profiles. Main results. In-vivo tests with the one-DoF systems demonstrated the feasibility to elicit muscle sensory neural responses in the median nerve of an animal model. Instead, in-vitro tests with the five-DoF prototype demonstrated high accuracy in producing directional and frequency selective vibrations along different magnet axes. Significance. These results provide evidence for a new technique that interacts with the native neuro-muscular anatomy to study proprioception and eventually pave the way towards the development of advanced limb prostheses or assistive devices for the sensory impaired.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Proprioception is the sense of one's position, orientation, and movement in space [1]. It plays a central role in motor control, because the information this sense helps to refine movement and balance. When proprioception is impaired, motor execution becomes error-prone, leading to clumsy and poorly coordinated movements [2, 3]. At the mechanistic level, muscle spindle afferents (found in skeletal muscles) and Golgi tendon organs (lying at the interface between muscles and tendons) have been identified as major sensory mediators to this perception [4] in addition to skin receptors that also contribute to proprioceptive sensation [4, 5].
Kinaesthesia in humans is often studied through the kinaesthetic or movement illusion [6, 7]. This perceptual illusion is elicited by non-invasively applying vibrations at frequencies between 70 and 115 Hz to muscles or tendons to generate sensations of movement at the joint crossed by the vibrated tendons [4, 6–8]. Efforts to provide insight into the neural basis of proprioception have been coupled with microneurography recordings [6, 9], and brain imaging [10–13] with the goal of contextualizing subjective perceptual descriptions with objective data. However, the difficulty associated with studies utilizing muscle vibration is that the mechanical displacements used to trigger proprioceptive percepts must be transmitted through the skin to the underlying muscle. This complicates disambiguating between proprioceptive percepts that arise from muscle sensation versus the mechanosensory and proprioceptive percepts that arise from skin sensation [11, 13]. From a perceptual perspective, in individuals with surgical separation between the muscle sensory receptors and the overlying skin, complex illusory kinaesthetic perceptions are clear and identifiable [14]. In contrast, able-bodied individuals with intact skin co-localized with the vibration of deep muscles appear to show kinaesthetic illusory percepts that are often unclear, difficult to describe, highly variable, and subject to bias and priming [15, 16].
An important avenue for exploration of proprioceptive function is developing methods for selective activation of muscle-based kinaesthesia without overtly activating skin mechanosensory receptors when pushing through to the deeper muscle or disrupting the native neuro-muscular anatomy [1, 17]. Here we propose the concept of a stimulation system to activate the muscle-sensory mediated aspects of kinaesthesia through the untethered application of localized vibration to muscles or tendons. The complete vibratory system would be composed of permanent magnets that are implanted in different muscles. External electromagnetic coils connected to current drivers and a control system would serve as the actuation interface (figure 1). Controlled magnetic fields (MFs) would be generated through the coils to apply forces and torques on the implanted magnets. The stimulation system would also include a localizer with MF sensors, to monitor the position and orientation of the implanted magnets for closed-loop vibration control (force or/and torque). By using the coils to impose specific temporal evolutions of force and torque on the magnets, they could be vibrated in a controlled fashion in the local environment of the deep muscle sensory receptors.
Figure 1. Overview of the stimulation system based on the untethered application of localized vibration to muscles or tendons. A localizer with MF sensors samples the compound MF generated by permanent magnets implanted in different muscles. The MF is processed to retrieve the magnets' poses. These poses are then used by the actuation interface as target locations where to generate, through a coil array, controlled MFs to apply forces and torques on the magnets.
Download figure:
Standard image High-resolution image2. Methods and materials
In order to explore the viability of a potential kinaesthetic stimulation system, we developed and investigated three different rat-sized proof-of-concept systems. Two were tested in-vivo using a permanent magnet implanted in a Sprague–Dawley rat animal model (figure 2). The first in-vivo system used a vibrating external magnet (linear permanent magnet system) to control one linear degree of freedom (DoF) vibration of a single permanent magnet implanted in muscle. The second in-vivo system used a single electromagnetic coil (single-coil system) to control one-DoF torsional vibration of an implanted magnet. The third in-vitro system was an eight-coil array (coil-array system) that was capable of controlling five DoFs (three-DoF linear vibrations and two-DoF torsional vibrations) of a permanent magnet in a silicone muscle tissue analog (phantom).
Figure 2. Working principles for the generation of one-DoF linear (A) or one-DoF torsional (B) vibrations in a single permanent target magnet, PM, used in the linear permanent magnet and single-coil systems, respectively. (A) Linear vibrations are generated using a remote magnet, PMR, attached to a voice coil motor, whose magnetic moment,  , is aligned with that of the magnet
, is aligned with that of the magnet  . The generated force depends by the distance
. The generated force depends by the distance  between the two magnets. (B) Torsional vibrations were generated using an electromagnetic coil with magnetic moment,
between the two magnets. (B) Torsional vibrations were generated using an electromagnetic coil with magnetic moment,  , perpendicular to the magnetic moment of the target magnet,
, perpendicular to the magnetic moment of the target magnet,  . (C) and (D) Linear permanent magnet and single-coil systems used in in-vivo tests with a Sprague Dawley rat model.
. (C) and (D) Linear permanent magnet and single-coil systems used in in-vivo tests with a Sprague Dawley rat model.
Download figure:
Standard image High-resolution image2.1. Mathematical framework and architecture of the three stimulation systems
2.1.1. One-DoF force control of a single magnet—linear permanent magnet system
To generate a linear vibration in a magnet suspended in an elastic medium, a remote vibrating magnet (e.g. connected to a linear actuator) can be used (figure 2(A)). If the magnetization axes of the two magnets are aligned, the oscillatory movement of the latter generates a MF gradient that induces a force (and, in an elastic medium, a movement) on the target magnet. Quantitatively, the force generated on the target magnet by a remote magnet, with aligned magnetization axes but opposite directions, reads:
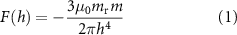
where  and
and  correspond to the magnitude of the magnetic moment of the remote and the target magnets, respectively, and
correspond to the magnitude of the magnetic moment of the remote and the target magnets, respectively, and  is their relative distance. Although equation (1) is nonlinear in
is their relative distance. Although equation (1) is nonlinear in  , for a small distance variation between the magnets (i.e.
, for a small distance variation between the magnets (i.e.  ) a linear approximation can be used:
) a linear approximation can be used:

where  is the force applied in the stationary condition, and
is the force applied in the stationary condition, and  is the change in the applied force. Hence, a linear vibration, proportional to
is the change in the applied force. Hence, a linear vibration, proportional to  , can be obtained by varying the distance between the magnets by
, can be obtained by varying the distance between the magnets by  (figure 2(A)). If only the vibratory stimulus is of interest, as the dynamic component of the force is entirely described by
(figure 2(A)). If only the vibratory stimulus is of interest, as the dynamic component of the force is entirely described by  , the term
, the term  in equation (2) can be neglected.
in equation (2) can be neglected.
Following this basic design, the linear permanent magnet system was implemented by means of a miniature permanent magnet (square cuboid 3.17 mm side × 9.5 mm length, N50, magnetic moment  0.0746 Am2; CMS Magnetics, Garland, TX, USA) attached to the axis of a position-controlled voice coil motor (VCM, Equipment Solutions, Sunnyvale, CA, USA). The latter could generate vibrations with amplitude larger than 50 µm (peak to peak) at 90 Hz, and included a 150 nm resolution optical linear displacement sensor (Equipment Solutions, VCS-10, SCA-824, Sunnyvale, CA). The VCM was controlled through a dedicated driver on a PC.
0.0746 Am2; CMS Magnetics, Garland, TX, USA) attached to the axis of a position-controlled voice coil motor (VCM, Equipment Solutions, Sunnyvale, CA, USA). The latter could generate vibrations with amplitude larger than 50 µm (peak to peak) at 90 Hz, and included a 150 nm resolution optical linear displacement sensor (Equipment Solutions, VCS-10, SCA-824, Sunnyvale, CA). The VCM was controlled through a dedicated driver on a PC.
2.1.2. One-DoF torque control of a single magnet—single-coil system
To implement a torsional vibration in a magnet suspended in an elastic medium, a single coil can be used (figure 2(B)). Quantitatively, the torque generated on the target magnet by a coil h distance from it, and with perpendicular magnetization axes, reads:
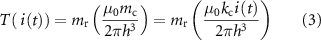
where  is the target magnet magnetic moment,
is the target magnet magnetic moment,  is a coil-dependent scale factor and
is a coil-dependent scale factor and  is the current supplied to the coil (with the equivalent dipole fitted on the coil surface). By applying a time-varying current, it is possible to induce a time-varying torque on the target magnet, thus generating a torsional vibration on the plane xz of the magnet (figure 2(B)).
is the current supplied to the coil (with the equivalent dipole fitted on the coil surface). By applying a time-varying current, it is possible to induce a time-varying torque on the target magnet, thus generating a torsional vibration on the plane xz of the magnet (figure 2(B)).
The design of the single-coil system was based on equation (3), and used an electromagnetic coil (ITS-MS 3025, Intertec Components GmbH, Germany; characterized as in [18], table 1), a dedicated current driver (MC5004 P RS/CO, Faulhaber Minimotor SA, Switzerland), and a PC. The latter hosted the controller, implemented as a C++ application running on a real-time OS (Ubuntu Linux with the RT-PREEMPT patch, 500 Hz control frequency).
2.1.3. Five-DoF control of a single permanent magnet—coil-array system
By generalizing the concept and the equations of the single-coil system it is possible to devise a coil-array architecture that provides full controllability over a target magnet. The force and torque generated on a target magnet with magnetic moment  , due to a MF produced by a coil/electromagnet
, due to a MF produced by a coil/electromagnet  , reads:
, reads:

where  is the magnet position vector [19].
is the magnet position vector [19].  depends linearly on the applied current
depends linearly on the applied current  , and by adopting the magnetic dipole model [18], it reads:
, and by adopting the magnetic dipole model [18], it reads:

where  ,
,  is the position vector of the coil,
is the position vector of the coil,  is a scale factor and
is a scale factor and  is the unit vector representing the coil orientation.
is the unit vector representing the coil orientation.
From equations (4) and (5), it follows that the force  (and the torque
(and the torque  ) can be modulated through the coil current. In the case of C coils operating under certain assumptions [20], the compound MF generated in a point in space is simply defined as the sum of the fields generated by each coil:
) can be modulated through the coil current. In the case of C coils operating under certain assumptions [20], the compound MF generated in a point in space is simply defined as the sum of the fields generated by each coil:
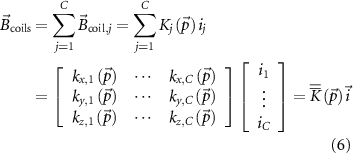
with  a
a  matrix and
matrix and  a C-dimensional vector. By substituting equation (6) in equation (4) we get:
a C-dimensional vector. By substituting equation (6) in equation (4) we get:

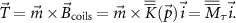
These two systems, of three equations each, can be arranged in a unique system of six equations:
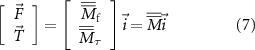
with  being a
being a  matrix. Thus, to generate specific forces and torques, e.g. vibration profiles, at a desired location within the workspace, it is sufficient to know (or identify) the entries of
matrix. Thus, to generate specific forces and torques, e.g. vibration profiles, at a desired location within the workspace, it is sufficient to know (or identify) the entries of  and to invert equation (7) to find
and to invert equation (7) to find  . Notably, this requires
. Notably, this requires  to range from
to range from  (when passive restoring forces are present, e.g. due to elasticity in the medium) to 8 (full independent control) [20, 21].
(when passive restoring forces are present, e.g. due to elasticity in the medium) to 8 (full independent control) [20, 21].
The design of the coil array system was based on these general equations. It consisted of  electromagnetic coils and drivers, two sensor boards each containing three 3D MF sensors (HMC5983, Honeywell; ±8 G max. range, 0.73 mG maximum resolution) (figure 3), and a PC with real-time OS. The electromagnetic coils were arranged around a hollow cylinder (d = 30 mm), representing the workspace of the device, and pointed towards its center (figure 3). The current of each coil was controlled by dedicated driver connected to the PC. As with the single-coil system, the latter hosted the controller, which both localized the magnet, based on the MF sampled by the sensors, and generated the current profiles to induce the desired forces/torques (periodic sinusoids, square waves, and generic polynomial signals). In this implementation, the desired signal shape, frequency, and amplitude could be chosen manually. The localization of the magnet was done once, to calibrate the system (or to identify
electromagnetic coils and drivers, two sensor boards each containing three 3D MF sensors (HMC5983, Honeywell; ±8 G max. range, 0.73 mG maximum resolution) (figure 3), and a PC with real-time OS. The electromagnetic coils were arranged around a hollow cylinder (d = 30 mm), representing the workspace of the device, and pointed towards its center (figure 3). The current of each coil was controlled by dedicated driver connected to the PC. As with the single-coil system, the latter hosted the controller, which both localized the magnet, based on the MF sampled by the sensors, and generated the current profiles to induce the desired forces/torques (periodic sinusoids, square waves, and generic polynomial signals). In this implementation, the desired signal shape, frequency, and amplitude could be chosen manually. The localization of the magnet was done once, to calibrate the system (or to identify  ) (equation (7)) [22–24]; during normal operation, the position of the magnet was assumed to be time-invariant.
) (equation (7)) [22–24]; during normal operation, the position of the magnet was assumed to be time-invariant.
Figure 3. Schematic representation (A) and physical implementation (B) of the coil array system. Two coil rings, consisting of four elements each, were distributed around a cylindrical workspace (d = 30 mm, h = 40 mm). Two sensor boards, each one comprising three 3D MF sensors, were placed around the workspace. (B) Eight coil drivers connected together with two motherboards were used to control simultaneously the coil currents.
Download figure:
Standard image High-resolution image2.2. In-vivo tests with the linear permanent magnet and single-coil systems
2.2.1. Neural recordings
All neural recordings were done using a 65 kΩ 51 μm diameter PFA-insulated stainless steel 'type 316 composition' wire hook electrode (A-M Systems, Carlsborg, WA). Neural signals were amplified (Bak Electronics Inc. Model A-1, Umatilla, FL) and passed to an oscilloscope (BK Precision 2120B, Yorba Linda, CA), filters, and audio speaker (Neurolog 125, 126, and 120, Digitimer, Ft. Lauderdale, FL), and recorded with a Cambridge Electronic Design (CED) Power 1401 computer interface (Cambridge, UK) sampling at 83.3 kHz. Single unit spikes from the stimulus presentations (described below) were counted using Spike 2 software (Cambridge, UK).
2.2.2. Animal preparation
The ability of the linear permanent magnet and single-coil systems to activate muscle receptors was assessed by measuring the neural activation signals in the median nerve, elicited by the vibration of a permanent magnet implanted in rat forelimb muscle. All surgical and experimental procedures were conducted in accordance with protocols approved by the Lerner Research Institute's Institutional Animal Care and Use Committee.
For the linear permanent magnet system, peripheral nerve recording experiments were performed in one animal (weight 457.4 g). The rat forelimb was de-gloved to expose the median nerve and to remove any cutaneous mechanoreceptors overlying the muscles that could potentially be mistaken for muscle-specific sensory responses. The median nerve was identified and then severed near the axilla. The distal nerve stump was arranged in a recording well formed with silicone grease as needed to prevent leakage of saline. Small groups of axons were teased from the distal nerve stump on a glass microscope cover slip placed at the bottom of the recording well, and single unit action potentials were recorded from the axons teased from the proximal median nerve stump.
To verify nerve integrity an axon was hooked and then cutaneous mechanosensory responses were identified by brushing and tapping the glabrous skin of the rat forepaw while listening to the audio output of the recording system through a speaker. Following affirmation of nerve integrity, the median nerve was cut at the wrist while recording to confirm silencing of the cutaneous mechanosensory nerves of the forepaw.
We then searched for muscle sensory-neural activity by pulling the forelimb tendons with forceps and also tapping the forceps with a padded probe [1]. Upon isolation of a well-defined single unit response that mapped to the forelimb muscle a small clip was attached to the distal tendon. Axonal responses were recorded time locked with the position sensor output of the VCM which was mounted to an adjustable armature. We collected responses to square wave applications of mechanical stimulation (2 mm displacement for 1 s repeated five times) to test the response to large amplitude static displacement, and applications of sinusoidal vibration ((low amplitude displacements at 20 Hz (23 µm), 50 Hz (21 µm), 70 Hz (20 µm), 90 Hz (20 µm), 100 Hz (20 µm), and 150 Hz (21 µm)) and (high amplitude displacements at 20 Hz (221 µm), 50 Hz (171 µm), 70 Hz (154 µm), 90 Hz (146 µm), 100 Hz (137 µm), and 150 Hz (97 µm)) for 100 cycles repeated five times).
2.2.3. Magnet implantation
After collecting the responses to these mechanical stimuli, the clip was removed from the distal tendon, and a cylindrical NdFeB permanent magnet (N50 magnetization, 1 mm diameter, 2 mm height, magnetic moment  0.0016 Am2) was inserted into a pocket created in the muscle belly and covered by filter paper affixed with Vetbond tissue adhesive (3 m, St. Paul, MN) (figure 4(A)). While the adhesive was drying, the tendon clip was substituted by the linear permanent magnet system (figure 4(B)). For the linear permanent magnet system, the VCM was positioned so that the movement of the remote magnet moved the implanted magnet within the muscle belly. We used a micro-positioner to adjust the initial distance between the remote and the implanted magnet (
0.0016 Am2) was inserted into a pocket created in the muscle belly and covered by filter paper affixed with Vetbond tissue adhesive (3 m, St. Paul, MN) (figure 4(A)). While the adhesive was drying, the tendon clip was substituted by the linear permanent magnet system (figure 4(B)). For the linear permanent magnet system, the VCM was positioned so that the movement of the remote magnet moved the implanted magnet within the muscle belly. We used a micro-positioner to adjust the initial distance between the remote and the implanted magnet ( ) until successful magnetic coupling was achieved, a distance of ≈2–3 mm. The same static and small/large displacement frequency stimulus presentations were run as described above. It is worth noting that the displacements presented as input stimuli did not necessarily match with those at the (remote) muscle/magnet site, as the latter depend on equations (1) and (2) and on the dynamics of the system.
) until successful magnetic coupling was achieved, a distance of ≈2–3 mm. The same static and small/large displacement frequency stimulus presentations were run as described above. It is worth noting that the displacements presented as input stimuli did not necessarily match with those at the (remote) muscle/magnet site, as the latter depend on equations (1) and (2) and on the dynamics of the system.
Figure 4. Representative images of the in-vivo characterization of the linear permanent magnet and single-coil systems. Above: (A) permanent magnet was implanted in a muscle of a Sprague–Dawley rat's forelimb (solid oval). Neural activity in the median nerve was recorded through teased axons placed on a hook electrode (dashed oval). (B) The configuration of the linear permanent magnet system. The permanent magnet attached to the linear motor (dashed oval) was placed over the implanted magnet in a single forelimb muscle to magnetically couple the movements of the two magnets remotely without having them in physical contact. (C) The configuration of the single-coil system. The permanent magnet implanted in the forelimb muscle was remotely actuated with the MF generated by the single-coil solenoid.
Download figure:
Standard image High-resolution image2.2.4. Static displacement and vibrational response characterization
The procedure to isolate single unit responses and perform full static displacement and sinusoidal vibrational characterizations for both the mechanical displacements and magnetic systems was complex. We were able to demonstrate a full characterization on one recorded response from the linear permanent magnet system and the single-coil system. For the remaining 31 units we were unable to complete full characterizations due to changes in background noise, loss of signal before all stimulus paradigms were completed, or inability to clearly sort and separate overlapping responses. These 31 recordings that did not rise to the most stringent criteria for signal clarity and completion of the full battery of stimulus inputs and as such were considered not fully characterized and removed from the sets of data. Any units that were found to be spontaneously active without responsiveness to stimulus input were not utilized for recording.
For the single-coil system the same general procedure (above) was followed. Following delineation of the mechanically-induced muscle sensory responses the tendon clip was removed and the single coil system was positioned near the forelimb and the magnet implanted in the muscle (figure 4(C)). We were able to complete full trials at 50 and 70 Hz sinusoidal displacements where the electrophysiological recording system was not overly saturated with coil-generated electromagnetic interference. The other trials at the remaining frequencies (100, 150, and 250 Hz) produced single unit neural signals that could not be reliably separated from the coil-induced background noise.
2.3. In-vitro tests with the coil-array system
In-vitro tests were performed to verify the ability of the coil-array system to induce torsional and linear vibrations with different waveforms, on a remote target magnet. These tests were carried out with visualization by a high-speed video camera (Sony DSC-RX10M4; 1000 fps maximum frame rate, with a 1080 p maximum resolution), which allowed to direct observation of the displacement induced on the remote magnet in a single plane (xy in figure 5). A LED was used to synchronize the video with the actuation. The magnet, a commercial cylindrical NdFeB permanent magnet (N48 magnetization, 3 mm diameter, 8 mm height, magnetic moment  0.0584 Am2), was rigidly attached on top of a cylinder (
0.0584 Am2), was rigidly attached on top of a cylinder ( ,
,  ) of viscoelastic material (Ecoflex 00–30, Smooth-On Inc., Macungie, Pennsylvania, USA), resembling the stiffness of muscular tissue [25] (cylinders mounted to each other on their curved side, figure 5, supplementary video 1 available online at stacks.iop.org/JNE/19/026048/mmedia).
) of viscoelastic material (Ecoflex 00–30, Smooth-On Inc., Macungie, Pennsylvania, USA), resembling the stiffness of muscular tissue [25] (cylinders mounted to each other on their curved side, figure 5, supplementary video 1 available online at stacks.iop.org/JNE/19/026048/mmedia).
Figure 5.
In-vitro characterization of the coil-array system. A permanent magnet was attached on top of a cylinder made of viscoelastic material and placed inside the workspace. A high-speed camera was used to record the motion ( and
and  ) of the magnet on the visible plane.
) of the magnet on the visible plane.
Download figure:
Standard image High-resolution imageOne-DoF sinusoidal, square, and sawtooth, torsional ( ) and linear (
) and linear ( ) vibrations were generated at 20 and 90 Hz on the plane observed by the camera. We tested (a) torsional vibrations with a peak to peak torque amplitude of 900
) vibrations were generated at 20 and 90 Hz on the plane observed by the camera. We tested (a) torsional vibrations with a peak to peak torque amplitude of 900  Nm, (b) linear vibrations with a peak to peak force amplitude of 64 mN, along the axis orthogonal to the magnetization axis of the magnet, and (c) of 40 mN along the axis parallel to the magnetization axis of the magnet. All vibrations had a zero mean (no DC component). For each experimental condition (three waveforms, two frequencies, and one amplitude) we ran five repetitions lasting for 3.5 s each (supplementary video 1). The videos, recorded at 500 fps, were processed frame by frame, using custom image processing algorithms developed in MATLAB (MathWorks, Natick, MA, USA) to reconstruct the magnet orientation and the position of its centroid, namely the planar absolute pose (blob analysis, figure 5). The position (
Nm, (b) linear vibrations with a peak to peak force amplitude of 64 mN, along the axis orthogonal to the magnetization axis of the magnet, and (c) of 40 mN along the axis parallel to the magnetization axis of the magnet. All vibrations had a zero mean (no DC component). For each experimental condition (three waveforms, two frequencies, and one amplitude) we ran five repetitions lasting for 3.5 s each (supplementary video 1). The videos, recorded at 500 fps, were processed frame by frame, using custom image processing algorithms developed in MATLAB (MathWorks, Natick, MA, USA) to reconstruct the magnet orientation and the position of its centroid, namely the planar absolute pose (blob analysis, figure 5). The position ( ) and angular displacements (
) and angular displacements ( ) relative to the initial planar pose (when no actuation was delivered) were computed. The correlation coefficient, r, between the normalized power spectral densities (PSD) of the vibration signal (actuation signal) and of the magnet displacement (input and output waveforms, respectively), along the desired axes, was computed to assess frequency distortions in the generated vibrations.
) relative to the initial planar pose (when no actuation was delivered) were computed. The correlation coefficient, r, between the normalized power spectral densities (PSD) of the vibration signal (actuation signal) and of the magnet displacement (input and output waveforms, respectively), along the desired axes, was computed to assess frequency distortions in the generated vibrations.
For sinusoidal-shaped vibrations, the PSD of the magnet displacement signals were also used to extract some parameters, to quantify the coherence of the induced vibrations [18]. These were: (a)  and
and  , namely the PSDs of the displacement signal along the desired actuation direction, in the whole spectrum and at the frequencies of interest (respectively), when a force was applied, (b)
, namely the PSDs of the displacement signal along the desired actuation direction, in the whole spectrum and at the frequencies of interest (respectively), when a force was applied, (b)  , the PSD of the displacement signal along the undesired (orthogonal) direction, in the whole frequency spectrum, when a force was applied, (c)
, the PSD of the displacement signal along the undesired (orthogonal) direction, in the whole frequency spectrum, when a force was applied, (c)  and
and  , the PSD of the angular rotation, in the whole spectrum and at the frequencies of interest, respectively, when a torque was applied. In particular, the values of
, the PSD of the angular rotation, in the whole spectrum and at the frequencies of interest, respectively, when a torque was applied. In particular, the values of  and
and  were computed as the area of the main lobe of the PSD, centered at the frequencies of interest (i.e. 20 or 90 Hz, figure 5). These were used to assess the efficiency of the system according to the following equations:
were computed as the area of the main lobe of the PSD, centered at the frequencies of interest (i.e. 20 or 90 Hz, figure 5). These were used to assess the efficiency of the system according to the following equations:
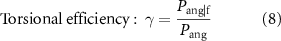
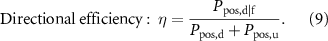
The maximum efficiency was expected when the vibration was generated at the selected frequency (unique frequency in a sinusoid) and along the specified direction only.
3. Results
The goal of designing an untethered vibratory system, capable of remotely activating muscle sensory receptors without activating skin mechanosensory receptors, was approached with three different systems. The linear permanent magnet and single-coil systems were used to examine our ability to generate the neurological indicators associated with vibration-induced kinaesthesia in the muscles of an animal model. The coil-array system was developed as a more generalizable proof-of-concept system and was tested in-vitro by generating vibrations using a variety of forces, torques, frequencies, and waveforms in a silicone phantom.
3.1. In-vivo tests with linear permanent magnet and single-coil systems
Using the linear permanent magnet system, we recorded activity from a muscle sensory afferent neuron in the median nerve of the rat forelimb. By surgically degloving the forelimb and denervating the forepaw of rats, we silenced any potential confounding sensory input from the cutaneous receptors. We found that the linear permanent magnet system was able to elicit neural responses that were indistinguishable from responses that were elicited with matched mechanical actuation (figure 6(A)). In this neural recording, the magnetic actuation was able to reveal slowly adapting responsiveness to static displacement over one second (figure 6(B)). Similarly, the magnetic actuation produced neural responses that tracked with sinusoidal displacements across all tested frequencies from 20 to 150 Hz (figures 6(C)–(H)).
Figure 6. Neural responses between direct mechanical displacement and remotely coupled magnetic actuation with static and sinusoidal displacements. (A) A direct comparison between action potential profiles of a single unit muscle sensory receptor recorded between direct mechanical displacements (top panel: 'Mechanical') and displacements that were mediated by remote magnetic actuation (bottom panel: 'Magnet'). (B) A representative slowly adapting neural response to magnetically actuated static displacements. (C)–(H) Representative single unit neural responses to 20, 50, 70, 90, 100, and 150 Hz sinusoidal displacements activated by remotely coupled magnetic actuation (note: grey traces in panel F represent electromagnetic interference delineated by thresholding).
Download figure:
Standard image High-resolution imageAt the 20 and 50 Hz displacements, the neurons fired at a rate close to one action potential per cycle (average of 104.0 spikes per 100 cycles at 20 Hz; and 100.0 spikes per 100 cycles at 50 Hz). This response activity reflected the spike-per-cycle activity of the large-amplitude mechanical displacements at the same frequencies (figure 7). As the frequency of the linear permanent magnet system actuation increased (70, 90, and 100 Hz), the number of average action potentials per cycle decreased steadily (average of 50.8 spikes per 100 cycles at 70 Hz; 61.9 spikes per 100 cycles at 90 Hz; and 35.8 spikes per 100 cycles at 100 Hz). At 150 Hz the spike-per-cycle activity of the linear permanent magnet actuated neural responses reflected the values obtained at 20 µm displacement with the direct mechanical displacements (15.6 spikes per 100 cycles at 150 Hz).
Figure 7. The relationship between vibratory stimulation frequency and the number of spikes per cycle for the same muscle sensory receptor response with respect to mechanical vibratory stimulation at small amplitude displacements (filled squares), large amplitude displacements (open squares), and remote actuation with the linear permanent magnet system (open circles). Numerals denote actual measured displacement distances (peak-to-trough in µm) through the calibrated voice coil linear motor optical position sensor voltages.
Download figure:
Standard image High-resolution imageUsing the single-coil system we were able record solenoid-induced neural signals from the same sensory receptor represented in the direct mechanical and linear permanent magnet displacement recordings. Although the neural activation signals obtained across multiple vibratory frequencies (50, 70, 100, and 150 Hz) were complicated by electromagnetic interference due to the solenoid's proximity to the recording electrode we were able to reliably separate the neural signal from the background noise for the 50 and 70 Hz vibrational displacements. We found that the solenoid was able to activate neural activity at an average of 1.2 action potentials per vibratory cycle (five banks of 100 cycles each) at 50 Hz, and an average of 1.6 action potentials per vibratory cycle (five banks of 100 cycles each) at 70 Hz.
3.2. In-vitro tests with the coil-array system
The several tests demonstrated that it was possible to produce directional and frequency selective vibrations along different magnet axes, using a five-DoF control, at both 20 and 90 Hz. When providing sinusoidal torsional vibration at 20 Hz as the control signal, the actual orientation  followed the sinusoidal shape almost perfectly with amplitude
followed the sinusoidal shape almost perfectly with amplitude  and oscillating frequency centered at 20 Hz (figure 8(A)). The torsional efficiency proved
and oscillating frequency centered at 20 Hz (figure 8(A)). The torsional efficiency proved  = 0.898, meaning that approximately 90% of the power was concentrated around the desired frequency. The (undesired) displacements of the centroid of the magnet were confined to less than ±50
= 0.898, meaning that approximately 90% of the power was concentrated around the desired frequency. The (undesired) displacements of the centroid of the magnet were confined to less than ±50  (∼10% the arc subtending
(∼10% the arc subtending  ), with a larger intensity on the direction orthogonal to the magnet axis.
), with a larger intensity on the direction orthogonal to the magnet axis.
Figure 8. (A) Representative example of the magnet angular displacement  and the (undesired) displacements
and the (undesired) displacements  ,
,  when a torsional sinusoidal vibration at 20 Hz,
when a torsional sinusoidal vibration at 20 Hz,  , is provided (the shaded area highlights a small transient which is observed at the beginning of the actuation). (B) Actuation efficiencies at 20 and 90 Hz (for sinusoidal waveforms). From left to right: torsional and linear efficiencies (for displacements orthogonal,
, is provided (the shaded area highlights a small transient which is observed at the beginning of the actuation). (B) Actuation efficiencies at 20 and 90 Hz (for sinusoidal waveforms). From left to right: torsional and linear efficiencies (for displacements orthogonal,  , and parallel,
, and parallel,  , to magnet axis, respectively).
, to magnet axis, respectively).
Download figure:
Standard image High-resolution imageWhen compared to the 90 Hz signals, the 20 Hz sinusoids generally led to a larger efficiency together with a smaller dispersion of values around the mean (figure 8(B)). For the torsional vibrations, γ was equal to 0.897 ± 0.002 (average ± std. dev.) along the z rotational direction. Its value slightly decreased to 0.872 ± 0.017 at 90 Hz. Regarding the linear vibrations,  proved equal to 0.897 ± 0.001 and 0.878 ± 0.006, respectively for the sinusoids at 20 and 90 Hz, for displacements orthogonal to the magnetization axis (
proved equal to 0.897 ± 0.001 and 0.878 ± 0.006, respectively for the sinusoids at 20 and 90 Hz, for displacements orthogonal to the magnetization axis ( ), while it proved equal to 0.797 ± 0.03 and 0.58 ± 0.071, respectively for sinusoids at 20 and 90 Hz, for displacements parallel to the magnetization axis (
), while it proved equal to 0.797 ± 0.03 and 0.58 ± 0.071, respectively for sinusoids at 20 and 90 Hz, for displacements parallel to the magnetization axis ( ).
).
With square wave and sawtooth waveforms, the comparison of the normalized theoretical and experimental PSD of the vibrations generated by the coil array system exhibited only minimal differences (representative examples are shown in figure 9). Irrespective of the shape (i.e. square wave, sawtooth) and frequency (i.e. 20 and 90 Hz), we retrieved a good overlap of the PSDs in the locations of the principal harmonics, for both torsional and linear vibrations. Indeed, the two signals appeared to share the same frequency peaks, although the output PSD exhibited a lower amplitude in the proximity of the harmonics at higher frequencies (figure 9).
Figure 9. PSDs of the actuation (input, blue) and vibration (output, orange) signals for square and sawtooth waveforms (representative cases). (A) Square wave torsional vibration at 20 Hz, (B) sawtooth torsional vibration at 20 Hz, (C) square wave linear vibration at 20 Hz, (D) square wave linear vibration at 90 Hz.
Download figure:
Standard image High-resolution imageFor square waves, irrespectively on the vibration frequency, the average correlation coefficient r proved 0.88 ( < 0.05) and 0.94 (
< 0.05) and 0.94 ( < 0.05), for linear and torsional vibrations, respectively.
< 0.05), for linear and torsional vibrations, respectively.
For sawtooth waveforms, r was dependent on the vibration frequency and the actuation direction (for linear vibrations). In particular, in the case of 20 Hz vibrations, we found that r = 0.94 ( < 0.05) and 0.92 (
< 0.05) and 0.92 ( < 0.05), for linear vibrations orthogonal to the magnetization axis and torsional ones, respectively. These values dropped to r = 0.8 (
< 0.05), for linear vibrations orthogonal to the magnetization axis and torsional ones, respectively. These values dropped to r = 0.8 ( < 0.05) and 0.65 (
< 0.05) and 0.65 ( < 0.05) in the case of 90 Hz vibrations. Conversely, the correlation coefficient for linear vibrations along the magnetization axis increased from r = 0.26 (
< 0.05) in the case of 90 Hz vibrations. Conversely, the correlation coefficient for linear vibrations along the magnetization axis increased from r = 0.26 ( < 0.05) to r = 0.75 (
< 0.05) to r = 0.75 ( < 0.05), respectively for 20 and 90 Hz.
< 0.05), respectively for 20 and 90 Hz.
4. Discussion
Proprioception is an important contributor to effective sensory-motor control [1]. Recent advances have shown the effectiveness of kinaesthetic feedback for improving motor control in individuals with amputation and individuals with stroke [14, 26, 27]. These approaches utilize vibration that is transmitted to the muscles in order to elicit kinaesthetic illusions of joint movement. However, there are limitations with applying surface vibration to the deep muscles. Although, best results are obtained with peak-to-peak displacements of 1 mm at 90 Hz [1, 15], the actuator displacement and power required to move dense muscle tissue serves as a barrier clinical implementation. Achieving high frequency with large displacement requires powerful actuators that cannot draw power from the on-board battery of a prosthetic limb without reducing available wear-time due to battery discharge. The large displacements and high frequency required to elicit vibration-induced kinaesthetic percepts also runs the risk of damaging the delicate hardware associated with implanted electrical prosthetic control and feedback systems [28]. Challenges are also faced when choosing between touch percepts or kinaesthetic percepts with limited space for applications utilizing targeted reinnervation as the neural-machine interface [14]. For applications involving individuals such as patients with stroke or patients with traditional standard of care amputations, passing deep muscle vibration through intact skin also confounds the perception of joint movement [15]. Developing approaches to provide vibration-induced kinaesthetic perception through means that can bypass some of the current limitations represents good progress forward over current advanced applications of prosthetic feedback.
The results presented here provide evidence that peripheral neural activity can be elicited within the muscle belly using micron-level displacements of untethered implanted magnets. We have termed this system the 'myokinetic stimulation interface'. For this study we developed and assessed three proof-of-concept systems; a linear permanent magnet actuator, a single-coil actuator, and a coil-array actuator. The linear permanent magnet system demonstrated that a wide range of frequencies applied through remote actuation were able to elicit muscle sensory neural responses in the median nerve. The single-coil system further built on this concept, demonstrating that a neural response could be achieved with electromagnetic-coil induced actuation; this approach being more scalable and practical. The coil-array system demonstrated a high degree of flexibility and control which allowed for selection of force, torque, frequency, amplitude, and signal waveform parameters to control five DoFs when vibrating a permanent magnet in an in-vitro assessment.
4.1. In-vivo tests with the linear permanent magnet system
The muscle sensory receptors, joint capsule receptors, and the skin around the joints all play a role in the sense of the movement and position of our limbs and bodies in space [4, 6]. In particular, the type Ia muscle spindle fibers are considered to be the sensory receptors responsible for signalling limb movement and position and are responsive to static displacements and a wide range of vibratory frequencies [29]. With the use of the linear permanent magnet system we were able to elicit neural responses from a slowly adapting muscle sensory receptor. We found that we could activate action potentials in a single afferent receptor with static displacements and vibration induced by the coupling between a permanent magnet that was implanted in the muscle and a remote permanent magnet attached to a linear actuator. The ability to apply a static displacement using magnetic actuation suggests that different onset velocities can be provided to signal rate of change of movement to proprioceptive afferents. Furthermore, the frequencies that we were able to achieve spanned from 20 to 150 Hz; frequencies that are known to activate the type Ia muscle spindle receptors [30]. Specifically, illusions of limb movement can be triggered with vibration of the muscle at frequencies above 70 Hz [7, 8, 30, 31]. In our previous work, we have shown that 90 Hz vibration of deep muscles in human patients with amputation and a neural-machine interface (targeted reinnervation) improves functional prosthetic outcomes through kinaesthetic perception of hand grasping movements [14, 27]. The linear permanent magnet system provides evidence that muscle sensory action potential responses can be remotely activated at 90 Hz frequency. These results provide evidence in an in-vivo animal preparation that remote actuation of permanent magnets implanted in the muscle can be utilized for effective kinaesthetic prosthetic feedback.
A key feature of the vibrational input from the remotely actuated implanted magnets was that the displacements required to trigger muscle sensory afferent responses appeared to be very small. We used the VCM optical position sensor to physically measure the displacements that were applied directly to the muscle tendon in the control experiments. We could then compare that to the number of action potentials activated by the movement of the implanted magnet for each bank of 100 sinusoids to the number of action potentials elicited in the same neuron by the measured mechanical displacement. With that information, we could infer the displacement range at which the magnets were likely operating (figure 10). At the lower frequencies (20 and 50 Hz) we were able to elicit action potential responses that tracked one to one with each sinusoidal cycle (see: figure 7 red line and open circles). This was similar to measured mechanical displacements eliciting one to one firing that ranged from 221 µm down to 97 µm (see: figure 10). It appears that at 20 and 50 Hz, the magnitude of the implanted magnet displacement was sufficient to achieve a one-to-one response. However, because that critical lower bound was not expressly identified, we can only infer that the amplitude of the implanted magnet is somewhere in the 21–97 µm range for frequencies of 70 Hz and above. Conversely, if the receptor had a response plateau of one impulse per cycle, we are equally unable to determine the upper bound of displacement to achieve the one-to-one response. Moreover, as the frequency of remote magnetic activation increased, the relative rate of firing per 100 cycles decreased, suggesting that the distance the magnet moved likely attenuated (see: figure 7 red line and open circles). At the highest frequency the spikes-per-100-cycles was equivalent to those elicited by the mechanical vibration at 21 µm displacement (see: figure 7 red line and open circles). Moreover, with frequencies higher than 150 Hz, activating Pacinian corpuscles in the muscle is a possibility. However, with magnet displacement attenuation, further experiments would need to be conducted to determine if these receptors could be activated ([32]). Together, these results suggest that the remotely actuated implanted magnets were potentially vibrating within the range of 20–100 µm (figure 10 light red band), although the implanted magnet could be vibrating at a higher displacement if the receptor response plateaued at one impulse per cycle. Similarly, this approach shows the potential for remotely actuating the deep muscle sensory afferents without the necessity of pushing through the skin [14, 27].
Figure 10. The potential working displacement range of the linear implanted magnet system in relationship to measured displacements of the voice coil linear actuator (VCM). The line of open squares denotes the number of action potentials triggered by 100 large mechanically-generated sinusoidal displacements. As frequency increases at the large displacements, the measured amplitude decreases (221 to 97 µm). The line of filled squares denotes the number of action potentials triggered by 100 small mechanically-generated sinusoidal displacements. The light red band denotes the measured displacement region (between 97 and 21 µm) where the implanted magnet was likely operating which was inferred from the spikes-per-100-cycles for each frequency as reported in figure 7.
Download figure:
Standard image High-resolution image4.2. In-vivo tests with the single-coil system
We were able to demonstrate that a remote solenoid could be used to actuate an implanted permanent magnet to induce single-unit neural responses within the muscle. Solenoid actuation alone is an important proof of concept result because of the increased flexibility of the stimulation parameters (discussed in further detail below). Most importantly, we were able to show neural activation at the 70 Hz frequency, which is the baseline frequency for activation of active kinaesthetic percepts [30]. Nonetheless, the proximity of the coil to the recording electrode made it difficult to effectively record the micro-volt level single unit responses from the axon hook electrode. Although the noise prevented similar recordings with the coil-array system, this work invites further studies in which electromagnetic interference is prevented by design, e.g. by using shields and/or appropriate materials, or robust localization algorithms. It is worth mentioning though, that electromagnetic emissions would not necessarily hamper the implementation of the myokinetic stimulation interface, since their (known) frequency content could be tracked and rejected. In addition, substituting the coils with air-core coils could largely reduce those emissions, favouring the integration of the two systems [21].
4.3. In-vitro tests with the coil-array system
The experimental conditions for the characterization of the coil-array system were chosen in order to gather overall insights on the capabilities of the system, and testing them represented an important comparison to the in-vivo tests. In particular, the 90 Hz vibration was motivated by the neurophysiology of the kinaesthetic illusion [14, 27], whereas the 20 Hz corresponded to the lowest frequency tested with the direct mechanical displacement in the in-vivo experiments. The chosen amplitudes (40 and 64  , for linear vibrations along the magnetization and the orthogonal axis, respectively, and 420
, for linear vibrations along the magnetization and the orthogonal axis, respectively, and 420  for torsional vibrations) were the maximum allowed by the coil-array system in all involved experimental conditions, in order to allow for direct comparison. Furthermore, due to visibility limitations of the camera setup (figure 5), we placed the remote magnet on top of the tissue phantom, rather than placing it inside. Finally, the sawtooth and the square waveforms were tested to generalize the physical capabilities of the coil-array system.
for torsional vibrations) were the maximum allowed by the coil-array system in all involved experimental conditions, in order to allow for direct comparison. Furthermore, due to visibility limitations of the camera setup (figure 5), we placed the remote magnet on top of the tissue phantom, rather than placing it inside. Finally, the sawtooth and the square waveforms were tested to generalize the physical capabilities of the coil-array system.
The efficiency of the torsional (γ) and linear (orthogonal to the magnetization axis,  ) sinusoidal vibrations, was large (∼85%–90%) and similar to our previous work [18] (figure 8(B)). This was also true with fewer coils (8 instead of 12, which implies a lower isotropy and controllability of the system), as well as with the different viscoelastic properties of the silicone gel (Ecoflex 00–30 vs. ballistic gelatine). The silicone gel (which was not modelled in the control equations) may have reduced the efficiency from altering the PSD distribution in correspondence to the main frequency components and also at low frequencies (<2 Hz, figure 9(A)). Such a measured spread may also have been an artifact of the video analysis, considering that the frequency resolution of the Fast Fourier Transform was inversely proportional to the size of the temporal window. In particular, having longer acquisitions of the vibrations, or faster acquisition rates, would allow for a better estimate of the PSD, reducing the size of the spectral side lobes. Thus, this limitation may be partially attributed to the available camera, which allowed high speed recordings (500 fps) for no more than 3.5 s. In addition, it is possible that other sources of noise in the video processing may have altered the measures. The main confound was the change in the lightning conditions from the different reflection of the light on the surface of the magnet while moving/vibrating. This was exacerbated by the uneven light across frames due to the 50 Hz power line switching, which led to additional distortion of the measured spectra.
) sinusoidal vibrations, was large (∼85%–90%) and similar to our previous work [18] (figure 8(B)). This was also true with fewer coils (8 instead of 12, which implies a lower isotropy and controllability of the system), as well as with the different viscoelastic properties of the silicone gel (Ecoflex 00–30 vs. ballistic gelatine). The silicone gel (which was not modelled in the control equations) may have reduced the efficiency from altering the PSD distribution in correspondence to the main frequency components and also at low frequencies (<2 Hz, figure 9(A)). Such a measured spread may also have been an artifact of the video analysis, considering that the frequency resolution of the Fast Fourier Transform was inversely proportional to the size of the temporal window. In particular, having longer acquisitions of the vibrations, or faster acquisition rates, would allow for a better estimate of the PSD, reducing the size of the spectral side lobes. Thus, this limitation may be partially attributed to the available camera, which allowed high speed recordings (500 fps) for no more than 3.5 s. In addition, it is possible that other sources of noise in the video processing may have altered the measures. The main confound was the change in the lightning conditions from the different reflection of the light on the surface of the magnet while moving/vibrating. This was exacerbated by the uneven light across frames due to the 50 Hz power line switching, which led to additional distortion of the measured spectra.
Although the system exhibited relatively large efficiencies for torsional (γ) and linear (orthogonal to the magnetization axis,  ) vibration, we failed to demonstrate similar efficiencies for linear vibrations parallel to the magnetization axis (
) vibration, we failed to demonstrate similar efficiencies for linear vibrations parallel to the magnetization axis ( ), especially at 90 Hz (figure 8(B)). This outcome was counterintuitive. Considering the geometrical configuration of the coils in the coil-array system, the lower surface offered by the cylinder base compared to the lateral face, and most of all, standing to equation (5), we expected a larger current/displacement efficiency along the axis of magnetization with respect to the orthogonal axis, as in [18]. Here instead, it was difficult to produce significant displacements along the magnetization axis (i.e. unexpectedly large currents were required, with values close to the maximum produced by the coils). This result may be reflected in an unexpectedly low efficiency
), especially at 90 Hz (figure 8(B)). This outcome was counterintuitive. Considering the geometrical configuration of the coils in the coil-array system, the lower surface offered by the cylinder base compared to the lateral face, and most of all, standing to equation (5), we expected a larger current/displacement efficiency along the axis of magnetization with respect to the orthogonal axis, as in [18]. Here instead, it was difficult to produce significant displacements along the magnetization axis (i.e. unexpectedly large currents were required, with values close to the maximum produced by the coils). This result may be reflected in an unexpectedly low efficiency  . The difficulty in producing displacements along the magnetization axis was the reason choosing a lower amplitude (40 mN vs. 64 mN, peak to peak) to be tested for that axis. However, since this displacement was rather small, and the resolution of the video camera was limited, any environmental noise, undesired movement, or inaccuracies in the video analysis likely had an outsized impact on that particular measurement. The tests with the saw-tooth and square waves generalized the capabilities demonstrated with the sinusoidal vibrations. The imperfect match between the input and output PSDs of the waveforms (figure 9) may be attributed (again) to the viscoelasticity of the medium but also because the input signal was represented in its ideal form (pure tones) rather than with its actual (measured) spectrum.
. The difficulty in producing displacements along the magnetization axis was the reason choosing a lower amplitude (40 mN vs. 64 mN, peak to peak) to be tested for that axis. However, since this displacement was rather small, and the resolution of the video camera was limited, any environmental noise, undesired movement, or inaccuracies in the video analysis likely had an outsized impact on that particular measurement. The tests with the saw-tooth and square waves generalized the capabilities demonstrated with the sinusoidal vibrations. The imperfect match between the input and output PSDs of the waveforms (figure 9) may be attributed (again) to the viscoelasticity of the medium but also because the input signal was represented in its ideal form (pure tones) rather than with its actual (measured) spectrum.
Motivated by the success of magnetic actuation in generating relevant neural activations in the frequency range of the kinaesthetic illusion, future refinements of the proposed stimulation system would allow the development of bidirectional interface for a prosthetic limb. As a next step we foresee the complete integration of the localizer in the stimulation interface, recalling that in the present work it was not used while inducing vibrations. This step is necessary to induce precise vibrations during muscle deformations caused by voluntary contraction or elongation. In this regard, system performance in terms of spatial resolution (submillimetre) and bandwidth (around 50 Hz for the tracking), seems to not represent a bottleneck for the clinical translation. Final refinements, as well as studies with human participants, could contribute in the future to a better understanding of proprioception.
Acknowledgments
This work was funded by the European Research Council under the MYKI project (ERC-2015-StG, Grant No. 679820) and by the Cleveland Clinic Research Program Committee (RPC) project #557. We also thank Courtney E Shell and Madeline D Newcomb for data collection, and Rick Rozic for his assistance.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.










