Abstract
Following the revision of the International System of Units (SI), that takes effect on 20 May 2019, the unit mole is defined by using a fixed number of elementary entities. This number is the fixed numerical value of the Avogadro constant, which is the defining constant of the unit mole. This definition was made possible because the determination of the Avogadro constant had reached a level of relative uncertainty that allowed its value to be fixed and, at the same time, safeguard continuity of measurement results before and after the definition. The motivation for the revision of the SI and the mole in particular will be explained and the experimental work that allowed it is summarized.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The definitions of the SI base units undergo a fundamental revision in May 2019 that puts the emphasis on the 'defining constants'. From the fixed numerical values of these defining constants, expressed in the units of the SI, the complete system of units can be derived. The revised definitions are based on these constants and are therefore inherently stable.
On this occasion, the definition of the mole, a centrepiece of measurements in chemistry, is also revised. This required extremely accurate measurements of the relevant defining constant, the Avogadro constant, in order to assure the continuity of measurement results before and after the revision. The revised definition of the mole is as follows [1]:
'The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol−1 and is called the Avogadro number.
The amount of substance, symbol n, of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles'.
The revised definition of the mole is based on a specified number of elementary entities (typically atoms or molecules) and does no longer depend on the definition of the unit of mass, the kilogram. Traceability to the mole can still be established via mass measurements, tables of relative atomic masses (IUPAC, the international Union of Pure and Applied Chemistry, uses the term relative atomic weights) and the molar mass constant Mu. Relative atomic masses are unaffected by this change in definition and Mu is still 0.001 kg mol−1 within the accuracy required for practical chemistry, although now with non-zero uncertainty.
This means that the changes to the unit mole are both revolutionary and reassuring at the same time. Our measurement results can be quantitatively expressed in the same way as before and measurements of the amount of substance of the same system will give the same result before and after the revision of the mole within any practical uncertainty.
In future, however, the definition of the unit will be based on a fixed number of elementary entities. The definition no longer makes any reference to mass, which should help to eliminate confusion between the quantities of amount of substance and mass. Practical realization of the unit will be described in the so-called 'mise en pratique' of the mole [2]. A mise en pratique for the definition of a unit is a set of instructions that allows the definition to be realized in practice at the highest level of accuracy. The mise en pratique describes a primary realization of a unit based on top-level primary methods. The mise en pratiques are appendices to the SI brochure and subject to change whenever technical improvements necessitate an update. Hence, no changes of the unit definition are expected in the foreseeable future.
In case of the mole, the link to mass (and, implicitly, the kilogram prototype), and reference to the element carbon is no longer part of the definition. The impractical consideration of 'unbound atoms of carbon 12 at rest and in their ground state' (see section 3) is no longer necessary. In contrast, with the 'Avogadro' or 'XRCD' (x-ray crystal density) experiment described below, we can now also provide a mise en pratique with a primary realization of the mole and also the kilogram.
The redefinition in this form also results from a very close and beneficial cooperation between CCQM, the Consultative Committee for Amount of Substance—Metrology in Chemistry and Biology, and IUPAC. Following two workshops organized on the redefinition of the mole by the CCQM, IUPAC established a task group to deliver a Technical Report reviewing possible definitions of the mole and the kilogram, as well as of the quantity amount of substance [3]. The CCQM established an ad hoc working group [4] to deal with the mise en pratique of a redefined mole, to create awareness with respect to a possible redefinition of the mole, and to lead discussions within the CCQM on these issues. The IUPAC task group outlined a recommendation for the redefinition of the mole and addressed it to two relevant consultative committees of the International Committee for Weights and Measures (CIPM): the CCQM and the Consultative Committee for Units (CCU). As a result of this initiative, the ideas of the task group are reflected in the new definition of the mole.
To appreciate the progress made in measurements since the first definition of the mole as an SI unit in 1971, it is interesting to refer to the wording used by Jan de Boer, Secretary of the CIPM, in 1971 to explain the definition of the mole at that time:
'Naturally, one might ask also in the case of the mole would it not be preferable to replace the definition of the mole given here by a molecular one; but as in the cases of the unit of mass and of electric current this would require determinations such as the absolute counting of molecules or the measurement of the mass of molecules that are not possible with the required precision'.
He did not even add: '...at the current time' as a qualifier at the end. This shows how far away from 'counting with the required precision' the 'state-of-the-art' was at that time and what a long way precision measurements have come since then.
This progress was made possible by the so-called 'Avogadro' or 'x-ray crystal density (XRCD)-experiment' conducted by the International Avogadro Coordination (IAC). It combines precision experiments on atomistic and macroscopic levels in a unique way. This is aimed at the determination of the Avogadro constant and the Planck constant with the required precision for the redefinition of the units kilogram and mole as described below. 'Chemical' measurements made a significant contribution to its progress by overcoming the probably most critical measurement challenge along the way: determination of the molar mass of silicon with the required precision.
One might consider that the words used in the IUPAC recommendation [5] respond to the statement of Jan de Boer from 1971:
'With the recent advances in science and measurement practice, our ability to determine the value of the Avogadro constant has now reached a level of relative uncertainty that allows a redefinition of the mole in terms of the explicit number of elementary entities. ... it realigns the definition of the mole with the way most chemists understand it'.
After the approval of the revised definition of the SI units at the CIPM meeting in October 2017, the final decision of the General Conference on Weights and Measures (CGPM) was made at the meeting at 16 November 2018. The revision comes into force from 20 May 2019.
2. Historical summary of the mole
2.1. The path to the SI unit mole
The concepts 'mole' and 'Avogadro constant' are based on the understanding of chemical processes as interactions between atoms and molecules. In 1808, John Dalton proposed that atoms of an element do not differ from one another and that they have a defined atomic mass. At this time, there was no experimental evidence demonstrating the existence of atoms and Dalton was very much aware that he was proposing an ambitious and speculative idea, born nonetheless out of many empirical indications. Cautiously he titled his publication 'A New System of Chemical Philosophy' in order to express his concerns [6]. In 1811, Amedeo Avogadro published his hypothesis that the same volumes of all gases contain—at the same temperature and the same pressure—the same number of molecules, with reference to Dalton's publication [7]. Initially, Avogadro's observation was forgotten until Stanislao Cannizzaro revived it. In 1858 he published a consistent system of chemical formulas and 'atomic weights' (relative atomic masses, see below) of all elements. Subsequently, terms such as atomic and molecular weight as well as other terms based on atomic theory were developed and used in chemistry [8].
The origin of the concept 'mole' is attributed to Wilhelm Ostwald (see, e.g. [9]). In his 'Hand- und Hilfsbuch zur Ausführung Physiko-Chemischer Messungen' of 1893, he writes: 'Let us generally refer to the weight in grams of a substance that is numerically identical to the molecular weight of that substance, as one mole...' [10]. Similar terms such as, for example, 'g-Molekel' or 'g-Mol' with comparable meaning were also used contemporaneously by others such as Walther Nernst (see, e.g. [11]). According to this definition, the unit 'mole' was, therefore, closely connected with mass and for a long time it was interpreted as a 'chemical mass unit' although the atomic perception that links the mole with a number of particles had existed since Dalton and Avogadro. There was, however, a lack of experimental evidence, which could unequivocally confirm these models [11]. The experimental confirmation of the atomic theory finally came from the complete explanation of Brownian motion by Perrin and Einstein in 1909 [12] that also led to the definition of the Avogadro constant. It was followed by the discovery of x-ray crystal diffraction in 1912 by von Laue, Friedrich and Knipping [13] that was essential in fixing the numerical value of the Avogadro constant. This finally led to two different perceptions of the mole, which Stille differentiated by the concepts 'mole' (as a chemical mass unit) and 'mole number' as a unit related to a number of particles which is defined by the Avogadro constant and, therefore, suggesting the introduction of an additional base quantity, the 'amount of substance' [14, 15].
The integration of the unit 'mole' into the SI system of units resolved this ambiguity and made a differentiation of the concepts superfluous. It took place at a very much later date, in October 1971, after the 14th General Conference on Weights and Measures had decided to introduce the mole as a base unit of the SI. The English term 'amount of substance', the quantity of which the mole is the unit, was derived from the German term 'Stoffmenge', introduced by Stille [14, 15]. The decision had been preceded by a corresponding recommendation of the International Union of Pure and Applied Physics (IUPAP), the International Union of Pure and Applied Chemistry (IUPAC) and the International Organization for Standardization (ISO), together with the note to select the carbon isotope 12C as the reference point [16]. The CIPM committee that deals with issues related to the base quantity 'amount of substance' (Consultative Committee for Amount of Substance—CCQM) was—again—established much later, in 1993 [17].
2.2. The mole as an SI unit in chemistry
The mole establishes a connection between the SI and chemistry. With the aid of the mole, quantitative relations in chemistry can be made traceable to SI units and measurements internationally compared. Based on the mole, a large number of national standards for important measurands in chemistry have been introduced in recent years so that these measurements are now traceable to the SI. Generally these are for derived quantities such as the amount of substance concentration.
In usual practice (e.g. in the performance of chemical reactions in the laboratory or in the chemical industry), a very large number of atoms and molecules is involved in any reaction. The unit 'mole' therefore considers together many particles, such that reference to other units (the kilogram) can be made relatively easily. The mole is important in chemistry because it recognizes that atoms and molecules react together on an amount of substance basis and not on a mass basis—chemists call this stoichiometry.
The first definition of the SI unit 'mole', which remains in place until 20 May 2019 is [16]:
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol'.
When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.
In this definition, it is understood that unbound atoms of carbon 12, at rest and in their ground state, are referred to [16]. Unlike Ostwald, whose definition related only to the mass, reference is now also made to a number of particles. The number is derived in this definition, however, from a mass measurement, namely the mass of 0.012 kg of 12C and, hence, was still directly dependent on the SI unit kilogram. Traceability to the mole still ultimately required a mass measurement at this stage.
In practice, traceability to the mole is (and will be) in most cases realized via a weighing process and reference to the relative atomic mass. The connection between the amount of substance nA of an analyte A and its mass m (measured in kilogram) is performed via its molar mass M(A) (measured in kg mol−1):

whereby the molar mass of 12C, M(12C) = 0.012 kg mol−1, serves as a reference basis for M(A). The molar mass of a substance can be calculated from the mean, relative atomic masses Ar of the elements involved, which are usually well known. These also have 12C as the reference point; Ar(12C) = 12 (a dimensionless ratio) by definition. The mean relative atomic masses take into account the relative atomic masses of all isotopes of an element and their abundance on earth [16, 18]. The molar mass M(A) of the substance A is the sum of the mean relative atomic masses Ar of all elements of a molecule of the substance A in accordance with its stoichiometric composition Ar(A) multiplied by the molar mass constant Mu. In the definition of the mole from 1971 it follows that Mu is exactly 0.001 kg mol−1:
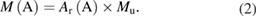
In the new definition the mole is defined by a fixed number of particles. This required the determination of the Avogadro constant with an accuracy that preserves continuity of measurement results related to the mole before and after the redefinition. This was made possible by the Avogadro experiment, described in more detail below. It allowed the numerical value of the Avogadro constant to be fixed and a definition of the mole based on a number of elementary entities without dependence on mass.
After redefinition, experiments for realizing the mole without reference to mass via determination of the number of elementary entities are conceivable, based on the fixed value of the Avogadro constant. Obviously, the Avogadro experiment itself is then best suited for this purpose as referred to in the mise en pratique of the mole [2] and explained in more detail in section 5. It then also allows for a realization of the kilogram based on counting a number of elementary entities [19].
Further to this, other properties of an elementary entity such as its charge might also be suitable for counting and the realization of the mole [20]. Concepts for measurements of the number of elementary entities have been available for a long time, although this had been limited to special cases. The principle utilized in the Avogadro experiment, namely the measurement of the amount of substance of a crystalline solid with the aid of its microscopic, crystallographic lattice parameter and its macroscopic volume, is not new. Similar—albeit much simpler—experiments have been carried out since the discovery of x-ray diffraction. Traceability to the SI was, however, not possible before the advent of x-ray interferometry [21]. For the redefinition of the SI much more sophisticated experiments were required in order to achieve improvements in uncertainty by several orders of magnitude. There are also experiments for the (direct or indirect) counting of elementary entities, e.g. of ions [22] and electrons (single-electron-tunneling—SET circuits). The latter is relevant to the realization of the revised SI base unit ampere [23]. Counting rates of several GHz have been achieved with this approach [24].
In addition, there are numerous procedures for the observation of single atoms (scanning probe microscopy) and also chemical-analytical procedures for the identification and detection of single molecules [25]. Except for the Avogadro experiment none of these procedures is, however, currently suited to quantify macroscopic sample quantities of the order of 1 mol. Some application fields such as nanotechnology do not require this though.
Unlike the other base units, the mole was, and still is, defined by two clauses. The first one defines a number of elementary entities. The second one requires the identification of the elementary entities and establishes—beyond the field of physics—the connection to analytical chemistry. The complete description of a measuring operation for the determination of the amount of substance of a measurand in the unit mole thus requires the identification and quantification of a specified entity (analyte). The amount of substance 1 mol always contains the same number of specified entities. This number is identical to the Avogadro number, the numerical value of the Avogadro constant.
As regards the identification of the entities, the additional notes in the SI Brochure also explain: 'It is important to give a precise definition of the entity involved (as emphasized in the second sentence of the definition of the mole); this should preferably be done by specifying the molecular chemical formula of the material involved' [1]. This implies that—in addition to the chemical formula—other information may be required for their complete description such as, for example, information about their structure.
3. Motivation for the revision of the SI system
3.1. Choices for a change
Prior to SI revision the kilogram remained the only base unit defined and realized as a single material artefact to which all mass measurements across the world were ultimately traceable [16]. In this respect mass measurement had the benefits of precision and the drawbacks of lack of resilience that are usually associated with a non-SI measurement scale: but in this case a special measurement scale that defined the SI unit of mass [26]. The drawbacks of a unique material artefact defining a unit are well known and outweigh the benefits. For a long time metrologists had been keen to redefine the kilogram in terms of constants of nature. The development of the Kibble balance [27] brought this possibility into sharper focus and also prompted additional proposals to redefine with respect to fixed numerical values of constants three other base units of the SI: the ampere, the kelvin and the mole [28, 29]. (The metre was already defined with respect to the speed of light in vacuum and the second depends on the material property of an atom and until the advent of definitions in terms of Planck units it always will. The candela, related to the luminous efficacy technical constant related to a spectral response of the human eye, is a rather separate issue and was not part of these discussions.)
One can consider that the definition of the kilogram, the ampere, the kelvin and the mole in the SI prior to revision are statements of the fixed nature of the mass of the international prototype of the kilogram (IPK), the magnetic constant, the triple point of water and the molar mass of carbon-12. With the exception of the magnetic constant these are related to material properties, and in one case a unique material artefact. The proposals for revision of the SI considered a number of options for combinations of constants that were more universal, allowed realization across the quantity scale not just at one fixed point, and were not related to material properties [29, 30]. The number of constants with fixed values could not over-constrain the system, for instance there could not be two independent constants or groups of constants defining the same SI unit. Equally all proposals needed to ensure that the relationships between quantities was unaltered, regardless of the choice of unit definitions. In particular it is worth noting two such quantity relations relating to the mole:


where m(12C) is the mass of one atom of 12C, M(12C) is the molar mass of 12C, NA is the Avogadro constant, h is the Planck constant, mu is the atomic mass constant, Mu is the molar mass constant, c is the speed of light in vacuum, α is the fine structure constant, R∞ is the Rydberg constant and Ar(e) is the relative atomic mass of the electron. This highlights that the values of h and mu cannot both be fixed, nor the three values of h, Mu and NA. The Planck constant and the Avogadro constant are directly linked via the Kibble balance experiment that links h to macroscopic mass and the Avogadro experiment to count atoms in a near perfect silicon sphere that links NA to macroscopic mass. It is possible to define mass in terms of either of these constants.
It soon became clear as these proposals developed that a fixed numerical value of NA was a likely outcome in a revision of the SI, and could also be used to provide a new definition of the mole based on a fixed number of elementary entities. The main options for fixing the numerical values of constants and their effect on the relative uncertainties most relevant to chemistry are given in table 1.
Table 1. The relative uncertainties, ur, that result from different options for defining the units most relevant to chemistry (adapted from [31] and updated [32]). The value of ur(mIPK) in the revised SI will be approximately equal to the value of ur(h), given in the table [32]. The relative uncertainties given as 0.45 × 10−9 are estimates based on the information available in [32].
| Option | ur(mIPK) | ur(h) | ur(NA) | ur(Mu) | ur(mu) |
|---|---|---|---|---|---|
| SI prior to revision | 0 | 10 × 10−9 | 10 × 10−9 | 0 | 10 × 10−9 |
| Revised SI | ≈10 × 10−9 | 0 | 0 | ≈0.45 × 10−9 | ≈0.45 × 10−9 |
| Fixed h, mole unchanged | ≈10 × 10−9 | 0 | ≈0.45 × 10−9 | 0 | ≈0.45 × 10−9 |
| Fixed mu, Mu, NA | ≈10 × 10−9 | ≈0.45 × 10−9 | 0 | 0 | 0 |
Considering table 1, in the SI prior to revision the mass of the IPK had no uncertainty and the molar mass constant is 0.001 kg mol−1 exactly: values of the other quantities are experimentally determined [32]; from (4), ur(NA) = ur(mu). After the revision, the numerical values of h and NA are fixed and thus the molar mass constant and the atomic mass constant have equal relative uncertainties, again inferred from (4). Another viable option, not chosen, would have been to fix the numerical value of h and leave the definition of the mole unchanged. This would have resulted in reduced uncertainties for ur(NA) and ur(mu), although would still have required the 1980 clarification appended to the definition of the mole concerning unbound atoms in their ground state. A further option would have been to fix the values of mu, Mu and NA. This proposition, which results in the kilogram being defined by mu, is very appealing to chemists but the value of ur(h) under these circumstances is not considered negligible by physicists. Relative atomic masses and relative molecular masses are ratios, not dependent on the definition of the kilogram or the mole, and are unaffected by the new definitions of the kilogram and the mole.
When considering a change of definition (for any SI unit, not just the mole) it is essential that at the point of re-definition the unit system remains coherent and consistent without any step changes in unit size. (The re-definition of the ampere resulted in a change of about 1 part in 107 but this is because under the previous definition the most precise electrical measurements are defined by non-SI conventions. The new definitions of the ampere and kilogram will bring electrical metrology within the SI once again). In particular, in the SI prior to revision Mu is exactly 0.001 kg mol−1 and M(12C) is exactly 0.012 kg mol−1. The continuity conditions agreed for the SI must ensure that these quantities be consistent with their historic values to within their newly acquired uncertainties, at the time of redefinition.
Equations (3) and (4) can be combined to yield (m(e) denotes the electron mass):
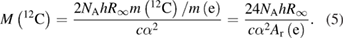
As may be deduced from equation (5), the relative uncertainty of the fine structure constant will dominate the experimental uncertainty of M(12C)—which will essentially be equal to 2ur(α) ≈ 0.45 × 10−9 since the relative uncertainties of the relative atomic mass of the electron and the Rydberg constant are significantly smaller. (As in the past, the value, and uncertainty, of α will continue to be updated as new data become available, the next occasion being the adjustment of the Committee on Data of the International Council for Science (CODATA-2018) in May 2019. The value of α is a pure number which is identical in all unit systems.) This consideration in turn informs the debate on the number of significant digits preferable for NA [33]. Nine significant figures were decided, such that NA is exactly equal to 6.022 140 76 × 1023 mol−1 in the SI after the revision [32].
3.2. The SI prior and after the revision
There are a number of benefits to the presence of the mole, and therefore chemical measurement, within the SI. These are as relevant now as they were when the mole was introduced into the SI in 1971, and remain so after revision of the SI [34]. In particular:
- It formally described a unit which was directly proportional to the number of elementary entities in a sample of a substance. This was an important requirement for chemists in order to describe chemical relationships in stoichiometric terms, without recourse to mass.
- It introduced amount of substance as having its own dimension [16], thereby distinguishing 'number of entities' from a pure number and extending the power of quantity calculus to chemistry.
Indeed, the definition of the mole prior to revision makes it clear that a number of elementary entities are specified: 'The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kg of carbon 12...'. The problem is that the quantitative statement is one relating to mass, not to what this number of elementary entities might be. There is evidence that this is bypassed by most educators who use a proxy definition that one mole is equal to an 'Avogadro number' of elementary entities [31]. Apart from not explicitly mentioning the Avogadro constant in its definition (or indeed the molar mass of carbon 126) we might consider that the definition prior to revision has other drawbacks. First, its reliance on mass therefore incurring all the drawbacks of traceability to the IPK. This still has the potential for some to confuse amount of substance with mass. Second the definition was closely linked to the realization, but in practice the mole cannot easily be realized in the way described. This is a problem with other SI units which are being redefined—although we should recognize that the definition of a unit in no way implies its realization. It is common practice to make small correction between real experiments and idealized definitions. This is necessary for the definition of the second, which specifies that the frequency measured must be 'unpertubed'—an impossibility. More important the former definition of the mole specifies a specific material property at a particular point on the amount of substance scale.
The new definition relates the mole to a fixed number of elementary entities, explicitly based on a fixed numerical value of the Avogadro constant: 'The mole, symbol mol, is the SI unit of amount of substance. One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol−1 and is called the Avogadro number' [1]. A diagrammatic representation of the relationships between relevant chemical quantities and how their uncertainties change as a result of the redefinition of the mole is given in figure 1.
Figure 1. Diagrammatic relationship between the quantities: atomic mass constant, mu; molar mass constant, Mu; relative atomic mass of 12C, Ar(12C); molar mass of 12C, M(12C); mass of one atom of 12C, m(12C); and the Avogadro constant, NA, before and after SI revision. The direction of the arrow indicates multiplications (for instance, mu × Ar(12C) = m(12C)). Those quantities with a fixed numerical value and zero uncertainty are shown with a red box around them (for instance, Ar(12C) ≡ 12 is unchanged by the revision of the SI). Adapted from [35]. © BIPM and IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageThe uncertainty in NA prior to redefinition moved to Mu and M(12C), albeit much reduced in relative terms thanks to h now having a fixed value, and following redefinition the numerical value of NA has no uncertainty. This has the effect of linking the microscopic and macroscopic worlds with zero uncertainty: this more clearly displays the nature of the Avogadro constant as a concept synthesizer [36] relating amount of substance to a number of defined elementary entities. A further consequence of an exact numerical value of the Avogadro constant is that atomic mass scales and the molar mass scales have equal relative uncertainty: a pleasing symmetry for chemical metrologists. There is also the benefit that the definition of the mole is no longer tied to the definition of the kilogram, although for the foreseeable future the vast majority of practical realizations of the mole and its derived units will still involve weighing via n = m/M where n is the amount of substance, m is the mass of a pure sample and M is the molar mass (mass per amount of substance). It is noteworthy that this equation can be used to determine the amount of substance without knowledge of the value of the Avogadro constant. This is useful because relative atomic masses were known accurately long before similarly accurate measurements of the Avogadro constant were made, and it will still be useful after redefinition [37].
The major benefit of the new definition is that it explicitly defines the mole in the way that most chemists think about it [31]. It also provides a definition not dependent on a material artefact that is more universal in its accessibility and applicability across the amount of substance scale. It conforms to a format similar to the other unit redefinitions, but was adapted to make it more understandable for the chemistry community, as requested by the International Union of Pure and Applied Chemistry [38]. The new definition says nothing about its realization, thereby making the new definitions a better fit to the technologies of the 21st century which will be used to realize them. This is especially true at very low amount of substance levels, where techniques such as single molecule counting may be employed [34].
A drawback of the new definition is the diminished importance of the molar mass constant and the molar mass of 12C, both becoming experimentally determined quantities. It has been argued that this change is one that could be seen as risking a lack of coherence in the system [30]. Although the situations where the newly acquired uncertainty in the molar mass constant might need to be considered, for instance for isotopes with very precisely known relative atomic masses, are currently only hypothetical because purity considerations dominate by many orders of magnitude. It has also been argued that an inexact molar mass constant might be difficult to accommodate in teaching. However, the uncertainty in Mu is still an order of magnitude lower that the most accurate realization of the mole—via the silicon sphere experiment—and several orders of magnitude smaller than the relative uncertainties in more common realizations of the mole, for instance in the mise en pratique for the mole. A more fundamental way to consider the newly acquired uncertainty of the molar mass constant in the new SI is that this is the level at which the assumption about the conservation of mass in chemical reactions begins to breakdown. This is insignificant for normal analytical chemistry but not for other areas of science. The final option in table 1, where mu, Mu and NA are all fixed, does not confer enough benefit to chemistry to warrant imposing it on the non-chemical community [31].
Concerns about teaching are still most acute when it comes to distinguishing counting quantities from amount of substance. In this respect the new definition will not help, and may cause even more confusion—this area needs attention from the chemical measurement community in future. The new definition also will not help to resolve the awkward historical relationship the mole has had with the quantity of which it is the base unit, amount of substance. This has mainly arisen because, unlike in physical metrology where we first conceive of a quantity and then of its unit, this happened in reverse for chemistry.
Overall the change is one of clear net benefit for chemistry, even if in the short term there are no practical implications and any improvements may take some time to realize. It is also a necessary change to keep metrology in chemistry aligned with the rest of metrology.
3.3. Other implications of the redefinition of the mole
The new definition of the mole will remove any link between mass and amount of substance: the mole will not rely on any other units or defining constants for its definition. The mole will also be clearly linked to a count, in such a way that the Avogadro constant acts as the constant of proportionality linking counting of elementary entities with amount of substance. It has also been highlighted that the new definition of the mole may also find use in the emerging areas of ultra-low chemical and biological quantification [34]. However, extra vigilance will need to be used to avoid confusion on whether measurement results are being reported in terms of the number of entities or amount of substance.
4. IUPAC and the redefinition of the mole
Unlike most of physical metrology which has a long history of oversight by CIPM, the metrology infrastructure for chemistry developed much later and with significant input from other organisations. Key in the evolution of chemical metrology was the International Union of Pure and Applied Chemistry, one of the oldest international science unions and an important organisation to obtain support from for any redefinition of the mole. IUPAC was established in 1919 by chemists from industry and academia, who recognized the need for international coordination and standardization in chemistry. The standardization of measures, names and symbols is essential to the success of the scientific enterprise and to the development of international trade and commerce. International cooperation among chemists and facilitation of the work of the fragmented chemistry community were the earliest characteristics of the Union. Even before the creation of IUPAC (1919), the International Association of Chemical Societies (IACS), had met in Paris in 1911 and produced a set of proposals for the standardization work that the new Association should address. The first international attempt at organizing organic chemical nomenclature—the Geneva Nomenclature of 1892—grew out of a series of international meetings, the first of which was organized by Kekulé in 1860, the 'Karlsruhe Congress'.
Among other activities IUPAC has been providing advice on issues related to (primarily) chemistry nomenclature, standards via a provision of evaluated scientific data (a role similar that of CODATA) such as atomic weights, solubility, thermodynamic data sets etc and contribution to the development of documentary standards and conventions.
As the obvious source of chemistry advice IUPAC was active in 1971 in the introduction of the unit mole to the SI. Currently IUPAC is represented at two consultative committees of the CIPM at two consultative committees CCU and CCQM and in such capacity has been part of the redefinition process, representing its national adhering organisations (NAOs) and the wider chemistry community.
The formulation of the new definition for the mole has been of a great concern for the chemistry community. Consequently in 2013 IUPAC launched the Mole Project (IUPAC Project Number 2013-048-1-100) [3] to examine the scientific, linguistic and ease of teaching issues surrounding the definition of the unit mole.
In order to prepare a critical assessment of facts and opinions about the new definition of the mole, the Physical and Biophysical Chemistry, Inorganic Chemistry and Analytical Chemistry Divisions of the Union along with the Committee for Chemical Education (CCE) and the Interdivisional Committee for Terminology, Nomenclature and Symbols (ICTNS), joined forces to examine in detail the redefinition [5, 31]. The membership of this Task Group consisted of experts from the above-mentioned bodies.
The work culminated in the publication of the technical report which contains the scientific assessment of the issues surrounding the (re)definition of the mole published in 2017 [31]. This was followed by an IUPAC 'Recommendation' (which is the IUPAC parlance for a document providing definitive language to be used in scientific literature, legal documents etc) which contains the wording for the definition of the mole [39].
(The final wording for the mole definition was agreed upon after vigorous debate in April 2017 at the CCQM and in September 2017 at the CCU.)
As part of the project more than 100 published documents (peer reviewed papers, opinion pieces, editorial and online discussions) related to this matter were reviewed and debated by the expert team. Additionally, an international scientific consultation process involving all NAOs of IUPAC was designed and carried out. The questions posed to the NAOs are presented in appendix. All information associated with consultation and the deliberation of the task group are available as electronic supplementary files to IUPAC Technical Report [31] to promote transparency. The Task Group concluded that the proposed new definitions have been studied sufficiently well to be successfully implemented. The opinions expressed by members from educational and metrological communities, as well as by chemistry practitioners were that the new definitions are desirable. A majority of opinions from the published material analyzed in this work were in accord with the results from the questionnaire study.
5. The Avogadro experiment
Measuring the Avogadro constant with unprecedented accuracy was essential to the redefinition of the mole and the kilogram. This was done by an international experiment known as the International Avogadro Coordination.
The accurate determination of the Avogadro constant using silicon crystals became possible only when Bonse and Hart [21] invented in the 1960s a method to determine the lattice plane spacing by combined x-ray and optical interferometry (XROI) (see section 5.1). Thus, the lattice parameter a can be measured very accurately in the length unit, metre. The cube with corner length a, the unit cell, contains in silicon 8 atoms. If the volume, V, of a macroscopic silicon sample is measured, the number of atoms, N, in the sample can be calculated ('atom counting method'):

In the beginning, the Avogadro project aimed only at the accurate determination of the Avogadro constant, NA. For this aim the amount of substance, n, of the silicon sample was determined by measuring its mass m and molar mass M:

yielding for the Avogadro constant
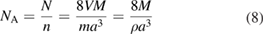
with the density ρ = m/V of the sample. That's why this method is usually called the x-ray crystal density (XRCD) method [40].
After reaching an uncertainty of 1 ppm in 1992 [41], plans were made to improve the method and reach an accuracy suitable for redefining the kilogram. Using nearly perfect silicon spheres for volume measurements (see section 5.2) and ingots highly enriched in the isotope 28Si, this goal was eventually achieved and a relative standard uncertainty of about 1 × 10−8 was reached in 2017 [42].
The equations (6)–(8) have to be corrected for two effects. First, the spheres are covered by a very thin oxide and possibly other layers. Second, the crystal contains a tiny amount of impurities and vacancies which have to be considered (section 5.4).
Whereas the Avogadro constant NA must be measured in conformity with the kilogram definition before the revision of the SI, the numerical value of NA is fixed to define the mole in the SI after the revision. This means that the mole can then be directly realized by the XRCD method using silicon spheres by

5.1. Lattice parameter
The XRCD method exploits the existence of large silicon single crystals where the atoms are perfectly ordered over macroscopic ranges. The principle set-up for measuring the distance of lattice planes is shown in figure 2 [43]. It consists of three lamellas, cut out of the Si crystal, oriented in direction of the {2 2 0} lattice planes. The first, the 'splitter' lamella ('S') splits the x-ray into two beams. In the second lamella, the 'mirror' ('M') the two beams are reflected and finally recombined in the third lamella ('A'). This analyzer lamella can be moved transverse to the beam yielding in a periodic intensity variation of the transmitted x-rays with the period of the diffracting-plane spacing. The movement of the analyzer is measured by an optical interferometer, so that the period of the x-ray fringes (i.e. the lattice spacing) is measured in units of optical fringes and thus traced back to a wavelength standard.
Figure 2. Principle of lattice spacing measurements using three lamellas. Reproduced with permission from [43].
Download figure:
Standard image High-resolution imageAs the analyzer lamella is separated from the crystal with the other two interferometer lamellas, the adjustment is very difficult and requires exact positioning [42]. An optical interferometer with polarization encoding and phase modulation is used to monitor the displacement with picometre accuracy and the rotation in pitch and yaw with nanoradian resolution.
The x-ray interferometer (XINT) is cut out of the crystal in an axial position near to the spheres. Except for carbon, oxygen and nitrogen, the contents of which are measured (see section 5.4), the contaminant concentrations are significantly less than one atom in 109 Si atoms (compare table 3). Since the contaminants in the silicon slightly change the mean atomic distances, the measured lattice parameter a(XINT) has to be corrected to calculate the mean lattice parameter of the sphere

using the strain coefficient βi of the point defect i and the concentration difference ΔCi between the x-ray interferometer (XINT) and the sphere [42].
Apart from the strain due to contaminants and vacancies, it must be ensured that the lattice parameter is determined for the same temperature and pressure as the volume (20 °C in the International Temperature scale of 1990 (ITS-90) and 0 Pa).
5.2. Volume measurement
For the determination of the sample volume in equation (6) spheres are used, since they are less susceptible to damage than cubes or cylinders, and only one parameter—the mean diameter—has to be determined. The diameter of the silicon sphere is measured by optical interferometry from many different directions [40]. If the deviation from a perfect spherical shape is very small, the volume can be calculated from the measured mean diameter D with high accuracy: V = (π/6)D3.
In the interferometer the sphere is placed between two reference surfaces (the etalon) and the distances d1 and d2 between the sphere surface and the reference surfaces are measured. The fractional orders of interference are measured by phase-shifting interferometry using optical frequency tuning. With an additional measurement of the length L of the empty etalon, the (apparent) diameter is given by D = L − d1 − d2.
Two different types of optical configuration are used (figure 3): At the Physikalisch-Technische Bundesanstalt (PTB, Germany) the reference surfaces are spherical, which enables diameter measurements in numerous directions without rotating the sphere [40]. This interferometer can measure about 10 000 diameters of the sphere simultaneously in an aperture angle of 60°. In the set-up of the National Metrology Institute of Japan (NMIJ) an etalon with flat reference surfaces is used (figure 3(b)) [40]. By rotating the sphere, the diameter topography of the entire sphere surface is available, and a topography can be determined (figure 4).
Figure 3. Principle set-ups of interferometric diameter measurements, (a) with spherical reference surfaces and (b) with a flat etalon. Reproduced from [44]. © IOP Publishing Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageFigure 4. Radius topography of the PTB Si-28 sphere Si28kg01a. The maximal shape deviation from a perfect sphere is only 29 nm.
Download figure:
Standard image High-resolution imageAs in the lattice parameter measurements, the volume is measured in vacuum and at 20 °C. Standard platinum resistance thermometers are used calibrated traceable to the ITS-90 at the triple point of water (0.01 °C) and at the melting point of gallium (29.7646 °C) [40].
The sphere is covered by surface layers, which cause a small phase retardation in the reflected light beam. The diameter measured by the interferometer therefore provides information only on the 'apparent diameter'. To deduce the diameter of the silicon core or the diameter of the whole sphere, the phase retardation on reflection from the sphere surface is evaluated from surface layer measurements (section 5.4) [40].
5.3. Isotopic composition
The determination of the Avogadro constant using silicon with natural isotopic composition failed to reach uncertainties of 10−7 or below. To reach the highest accuracy with the XRCD method, the enrichment of the 28Si isotope should be higher than 99.99%. Gaseous silicon tetrafluoride SiF4 is used to enrich the 28Si isotope by centrifuges, since fluorine consists of only one isotope [45]. Then the enriched SiF4 is transformed into silane (28SiH4) using calcium hydride (CaH2):
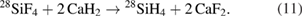
Since the silicon material must be also chemically extremely pure, the silane is cleaned in a very sophisticated procedure by cryofiltration with subcooled boiling and rectification [45]. Eventually, the enriched silicon is deposited by pyrolytic vapour deposition on a slim, electrically heated Si-28 rod. The produced polycrystalline silicon rod is purified by the float zone technique, first in vacuum to remove oxygen and later in argon [45].
The measurement of the isotopic composition and molar mass by 'virtual element isotope dilution mass spectrometry' is described in detail in section 6.
5.4. Surface layers and point defects
The surface of a silicon sphere is typically covered with a 1 nm oxide layer and additional carbonaceous and water layers which have a total thickness of about 0.5 nm to 1 nm (see table 2). Measurements by spectroscopic ellipsometry (SE), x-ray reflectometry (XRR), x-ray photoelectron spectroscopy (XPS), and x-ray fluorescence (XRF) spectrometry can be performed to characterize the surface layers [40]. XPS is most suitable for the thin layers on silicon, since it cannot only measure the amount of the chemical elements on the surface but also the chemical binding state of the elements. This allows the stoichiometric composition of the layers to be determined. Table 2 lists the amounts of substance on the Si-28 sphere Si28kg01a [46]. The amount of the element X can be calculated from the measured mass deposition md(X) by nS(X) = md(X)A/M(X), where A = πD2 is the surface area of the sphere with the diameter D and M(X) the molar mass of the element X. The number of silicon atoms in the oxide
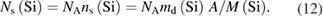
has to be considered for the calculation of the total amount of silicon in a silicon sphere, see section 5.5.
Table 2. Chemical elements on the surface of the 1 kg sphere Si28kg01a measured at PTB by XRF/XPS (in brackets: standard uncertainty) (appendix of [46]).
| Surface layer | Mass deposition in ng cm−2 | Amount of substance in µmol |
|---|---|---|
| Oxygen | 125.3(11.9) | 2.16(0.21) |
| Silicon | 94.1(19.0) | 0.93(0.19) |
| Carbon | 25.1(13.0) | 0.58(0.30) |
| Hydrogen | 7.2(2.4) | 1.97(0.66) |
Due to the sophisticated cleaning procedures, the crystals contain only very few impurities. Most important are carbon and oxygen which can be quantified by Fourier transform infrared absorption spectroscopy (FTIR) measurements [40]. The point defects in silicon influence the amount of silicon in the sphere. For example, carbon is incorporated substituting silicon atoms, thus reducing the amount of silicon. On the other hand, oxygen atoms are present on interstitial sites and do not change the number of lattice places as calculated by equation (6). All impurities and vacancies affect the lattice parameter and the density of the silicon, but these effects cancel each other. Vacancies in the lattice can be interpreted as substitutional atoms with zero mass. Table 3 lists the concentrations of the main point defects (impurities and vacancies) in the sphere Si28kg01a together with the relative amounts of substance C(X)/C(Si) with the 'concentration' of the silicon atoms: C(Si) = 5.0 × 1022 cm−3. The impurity atoms which substitute lattice places in the crystal have to be subtracted from the number of lattice places calculated by equation (6). The same is true for empty lattice places, i.e. for vacancies. This yields the correction Ncorr for substitutional point defects:
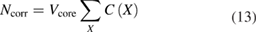
where 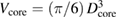 is the volume of the sphere without surface layers and the sum has to be taken over all substitutional impurities and the vacancies. The point defects change the amount of silicon atoms usually by less than 50 nmol mol−1 (compare table 3).
is the volume of the sphere without surface layers and the sum has to be taken over all substitutional impurities and the vacancies. The point defects change the amount of silicon atoms usually by less than 50 nmol mol−1 (compare table 3).
Table 3. Concentrations of the main point defects (impurities and vacancies) in the Si-28 sphere Si28kg01a [42] and their amounts of substance (in brackets: standard uncertainty).
| Point defect | Concentration in 1015 cm−3 | Rel. amount in nmol mol−1 | Amount of substance in nmol |
|---|---|---|---|
| Carbon | 0.89(14) | 17.8(2.8) | 637(100) |
| Oxygen | 0.132(21) | 2.64(42) | 94(15) |
| Vacancy | 0.33(11) | 6.6(2.2) | 236(80) |
5.5. Amount of substance formula
The number of silicon atoms in a silicon sphere can roughly be calculated by equation (5). Subtracting the substitutional impurities and adding the silicon atoms of the oxide layer yields:
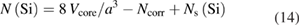
with Ncorr from equation (13) and Ns(Si) from equation (12). Thus, the amount of silicon in the sphere reads

Table 4 shows that the realization of the mole in the SI after the revision with the sphere Si28kg01a can reach a relative uncertainty of about 1 × 10−8. Thus, the XRCD method yields the lowest relative uncertainty for the realization of the mole, although we still have to wait until this finds practical applications.
Table 4. Foreseen uncertainty budget for the realization of the mole by the XRCD method after the revision of the SI as measured and calculated for the sphere Si28kg01a at PTB in 2017.
| Quantity | Relative standard uncertainty (10−9) |
|---|---|
| Avogadro constant NA | 0 |
| Lattice parameter | 5 |
| Volume of the sphere | 7 |
| Point defects | 4 |
| Surface layer | 5 |
| Total uncertainty | 11 |
The mass fraction of silicon in the sample can be calculated by
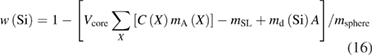
where the sum has to be taken over all impurities X, ma(X) is mass of the atom X and mSL is the total mass of the surface layers. 1 − w(Si) for the sphere Si28kg01a is about 5 × 10−8 proving the high purity of the sphere.
6. The molar mass of silicon
Even after the decision to use enriched silicon rather than using silicon with a natural isotopic abundance [47], the uncertainty associated with the determination of the molar mass M was the single largest contribution (approx. 60%) to the overall uncertainty of the Avogadro constant NA. Therefore, a desperate search for alternative methods started, trying to improve the uncertainty associated with M obtained using gas phase isotope ratio mass spectrometry (IRMS) by at least one order of magnitude. The alternative method would need to fulfil two important requirements: it should avoid the measurement of isotope ratios exceeding the linear range of available detectors and it should offer a much better way to handle the ubiquitous natural silicon blanks. In 2007, at PTB the idea was born to utilize the method of isotope dilution mass spectrometry (IDMS) which has been a tried and tested primary ratio method in chemistry for decades, especially for measurement in challenging matrices [48]. Initially, an apparently strange idea, because mass fractions and molar masses seemed to have nothing in common, the so-called 'virtual element' (VE) was defined. The VE consists only of the isotopes 29Si and 30Si—regarded as an impurity in the matrix of pure 28Si [49]. Because of its unsurpassed chemical purity, the 'Avogadro silicon' could be assumed—at least within the limits of the target uncertainty—to be a binary mixture of the VE and the 28Si. Thus, it was possible to determine the mass fraction w(VE) of the VE in the 28Si matrix. But why should this improve the situation? It was shown that the mass fraction w(VE) could be used to calculate the isotope amount-of substance-fractions x(iSi) of all three isotopes:
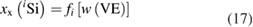
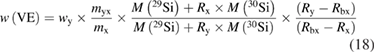
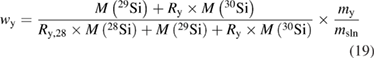
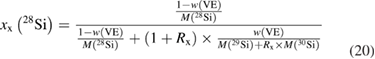

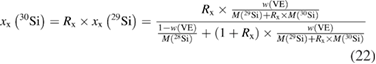
and therefore, this paved the way to determine also the molar mass of the silicon by applying the principle of IDMS [49, 50]. This complex set of equations was combined to yield a highly compact form, which simplified the estimation of uncertainties [51]:
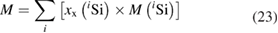
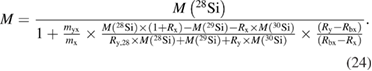
This way, the first important requirement was met, because the range of isotope ratios that needed to be covered was decreased from seven orders of magnitude down to two orders of magnitude. What was even more, the largest contribution to the uncertainty of the molar mass, namely the isotope ratio Rbx in the IDMS blend (prepared from the highly enriched 'Avogadro silicon' x by mixing it with a highly enriched 30Si material y) could now be arbitrarily adjusted to a one-to-one ratio with all the advantages this offered, such as robustness against detector dead-time correction and linearity. The second important requirement was fulfilled by implementing the virtual element IDMS method (VE-IDMS) using a multi-collector mass spectrometer with an inductively coupled plasma ion source (MC-ICP-MS). Even though MC-ICP-MS has never before been used to determine a molar mass with a relative uncertainty well below 10−8, it opened up the possibility to measure the blank caused by the preparation (solvent, containers, etc) but also by the measurement principle itself (background, memory from the sample introduction and ion optics, etc) directly before and after the sample solutions, so that all measurements could be corrected for the blank. No additional offline blank measurements like AAS in case of the gas phase IRMS were needed [52]. This in turn, combined with the parallel detection, helped to dramatically reduce the overall uncertainty associated with M. Unfortunately, the ratios in the VE-IDMS equation are true amount of substance ratios Ri = ni/n1 rather than measured signal intensity ratios r = Ii/I1. Because of the mass discrimination/fractionation effect inherent in any mass spectrometer regardless of the ion source, the measured intensity ratios deviate (in case of ICP-MS) in the percent range from the true ratios and had to be corrected for these effects. This is usually done by using a so-called mass bias correction factor K (usually called K factor):

In almost all cases certified isotope reference materials (iRM) are used to determine K factors. In the current example, this was not a feasible option because of the extremely demanding requirements concerning the uncertainty associated with M. Therefore, the K factors had to be determined ab initio without any simplifying assumptions using gravimetrically prepared binary mixtures of starting materials highly enriched in each of the three Si isotopes [49, 53, 54].
Combining the VE-IDMS method with the K factor determination based on binary isotope mixtures and further improved experimental procedures [55], yielded unsurpassed accuracies associated with the molar mass of the highly enriched silicon used to re-determine NA constant within the aimed at uncertainty (see figure 5 [51]).
Figure 5. Molar masses determined on 14 different samples from one silicon single crystal (Si28-10Pr11 'AVO28' [51]).
Download figure:
Standard image High-resolution imageAn uncertainty of u(M) < 5 × 10−9 can now routinely be obtained [51]. Subsequently, with an even higher enrichment of the silicon used, uncertainties below 10−9 were determined [56]. Based on these improvements, the relative contribution of the uncertainty associated with M to the overall uncertainty of NA was reduced to approximately 6% [57], which reduces the overall uncertainty associated with NA sufficiently so that the requirements of the revised definition of the base unit mole realized by NA are fulfilled.
7. Conclusion
The mole is an essential member of the set of SI base units. Since its introduction in 1971 it has enabled chemistry to become increasing integrated into the global metrology infrastructure. The definition of the mole as part of the 2019 revision of the SI completes the evolution of chemical measurement. Now there is a definition of the mole in terms of an explicit number of elementary entities: the reliance of the old definition on the unit of mass and on a material property of a specific isotope of carbon is no longer required. The mole and the second are now the only SI base units in the revised system defined by a single defining constant.
The new definition of the mole has been made possible through:
- strong engagement with stakeholders in chemical measurement, especially those outside the traditional metrology sphere;
- an increasing understanding of the distinction between amount of substance and counting quantities ensuring the new definition of the mole is not treated as 'just a number';
- the amazing advances in chemical and physical metrology over many years that have underpinned the current Avogadro and Kibble balance experiments.
Similarly to users of the other SI base unit definitions, no immediate effect of the change will be felt by practicing chemists, although there will be a continued need to inform and educate the chemical community on the changed definition. An immediate benefit of the new definition is that it is expected to be more readily understood, and will lead to a better appreciation and understanding of both the quantity amount of substance and its unit, the mole.
Appendix
IUPAC National Consultation questionnaire on the redefinition of the mole [5].
IUPAC NAOs are hereby asked the following:
- 1.Are you (as NAO representing your members) satisfied with the current definition of the mole?
- a.YES or NO?
- b.If NO, please specify in a few sentences why you opted for NO.
- c.If NO, please provide some suggestion on what to change.
- 2.Are you (as NAO representing your members) satisfied with the new definition of the mole as proposed by the 24th General Conference of Weights and Measures?
- a.YES or NO?
- b.If NO, please specify in a few sentences why you opted for NO.
- c.If NO, please provide some suggestion on what to change.
- 3.Are you (as NAO representing your members) satisfied with the current definition of the quantity amount of substance?
- a.YES or NO?
- b.If NO, please specify in a few sentences why you opted for NO.
- c.If NO, please provide some suggestion on what to change.
- 4.Are you (as NAO representing your members) satisfied with the current name of the quantity amount of substance?
- a.YES or NO?
- b.If NO, please specify in a few sentences why you opted for NO.
- c.If NO, please provide a suggestion for a new name.
Footnotes
- 6
One may suppose that the reason for this omission is that the quantity 'molar mass' breaks the SI convention of not referring to a unit in the name of a quantity. In addition, the example of the ampere was followed, where the definition defined a fixed value for the magnetic permeability of vacuum without ever mentioning the magnetic permeability of vacuum.