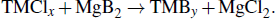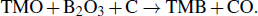Abstract
In recent years, transition metal borides (TMBs) have attracted much attention because they are considered as potential superhard materials and have more abundant crystal structures compared with traditional superhard materials. So far, however, no superhard materials have been found in TMBs. A large number of structures and potential new properties in TMBs are induced by the various hybridization ways of boron atoms and the high valence electrons of transition metals, which provide many possibilities for its application. And most TMBs have layered structures, which make TMBs have the potential to be a two-dimensional (2D) material. The 2D materials have novel properties, but the research on 2D TMBs is still nearly blank. In this paper, the research progress of TMBs is summarized involving structure, mechanical properties, and multifunctional properties. The strong covalent bonds of boron atoms in TMBs can form one-dimensional, two-dimensional, and three-dimensional substructures, and the multiple electron transfer between transition metal and boron leads to a variety of chemical bonds in TMBs, which are the keys to obtain high hardness and multifunctional properties of TMBs. Further research on the multifunctional properties of TMBs, such as superconductors, catalysts, and high hardness ferromagnetic materials, is of great significance to the discovery of new multifunctional hard materials.
Export citation and abstract BibTeX RIS
1. Introduction
Transition metal borides, carbides and nitrides are of great interest in both fundamental science and technical applications.[1–3] In 2005, Kaner et al. suggested that, in addition to the existence of superhard materials in the traditional covalent crystals, transition metal borides, carbides, and nitrides may have high hardness and even superhard materials.[4] Henceforth, there has been a new surge of research interest in searching for ultra-incompressible and superhard transition-metal (TM) borides, carbides, and nitrides. Transition metals have high valence electron density and high ultra-incompressibility. The covalent bond between the light elements has a high shear modulus. Preparing borides, carbides, and nitrides with transition-metals may result in a compound with high ultra-incompressible and hardness. Transition-metal borides, carbides, and nitrides have not only covalent bonds, but also ionic bonds and metallic bonds. Thus, transition-metal borides, carbides, and nitrides also have other properties, such as thermal, electrical, magnetic, and so on.
Boron is a special element. Boron, as an electron-deficient element, can not only form a variety of covalent bond hybridization ways (sp1, sp2, sp3) but also has the ability to gain and lose electrons. Thus, boron and transition-metals can form rich and varied compounds. And transition-metal borides (TMBs) can also form rich and varied crystal structures. Transition-metal borides not only have good mechanical properties but also perform well in thermal, electrical and magnetic fields. So far, a great deal of investigation has been carried out on TMBs, which have been found to have abundant crystal structures, and most of the TMBs are high hardness materials. At the same time, some new functions have been explored on this basis, and a preliminary understanding of its multifunction has been obtained.
In this paper, the research progress of transition metal borides in recent years is summarized from three aspects: structure, versatility, and synthesis.
2. The crystal structure of transition metal borides
There are abundant compounds of TMBs with a wide variety of crystal structures. Boron is a unique element. Boron sits at the boundary between metals and nonmetals on the periodic table, which allows it to gain and lose electrons. Transition metals have high electron density and can form abundant valence states. Boron and transition metal can form covalent bond, metal bond, and ionic bond. In the TMBs, boron atoms can exist in four structural types: isolated boron atom, one-dimensional (1D) boron chain, 2D boron structure, and three-dimensional (3D) boron structure. Isolated boron atoms are commonly found in TMBs with a higher proportion of transition metals, such as Cr2B and Mo2B.[5,6] This paper mainly introduces the structures of transition metal borides in four classes: monoboride (TMB), diboride (TMB2), tetraboride (TMB4), and dodecaboride (TMB12).
2.1. Transition metal monoborides
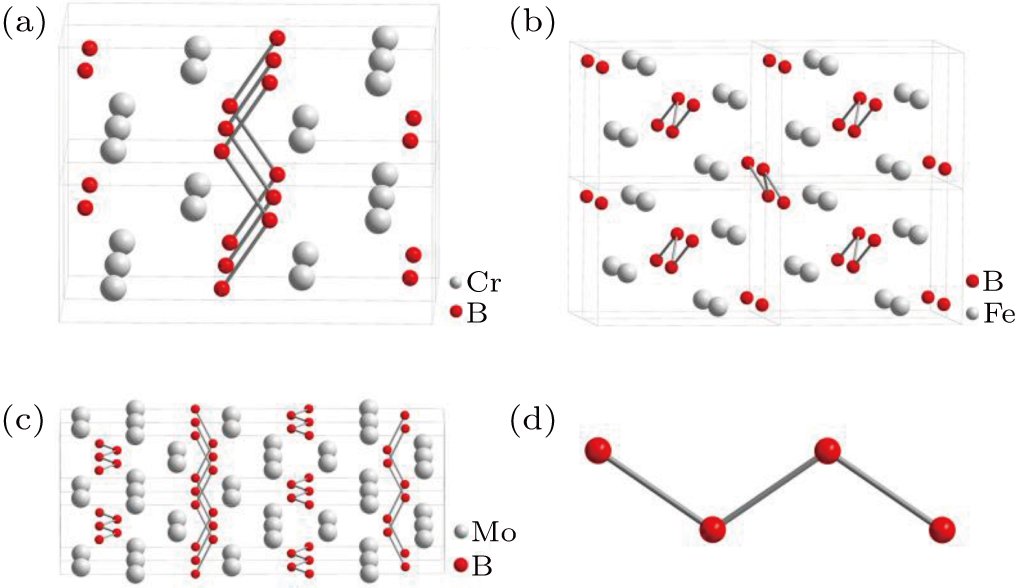
In monoborides, there are three main types of crystal structure, namely CrB-type, β-FeB-type, and α-MoB-type. The crystal structure of transition metal monoborides is shown in Fig. 1. The crystal structure of CrB, β-FeB, and α-MoB belong to the orthorhombic with space group Cmcm (No. 63),[7] the orthorhombic with space group Pnma (No. 62),[8] and the tetragonal with space group I41/amd (No. 141),[9] respectively. The main difference between the three crystal structures is the position of the boron zigzag chain. In CrB, boron zigzag chains are parallel along the a axis. There are seven monoborides belonging to the CrB-type structure, which are respectively VB, CrB, α-FeB, NiB, β-MoB, TaB, and β-WB. The boron zigzag chains of β-FeB are similar to those of CrB in that they are parallel along the a axis, but the inclination of boron zigzag chain is different. The structure of TiB, MnB, β-FeB, CoB, and IrB is β-FeB-type. In contrast to CrB, α-MoB possesses the perpendicular boron zigzag chain skeleton. So far, only two monoborides with α-MoB-type structure have been found, namely α-MoB and α-WB.
Fig. 1. Structure of monoborides: (a) the crystal structure of CrB; (b) crystal structure of β-FeB; (c) crystal structure of α-MoB; (d) boron atom chain. Boron atoms are in red, metal atoms are in gray.
Download figure:
Standard image2.2. Transition metal diborides
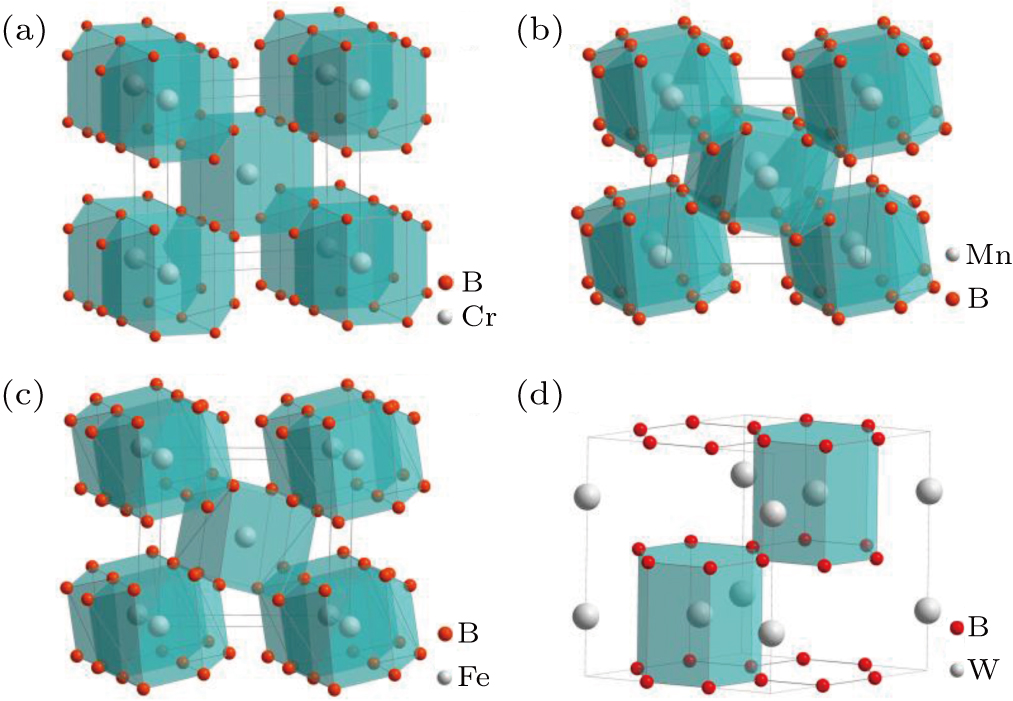
The structures of diborides can be roughly classified into five structural types: AlB2-type, β-MoB2-type, RuB2-type, α -WB2-type, and ReB2-type. The crystal structure of transition metal diborides is shown in Fig. 2. Boron atoms form 2D boron layers or quasi-3D wrinkled boron layers in these structures. Among diborides, the number of TMB2 with AlB2-type structure is the largest.[10] So far, twelve of TMB2 with AlB2-type structure have been prepared, namely, ScB2, TiB2, VB2, CrB2, MnB2, YB2, ZrB2, NbB2, α-MoB2, HfB2, TaB2, and α -WB2. In the AlB2-type structure, boron atoms form graphite-like boron layers and transition metal atoms compose close-packed layers. Each transition metal atom sits at the center of a regular hexagon of boron atoms. Each boron atom has six nearest neighbor transition metal atoms which form a trigonal prism. The boron layer and the transition metal layer alternately arranged.
Fig. 2. Structure of diborides: (a) the crystal structure of CrB2; (b) crystal structure of β-MoB2; (c) crystal structure of WB2; (d) crystal structure of W2B5; (e) crystal structure of ReB2; (f) crystal structure of OsB2. Boron atoms are in red, metal atoms are in gray.
Download figure:
Standard imageAmong the TMB2 structures, other structures can be considered as modifications of the AlB2-type structure. The alternating arrangement of boron and transition metal layers is the same, but the 2D structural units that boron atoms form are different. In these structures, boron atoms form a quasi-3D wrinkled boron layer, which is mainly caused by the amount of charge transfer and the directivity of the B–B covalent bond. In the structure of β-MoB2, there are two kinds of structure units: graphene-like boron layer and wrinkled boron layer, which also arranged alternately.[11] Different from the boron layer in the β-MoB2 structure, the boron atom formation in the RuB2 structure is pseudo-hexagonal corrugated boron layers.[12] Only RuB2, OsB2, and IrB2 have RuB2-type structure.[12,13] The boron layers of ReB2 are all quasi-3D wrinkled boron layers, which is due to the special electron structure of rhenium formed a special electron transfer.[12,14–19] This quasi-3D boron structure has an important effect on the properties of ReB2, which makes it more difficult to compress in the c-axis direction.[20]
There are some controversies about the structure of MnB2, MoB2, and WB2. Manganese diboride has two crystal structures, AlB2-type and ReB2-type. Theoretical calculations showed that the ReB2-type MnB2 was more stable than the AlB2-type MnB2 and was predicted to be a superhard material,[21,22] but only AlB2-type MnB2 was synthesized experimentally up to now.[23] MoB2 has two phases with α-MoB2 and β-MoB2. The theoretical calculations suggest the α-MoB2, which has a hexagonal crystal structure with space group P6/mmm (No. 191), is thermodynamically unstable.[24–27] But the α-MoB2 was obtained in the experiment and is stable structures at ambient conditions. β-MoB2 was originally considered to be the Mo2B5-type structure.[28,29] Okata et al. firstly reported that the ratio of molybdenum atom and boron atom is 1:2 in Mo2B5, which is actually β-MoB2.[30] β-MoB2 has a rhombohedral crystal structure with R-3m (No. 166) symmetry. As with the same group element molybdenum, the crystal structure of WB2 also has the so-called W2B5 phase. So far, three crystal structures of WB2 were reported in the experiment, Mo2B5-type (R-3m), AlB2-type (P6/mmm), and W2B4-type (P63/mmc).[31–41] However, the phonon calculations suggested the AlB2-type WB2 is dynamically unstable and was predicted to be a stable high-pressure phase above 65 GPa.[33] Another controversy is the stoichiometric ratio of W2B5.[42–44] Frotscher et al. reported that W2B5 is actually W2B4 (WB2) with a space group of P63/mmc (No. 194),[45] in which the crystal structures and composition were probed by x-ray diffraction (XRD), neutron diffraction, energy dispersive spectrometer (EDS), WDS, scanning electron microscope (SEM), and so on.
2.3. Transition metal tetraborides
In the past few years, TMB4 was paid attention extensively, because CrB4, MnB4, and FeB4 were considered as potential superhard materials.[21,46–54] The crystal structure of CrB4, MnB4, and FeB4 has many similarities, they all have a 3D network structure. The crystal structure of CrB4, MnB4, and FeB4 is shown in Figs. 3(a), 3(b), and 3(c). The crystal structure of CrB4 belongs to the orthorhombic. Initially, CrB4 was described in the space group of Immm (No. 71),[46,55] but the theoretical calculation shown that the Pnnm (No. 58) phase of CrB4 is more stable.[48,49,56,57] According to the results of XRD and TEM, Niu et al. believed the space group of CrB4 is Pnnm (No. 58).[48] Wang et al. reported that the structural difference between Immm and Pnnm is the site of the boron atom.[58] The powder x-ray and neutron diffraction patterns of Immm and Pnnm structures were simulated for CrB4. The results showed that XRD of Immm and Pnnm structures are the same, the neutron diffraction peaks are the difference. The structural controversy of CrB4 can be resolved by neutron diffraction. The structure of FeB4 is similar to that of CrB4. The symmetry of FeB4 obtained by the experiment is Pnnm (No. 58),[59] which is in agreement with the theoretical calculation results.[60] MnB4 was initially described in the space group of C2/m (No. 12).[61] The theoretical calculation suggests that the structure of MnB4 should be similar to that of CrB4 and FeB4, but the experimental results show that the structure of MnB4 is monoclinic with the space group of P21/c (No. 11).[52,62–64] This is mainly due to Peierls distortion, which causes the Mn–Mn atom dimers to appear in the MnB4 structure and thus distort the MnB4 crystal structure.
Fig. 3. Structure of tetraborides: (a) the crystal structure of CrB4; (b) crystal structure of MnB4; (c) crystal structure of FeB4; (d) crystal structure of WB3. Boron atoms are in red, metal atoms are in gray.
Download figure:
Standard imageThe crystal structure of MoB4 and WB4 is also a controversial issue. Theoretically, the structure of WB4 was considered to be thermodynamically unstable.[65] Some studies suggested that the reported WB4 is probably stoichiometric WB3. Although they have the same hexagonal crystal structure with P63/mmc (No. 194) symmetry,[66] the structure of WB3 is more stable in theory.[67,68] But Li et al. reported that the structure of WB4 and WB3 are thermodynamically stable and should be accessible to synthesis.[69] Because the boron atom is small, it is difficult that the site of boron atoms is detected by x-ray diffraction. The symmetry of WB4 and WB3 is the same, thereby the x-ray diffraction diagram is similar except for diffraction peak strength. The difference in the crystal structure of WB4 and WB3 is the boron–boron dimer. There is the boron–boron dimer in WB4. Cheng et al. reported that the results of Ac-HRTEM experiments suggested boron–boron dimer does not exist,[70] so WB3 was confirmed to exist. The crystal structure of WB4 (WB3) is shown in Fig. 3(d). Some other studies indicated that tungsten atom is the deficiency in WB3, the chemical formula of WB3 should be W1 – x B3.[71] The crystal structure of reported WB4 (WB3) needs further investigations in theory and experiments. The crystal structure of MoB4 is very similar to WB4. The reported MoB4 is not actually stoichiometric MoB4.[72,73] The general formula can be written as Mo1 – x B3, for example, Mo0.8B3 and Mo0.91B3. The crystal structure of Mo1 – x B3 is consistent with that of WB4 in Fig. 3(d). In addition, the calculations suggested that the R-3m structure of ReB4 is thermodynamically stable, but so far ReB4 has not been prepared.[74]
2.4. Transition metal dodecaborides
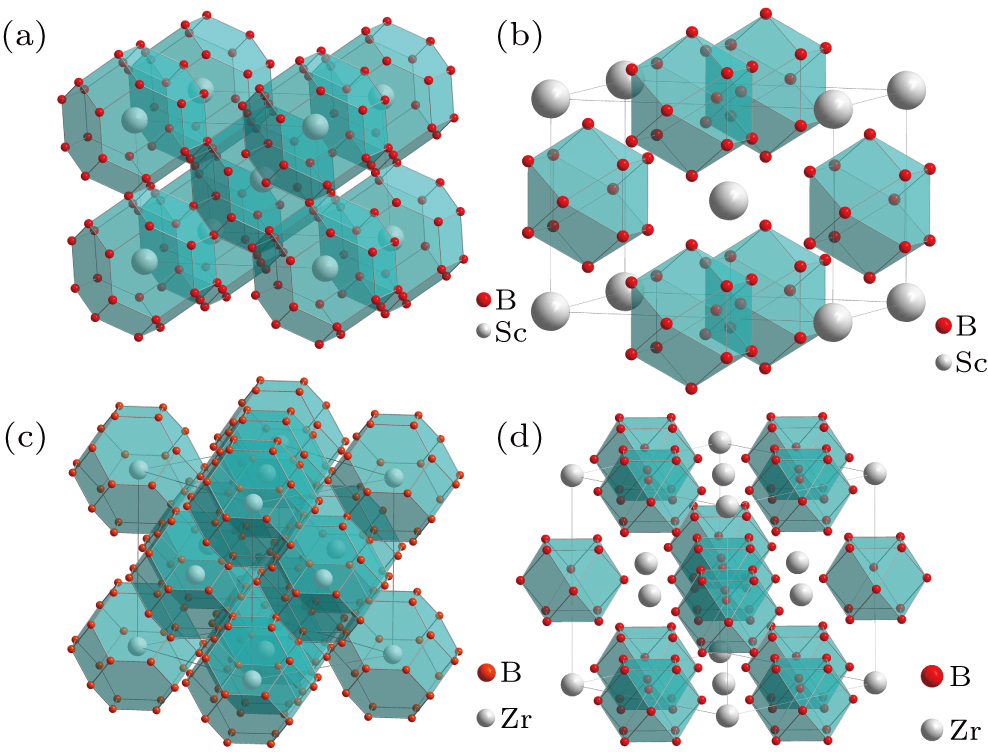
The crystal structure of transition metal dodecaborides (TMB12) can be divided into two structural types: UB12-type crystal structure,[75] which is the majority of TMB12 structure, and ScB12-type crystal structure,[76] to which only ScB12 belongs. The crystal structure of UB12-type and ScB12-type are shown in Fig. 4. In both types of crystal structures, there are the same structural units in which TM atoms are surrounded by B24 cages. In the UB12-type structure, the structural unit of B24 cages and TM atoms form the face-centered cubic structure, while in the ScB12-type structure, the structural unit of B24 cages and TM atoms form the body-centered tetragonal structure. In addition, B12 clusters also exist in both structures. The crystal structure of UB12-type is the same as that of NaCl, with B12 clusters at the Na sites and TM atoms at the Cl sites. Interestingly, the structure of both B24 cages and B12 clusters is cuboctahedral networks.
Fig. 4. Structure of dodecaborides: (a) the crystal structure of ScB12 with polyhedron of B24 cuboctahedra; (b) crystal structure of ScB12 with polyhedron of B12 cluster; (c) crystal structure of ZrB12 with polyhedron of B24 cuboctahedra; (d) crystal structure of ZrB12 with polyhedron of B12 cluster. Boron atoms are in red, metal atoms are in gray.
Download figure:
Standard image3. The multifunctional transition metal borides
Superhard materials are the material with Vickers hardness (HV) above 40 GPa. The most representative superhard materials are diamond and cubic boron nitride (cBN). However, both diamond and cBN have their own disadvantages. The thermal stability of diamond is poor, and diamond is easy to react with Fe-based materials. The hardness of cBN is lower than diamond, and the single crystal is prone to cleavage leading to damage. Therefore, it has always been an urgent need to find alternatives to superhard materials. Boron, carbon, nitrogen, and transition metals can form materials with high valence electron density, strong covalent bonds, and 3D crystal structure, which are the potential superhard materials. In 2007, ReB2 was reported as a superhard material with a hardness of 48 GPa under a load of 0.49 N by Chung et al.[20] This result sets off a new upsurge in the study of TMBs. In addition, TMBs are rich in crystal structure and can form the different amounts of charge transfer between transition metal and boron atoms, which determines the abundant properties of TMBs. This greatly expands the application range of TMBs.
3.1. Mechanical properties
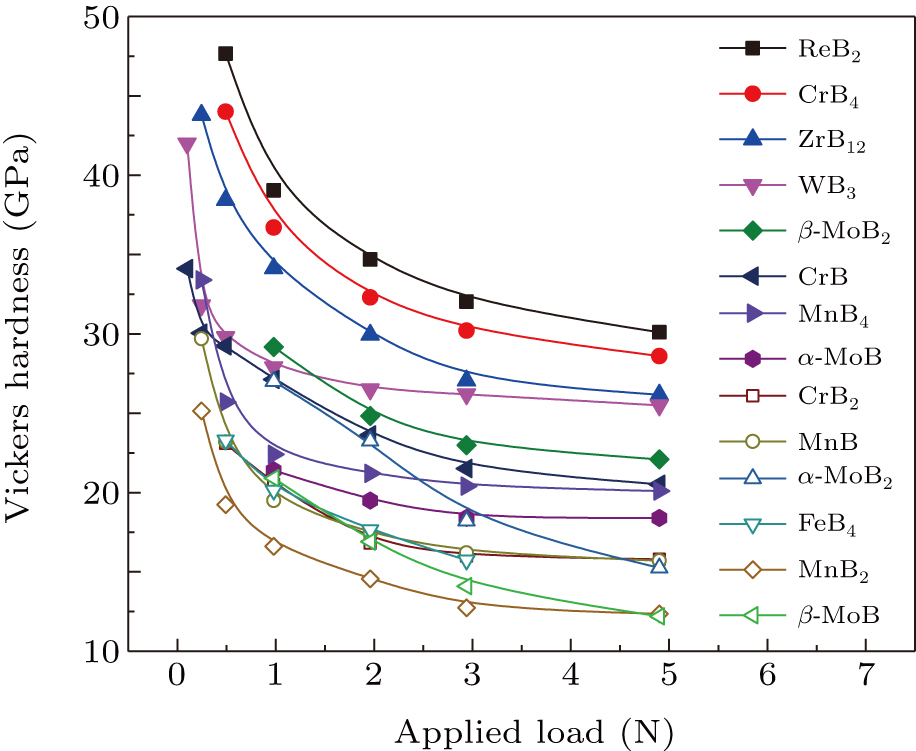
The ReB2 is particularly noticeable. Base on the first-principle calculations, Hao et al. reported the mechanical properties of ReB2.[77] Chung et al. reported that ReB2 was a superhard material experimentally.[20] So ReB2 was widely concerned. This is the beginning of the search for superhard materials in TMBs. The hardness of ReB2 is mainly due to the existence of wrinkle boron layers in the crystal structure of ReB2, which is a quasi-3D structure. However, asymptotic hardness is the true hardness of a material. ReB2 only exhibited superhard property (HV ≥ 40 GPa) under a load of 0.49 N.[20,78] The Vickers hardness of ReB2 is 31 GPa under a load of 4.9 N. The Vickers hardness of ReB2 is shown in Fig. 5. Gu et al. reported that the hardness of ReB2 is ∼ 27 GPa under a load of 4.9 N,[79] but Qin et al. reported that the hardness of ReB2 is only 18.4 GPa under a load of 4.9 N.[80] This result is consistent with the theoretical calculation of Yang et al.[81] The hardness difference of ReB2 is ∼ 13 GPa under a load of 4.9 N. This is mainly influenced by some extrinsic factors, such as boron content. Because it is usually necessary to add excess boron to prepare the sample, this allows excess boron in the product. Levine et al. reported that the excess boron distributed through the compact drastically reduces the hardness of the materials.[82] Because of the excess boron powder compact of ReB2, the hardness is substantially reduced. Actually, the effect of amorphous boron on the hardness of polycrystalline TMBs is still needed to be further investigated because amorphous boron also has a high hardness. The hardness of the single crystal is the intrinsic hardness of the sample. So far, although the hardness of singlecrystal of ReB2 has been reported, there are not the asymptotic hardness values under the high load.
Fig. 5. The hardness of ReB2, CrB4, ZrB12, WB3, β-MoB2, CrB, MnB4, α-MoB, CrB2, MnB, α-MoB2, FeB4, MnB2, and β-MoB (Refs. [7,9,11,20,23,58,59,94,100,101,103]).
Download figure:
Standard imageAlthough ReB2 is not a superhard material, hardness greater than 25 GPa is rare in layered structures. According to previous reports, TMBs with 3D boron atomic structure are more likely to have high hardness. The high hardness of ReB2 is mainly due to the wrinkled boron layer in its structure, which reflects the quasi-3D effect. This is also reflected in other TMB2. MoB2 has two phases with α-MoB2 and β-MoB2. The crystal structure of α-MoB2 is AlB2-type with space group P6/mmm (No. 191). The asymptotic Vickers hardness of α-MoB2 is ∼ 15 GPa. β-MoB2 has a rhombohedral crystal structure with R-3m (No. 166) symmetry, and its asymptotic Vickers hardness is ∼ 22 GPa. The graphite-like boron layers in α-MoB2 can transfer to puckered boron layers in β-MoB2 with enhanced the Vickers hardness of 7 GPa.[11]
In the OsB2 structure, boron atoms form another quasi-3D boron layer, a pseudo-hexagonal corrugated boron layer. The investigations suggest that the Vickers hardness of RuB2-type OsB2 reaches 37 GPa under a load of 0.245 N.[83] The value is close to the standard of superhard materials (HV ≥ 40 GPa). Hebbache et al.\ reported that RuB2-type OsB2 showed an asymptotic hardness of ∼ 29 GPa,[84] but Gu et al. reported that RuB2-type OsB2 showed only an asymptotic hardness of ∼ 17 GPa.[79] Although the hardness of RuB2-type OsB2 is a quite difference, both indicated that RuB2-type OsB2 is not a superhard material under high load. The investigations suggested that the hardness of RuB2-type OsB2 is highly dependent on the crystallographic orientation. The average hardness along the 〈 100 〉 direction is significantly higher than that in the orthogonal 〈 001 〉 direction. This is attributed to the stronger B–B bonds found along the 〈100〉 direction, which dominate the dislocation motion.[85] There have been many studies on the compressibility of RuB2-type OsB2 in experiments and theories,[33,86–91] and the results showed the bulk modulus of RuB2-type OsB2 was in the range of 365 GPa–395 GPa.[85,92] The compressibility of RuB2-type OsB2 is also anisotropy. The c direction of the crystal is the most incompressible. It is interesting that the incompressibility of RuB2-type OsB2 along the c axis is even larger than the analogous linear incompressibility of diamond.[84] This is because RuB2-type OsB2 belongs to the orthogonal crystal system with space group Pmmn (No. 59),[12,93] under the external force, the Os atom and B atoms squeeze each other, so that the electrostatic repulsion between atoms reaches the maximum value, thereby increasing the incompressibility of the c axis. The RuB2-type OsB2 exhibits metallic property, but there is a covalent bond of B–B and Os–B that leads to a high hardness, which is generated by the superposition of the d-electron layer of the Os and the p-electron layer of B.
High boron phases tend to form quasi-3D or 3D structures and thus exhibit high hardness, but some low boron phases also exhibit good hardness. These results provide a deeper understanding of the hardness mechanism of materials. Han et al. report that the higher Vickers hardness of CrB (∼ 20 GPa)[7] compared to CrB2 (∼ 16 GPa)[58] is mainly attributed to the strong 3D Cr–B and zigzag B–B bonding networks in CrB. The results indicate that the monoborides can have better mechanical behavior than the diborides counterparts. The boron content is hence not necessary criteria for the design of hard and superhard materials. The Vickers hardness of CrB and CrB2 is shown in Fig. 5. The symmetrical electronic partition of the 3D Cr–B and zigzag B–B bonding networks is the reason why CrB can have higher bulk moduli than CrB2 and CrB4. The asymptotic Vickers hardness of MnB, Mn3B4, and MnB2 are ∼ 16 GPa,[94] ∼ 16 GPa,[95] and ∼ 12 GPa,[23] respectively. Similar to CrB, MnB and Mn3B4 have a higher hardness than that of MnB2 due to the covalent zigzag or double zigzag B1–B2–B1 backbone. The experimental results indicated the asymptotic Vickers hardness of α-MoB (18.4 GPa) is higher than that of β-MoB (12.2 GPa).[9] Although the crystal structure of α-MoB and β-MoB has the boron zigzag chain (Bzc) skeletons (Bzcs), it is a different arrangement. The Bzcs of α-MoB has a higher shear modulus, and a higher grain boundary density, which resists the glide of dislocation. The results demonstrated modulating the covalent bond array and grain boundary density can also create high hardness in the low-boron content TMBs.
WB4 is the first tetraboride to be synthesized, and its hardness exceeds 40 GPa under 0.49-N load,[96] which has attracted a lot of attention. But the asymptotic Vickers hardness of WB4 was in the range of 28 GPa–32 GPa.[79,96,97] The crystal structure of WB4 does not exist, it should actually be WB3. The calculation indicated that the asymptotic hardness of WB4 (WB3) is lower than that of ReB2 (∼ 27 GPa).[79,98,99] Boron was excessive in reported experiments to prepare WB4 (WB3), and the hardness of amorphous boron is above 30 GPa,[100] so the reported hardness of WB4 (WB3) could be influenced by excess amorphous boron. If the hardness of WB4 (WB3) is to be obtained, the effects of amorphous boron must be excluded. Tao et al. restudied the hardness of WB3 and found that the hardness of WB3 excluding the effect of amorphous boron is just 25.5 GPa,[100] which is really lower than that of ReB2. The asymptotic hardness of WB3 is exhibited in Fig. 5.
Theoretical calculation showed that the Cr, Mn, Fe, and B in CrB4, MnB4, and FeB4 are covalently bonded to form a strong 3D network like as diamond. So there could be superhard materials in these materials. However, the asymptotic Vickers hardness of CrB4 is only ∼ 30 GPa.[58] The Vickers hardness of CrB4 is shown in Fig.5. Although it is the highest hardness of the Cr–B system, CrB4 is not a superhard material. The reasons why CrB4 with 3D crystal structure do not show superhard property are quantum-mechanical effect involving a transition between two-center and three-center bonding among the boron atoms that reduces the rigidity and directionality of the boron bonding and mechanistic effect caused by the pressure beneath the indenter that drives a lateral bond and volume expansion that further stretches and weakens the boron bonds in addition to the shear deformation in the CrB4 structure under Vickers indentation hardness tests.[50] Gou et al. reported that the Vickers hardness of MnB4 is ∼ 37 GPa under a load of 9.8 N,[64] but this value remains controversial. Knappschneider et al. also reported the nanoindentation hardness of MnB4 with ∼ 25 GPa,[63] which is well consistent with the reported by Ma et al., which the polycrystalline hardness of MnB4 is about 20.1 GPa.[101] FeB4 was reported to be superhard materials with a hardness of 62 GPa.[102] However, the other measurements of the hardness of FeB4 indicate that FeB4 is not a superhard material. Its asymptotic hardness is only 15.4 GPa, which is consistent with the calculated hardness (18.4 GPa).[59] The Vickers hardness of FeB4 is shown in Fig.5. Although this is still a contentious issue about its hardness, the point can be sure that FeB4 is not a superhard material.
The higher boron content of transition metal dodecaborides also forms the 3D structures, and the presence of B12 clusters in the structure suggests that TMB12 are likely to exhibit superhard properties. The hardness of both ZrB12 and ScB12 exceeds 40 GPa at 0.49-N load,[103,104] but unfortunately does not show superhard properties at high loads. The hardness of ZrB12 and ScB12 is 27 GPa[103] and 28 GPa[104] at 5 N, respectively. The Vickers hardness of ZrB12 is shown in Fig. 5. The hardness of the single crystal of ZrB12 is lower than that of polycrystalline samples. This may be caused by the effect of grain boundary in the polycrystalline samples.
3.2. Electrical properties
So far, most of the TMBs prepared are conductors, some of which have excellent conductivity comparable to good metal conductors even in the high boron phase. ZrB12 exhibits superior metallic behavior with ultralow electrical resistivity (∼ 18 μ Ω ⋅cm)[103] at room temperature, which is comparable to that of Pt (∼ 20 μ Ω ⋅cm). The reason why zirconium dodecaboride shows metallic behavior is the extensive B–B covalent network in ZrB12 can form delocalized π-bonds, which generate extensive conducting channels for valence electrons by contacting Zr 4d orbitals.
With the discovery of superconductivity of MgB2,[105] the electrical transport properties of TMBs have been paid more attention. So far, there are many TMBs with superconducting properties. The crystal structure of MgB2 is AlB2-type, but only ZrB2 has been reported to have superconductivity with Tc = 5.5 K among the AlB2-type TMB2.[106] However, these results were not observed in later studies. Stoichiometric NbB2 does not show superconductivity, but NbB2 + x with excess boron does show superconductivity with a critical temperature of 8.9 K–11 K.[107,108] Like NbB2 + x , adding excess boron to MoB2 will make it exhibits superconductivity. MoB2 + x also was reported to show superconductivity at Tc ≈ 7.5 K.[108] Tang et al. reported that a boron-rich molybdenum boride, the large single-crystal of nonstoichiometry molybdenum triboride (Mo0.757B3), was prepared under high-pressure high-temperature conditions, it exhibits superconductivity with a TC of 2.4 K.[110] The analysis of theoretical calculations indicated that the occurrence of its superconductivity is derived from the partial occupancy of Mo atoms. MoB4 is not a superconductor, but the Ti-doped and Nb-doped MoB4 were found to be superconducting near 6 K to 8 K.[111] Renosto et al. reported that the Zr0.96V0.04B2 specimen exhibited bulk superconductivity at a critical temperature of 8.7 K and explained that the superconductivity of the Zr0.96V0.04B2 specimen was related to multiple bands at the Fermi surface.[112,113] Gou et al. reported the experiment results that FeB4 shows the bulk superconductivity below 2.9 K.[102] However, the later experimental investigation showed that FeB4 did not exhibit the bulk superconductivity for temperature as low as 2.5 K.[59] FeB4 shows a metallic behavior from room temperature to 30 K. Further investigation is still needed for bulk superconductivity of FeB4. TMB12 with higher boron content has also been found to have superconductivity, such as ScB12 (0.39 K),[114] YB12 (4.7 K),[115,116] and ZrB12 (5.8 K).[103,117–120]
3.3. Catalysis
Energy and environment are the most important issues involved in the sustainable development of human society. Fossil fuels are a non-renewable resource, and their use will bring about environmental pollution, and the development of clean energy has become an urgent practical demand. Electrochemical water splitting is an important method for preparing clean fuel hydrogen with water, but the preparation of hydrogen is difficult and generally requires a catalyst. The most effective electrocatalysts are Pt-based materials for the hydrogen evolution reaction (HER), but the high cost and low earth abundance of noble metal based catalyst limit their wide use. It is very important to develop well-working nonprecious metal based electrocatalysts for HER.
α-MoB2 is a superefficient electrocatalyst for the HER and has excellent catalytic stability during HER.[121–124] It was demonstrated by Chen et al. that α-MoB2 efficiently catalyzes the HER, even at large current densities.[125] The theoretical and experimental results suggested the catalytic activity of α-MoB2 is due to its excellent conductivity, a large density of efficient catalytic active sites, and good mass transport properties. β-MoB2 is also the efficient electrocatalyst for HER, but the catalytic activities of β-MoB2 is lower than that of α-MoB2. In the Mo-B system, α-MoB2 is the most efficient catalyst for the HER. All the tungsten borides have the catalytic activity for the HER. Like α-MoB2, W2B4-type WB2, which has the same crystal structure with α-MoB2, is also the most efficient catalyst for the HER in the W–B system.[126,127]
3.4. Magnetic properties
With the rapid development of micro-electromechanical systems devices, magnetic sensors, high-speed motors, and magnetic springs, new requirements are put forward for ferromagnetic materials. The low hardness of traditional magnetic materials, such as Fe (1.4 GPa),[128] Fe3O4 (4 GPa),[129] FeNi (4 GPa),[130] etc., inevitably limits the application of traditional magnetic materials in harsh conditions. The demand for strong ferromagnetic materials with high hardness is constantly enhanced. Although the hardness of TMBs does not reach the hardness of superhard materials, most TMBs reach the hardness of cemented carbide. MnB exhibits high Curie temperature (546 K), high saturated magnetization (155.5 emu/g), and low coercive field (Hc = 15.9 Oe, 1 Oe = 79.5775 A⋅m−1).[94] The coercive field of MnB indicates that it is a soft magnetic material. The asymptotic Vickers hardness of MnB is ∼ 16 GPa, which is far higher than that of traditional ferromagnetic materials. Fe2B also exhibits ferromagnetism and the Curie temperature (1017 K) is comparable to that of Fe (1043 K). The asymptotic Vickers hardness of Fe2B (12.4 GPa) is far higher than that of traditional ferromagnetic materials.[131] Fe2B is a promising candidate for strong ferromagnetic and high hardness material.
3.5. Refractory materials
Refractory materials are widely used in industrial fields, such as aerospace, geological exploration, machinery manufacturing, etc. The majority of TMBs are refractory. ZrB2 has been widely paid attention to due to its excellent refractory properties. ZrB2 has a hexagonal crystal structure with the space group of P6/mmm (No. 191). Because ZrB2 has strong covalent B–B bonds, its melting point has a very high temperature of 3245 °C.[132–134] ZrB2 also shows outstanding oxidation resistance, surviving thermal cycling up to 2700 °C in air.[135] The thermal conductivity (k) of ZrB2 is its notable property. Kinoshita et al. reported that room-temperature k values for single crystals are 140 W⋅(m⋅K)−1 along the c axis and 100 W⋅(m⋅K)−1 along the a axis.[136] Lonergan et al. reported the high temperature thermal conductivity of ZrB2, which shows thermal conductivity of 127 W⋅(m⋅K)−1 at 298 K and 80 W⋅(m⋅K)−1 at 2273 K.[137] Besides, the high temperature flexure strengths of ZrB2 was measured by Neuman et al. The investigations indicated that strength between room temperature and 1200 °C was ∼ 390 MPa, decreasing to a minimum of ∼ 170 MPa between 1400 °C and 1500 °C and the strength increased to ∼ 220 MPa between 1600 °C and 2300 °C.[138] The increase in strength was mainly attributed to stress relief through plastic flow. As a kind of high temperature resistant material, ZrB2 has been used in the aerospace field.
4. Preparation of transition metal borides
The study of the physical properties of materials is inseparable from the preparation of materials. After years of development, there are many methods for the preparation of TMBs. Because transition metals and boron have very high melting points, and TMBs, especially the high boron phase, have strong covalent bonds, most preparation methods require high temperatures. At present, the synthesis methods of transition metal borides mainly include arc melting, solid-state sintering, high pressure and high temperature (HPHT), and so on.
Arc melting is one of the earliest and most widely used methods for synthesizing transition metal borides. Arc melting is conducted in a water-cooled copper hearth, with high purity argon as the protective gas, in which an electrode made of a material with a high melting point generates a high-temperature arc to melt and react the sample. Most TMBs with different boron contents can be synthesized by arc melting, such as MnB4, ReB2, WB4, and YB12, etc.[20,96,139,140]
Solid-state sintering is a widely used synthesis method, and can also be used to prepare TMBs. This method is relatively simple. Boron and transition metal powders are mixed in a certain proportion and sintered in a vacuum or an inert atmosphere to obtain the target products. But the drawbacks are also obvious. Due to the limitation of heating materials in equipment, this method is insufficient in preparing high boron phase, and the synthesis time is relatively long. Frotscher et al. reported that MoB2 was synthesized at 1373 K in an inert atmosphere within 14 d.[45] In the experiment, ball milling the precursor or adding fluxing agents is used to reduce the reaction temperature and increase the reaction rate.
Compared with other synthetic methods, HPHT has advantages in the synthesis of TMBs. In addition to the temperature, the HPHT method adds another adjustable parameter pressure. High pressure can shorten the distance between atoms, increase electron cloud overlap, and facilitate the formation of strong chemical bonds. So HPHT is the main way to synthesize diamond and cubic boron nitride. In addition, HPHT is also beneficial to the preparation of bulk materials with high density. This is very convenient to research the properties of bulk TMBs, like mechanical properties and electrical properties. Although many preparation methods can provide high temperatures, the presence of high pressures greatly facilitates the synthesis of TMBs. It takes 14 d–28 d to prepare the single crystal of MnB4 by solid-state sintering,[63] but only one hour to get a single crystal of MnB4 by HPHT.[64] In addition, HPHT can also obtain specimens that cannot be obtained by other methods. Studies have shown that it is difficult to prepare FeB4 under 3 GPa,[102] and the current reported preparation pressure of FeB4 is 10 GPa–15 GPa.[59] It can be seen that HPHT has more advantages in exploring new materials and new structures.
In addition, high-temperature electrochemistry, electron beam melting, spark plasma sintering, self-propagating sintering, magnetron sputtering, pulsed laser deposition, high-frequency induction, and floating zone can also be used to prepare TMBs. Magnetron sputtering and pulsed laser deposition are mainly used to prepare thin film samples. High-frequency induction and floating zone are often used to prepare single crystal samples.[141,142]
The synthetic routes of TMBs are also abundant. After boron purification technology is mature, the preparation of TMBs is mainly obtained by the reaction of boron and transition metal.[95,96] The chemical equation is as follows:
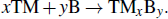
In addition, there are other synthetic routes, such as:
- 1)Borothermal reduction of transition metal oxides[143]

- 2)Solid state metathesis of borides[144]

- 3)Carbothermal reduction[145]

- 4)Boron carbide reduction of transition metal oxides[146]

- 5)Hydrothermal reaction in an autoclave[147]

Some are actually ways to synthesize nanomaterials. Because most synthetic routes are not preparation methods of bulk materials, they will not be listed here. Although there are many ways to synthesize TMBs at present, there are still many problems in the preparation of TMBs. Some TMBs do not readily form a single phase. For example, the synthesis of CrB4 is usually accompanied by the phase of CrB2.[58] The synthesis of FeB4 is also accompanied by the phase of FeB.[59] The synthesis of some transition metal borides requires an excess of boron, which affects the study of some properties.
5. Summaries
After more than 100 years of development, transition metal borides (TMBs) have obtained abundant crystal structure and physical properties experimentally. However, the research on TMBs is far from complete, and there are still many problems to be further studied. So far, the hardness of the synthesized TMBs is not ideal, and there is still a certain gap with the superhard materials. This is mainly because the TMBs have some metal bonds, so the number of covalent bonds is reduced, and the covalent bonds in the TMBs are not strong in direction, and the bonding strength is not enough. Therefore, the research on the hardness of TMBs needs to be improved in the material design concept. The key to the design and preparation of high hardness TMBs is to increase the electron transfer between the transition metal and boron atoms and to construct a network of quasi-3D or 3D strong covalent bonds. At present, the research on TMBs mainly focuses on crystal structure, mechanical properties and oxidation resistance and so forth, and many potential multifunction properties have not been explored in depth, such as strong ferromagnetic materials and electrocatalytic materials. Among all kinds of TMBs preparation methods, the HPHT method has unique advantages for the preparation of TMBs, which can effectively induce the electron transfer between the transition metal and boron atoms, so as to construct a more abundant crystal structure, and is an effective method for the preparation of hard multi-functional new TMBs materials. TMBs are still a candidate material for studying new materials, new structures, and new properties due to the existence of multiple electron transfer modes between boron and transition metal. In the future research of TMBs, one is to design and prepare TMBs with high hardness, and the other is to strengthen the research and development of other multifunction properties. In addition, there are a lot of layer structures in TMBs, which are the basis for studying two-dimensional materials. Therefore, in recent years, two-dimensional materials for the study of TMBs begin to rise. For example, the two-dimensional MoB has been prepared,[148] which greatly expands the research scope of TMBs.
Footnotes
- *
Project supported by the National Key Research and Development Program of China (Grant Nos. 2016YFA0401503 and 2018YFA0305700), the National Natural Science Foundation of China (Grant No. 11575288), the Strategic Priority Research Program and Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (Grant Nos. XDB33000000, XDB25000000, and QYZDBSSW-SLH013), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (Grant No. Y202003).