Abstract
The architecture of an organ is built through interactions between its native cells and its connective tissue consisting of stromal cells and the extracellular matrix (ECM). Upon transformation through tumorigenesis, such interactions are disrupted and replaced by a new set of intercommunications between malignantly transformed parenchyma, an altered stromal cell population, and a remodeled ECM. In this perspective, we propose that the intratumoral heterogeneity of cancer cell phenotypes is an emergent property of such reciprocal intercommunications, both biochemical and mechanical-physical, which engender and amplify the diversity of cell behavioral traits. An attempt to assimilate such findings within a framework of phenotypic plasticity furthers our understanding of cancer progression.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
An eloquent description in the medical literature on intratumoral heterogeneity (ITH) comes from an influential essay by the renowned oncologist Paul Calabresi, published in the Transactions of the American Clinical and Climatological Association in 1981. He stated, 'For many years experimental and clinical oncologists have considered cancer a monotonous and homogeneous disease characterized, like the crab, by propagating daughter cells identical in most respects to their progenitors.... By contrast, the clinical observations of some oncologists and pathologists have provided a totally different picture of neoplasia. Human tumors have been described as consisting of heterogeneous subpopulations of neoplastic cells that are morphologically and probably functionally distinct.... Considerable evidence suggests that, in fact, most solid tumors are heterogeneous and, therefore, the chimera might well be a more appropriate symbol for cancer than the crab' [1]. Such an articulation was reflective of advances in experimental cancer research at that time, such as flow cytometry, which began to discern quantitative variations within cellular traits. Since then, molecular techniques such as deep sequencing of genomes have built on the ubiquity of ITH by adding rich details of underlying genetic variegation within cancer cell populations.
Cancers are now known to represent rich quilt works of coexistent cellular niches with unique signatures of genomic aberrations, ranging from specific point mutations to global chromosome-level alterations [2, 3]. Furthermore, mechanisms of ITH have been also sought to be located within intercellular differences in epigenetic regulation of gene expression [4]. An epigenetics-based theory of ITH copiously borrows from developmental biological ideas of gene regulatory networks with unique and diverse biochemical states arising due to stochasticity in intercellular signaling and feedback-based bifurcations in phase spaces of network topologies [5]. Such state differences have important consequences on phenotypic traits: cells with unique molecular profiles show differential propensities for proliferation, metastasis, and survival against chemotherapy [6, 7].
However, tumors are not mere ensembles of transformed cells; the latter comprise a biological environment along with untransformed parenchymal and stromal cells, and an extracellular matrix (ECM) that abuts, and connects, these cellular populations. In doing so, the ECM and cells constantly exchange chemical and physical-mechanical cues with each other [8]. In homeostatic organs, the ECM acts not just as a structural scaffold, but actively maintains cells in their state of quiescence and differentiation, allowing them to perform their physiological function [9]. Upon malignant transformation, the loss in quiescence and the reversal in their differentiated states is associated with the restructuring of the biological, architectural, and physical-mechanical properties of the ECM. In fact, aberrations in ECM are now known to contribute to all the listed molecular-cellular hallmarks of cancer (reviewed extensively by Pickup and co-workers [10]).
Notwithstanding the progress in understanding the contributions of ECM to tumorigenesis, its specific role in the modulation of phenotypic ITH remains poorly assimilated with existent literature. Does the ECM enhance or dampen variations in genotypes and phenotypes of cancer cell populations? Can ECM give rise to clonal heterogeneity beyond cellular spatial scales?
In this essay, we review published literature that has framed and investigated these problems and showcase where the community is poised in its quest for solutions. The first two sections discuss how cancer cells and ECM modulate each other's properties: the cells through remodeling of the ECM, and the latter, as modulators for cellular gene expression and migratory behavior. In the third section, we invoke the concept of phenotypic plasticity to discuss how these reciprocal influences establish phenotypic heterogeneity within tumors. Our conclusion identifies challenges in the field and approaches that could help answer them. Developing an appreciation of the contribution of the ECM to ITH is an important step towards devising novel strategies aimed at controlling the same towards a better management of cancer.
2. The effect of cancer cells on their extracellular matrix
Tumorigenesis begins with the dissolution of a homeostatic architecture that typifies the tissue or organ [9]. Existent cell–ECM interactions (figure 1(A)) give way to a novel set as progressing cancer cells build a new architecture (known as the tumor microenvironment) by altering the ECM themselves, and through non-cancerous stromal cells recruited to such locales (figure 1(B)). The effect of cancer cells on their surrounding ECM is multimodal in nature: dynamics of synthesis, degradation, and modification of the ECM play out simultaneously to create the tumor microenvironment that is hospitable to multiple cell niches and aids progression. In the following subsections, we briefly review each of these dynamical processes.
Figure 1. Interactions within tissue and tumor microenvironment. (A) A stereotypical depiction of an untransformed epithelial tissue microenvironment. (B) Depiction of a tumor microenvironment with an emphasis on the various ways by which cancer cells and recruited activated stromal cells remodel the surrounding extracellular matrix (ECM). (C) Depiction of a tumor microenvironment with an emphasis on the ways by which the ECM modulates the phenotype of cancer cells. (D) A confining tumor microenvironment acts on a population of tumor cells to amplify differences in physical properties such as size and stiffness, leading to cell sorting and mesoscale intratumoral heterogeneities.
Download figure:
Standard image High-resolution image2.1. Synthesis
All cells within an organ cooperate to constitute its organotypic ECM. Therefore, ECMs of different organs differ in their chemical composition and physical properties. Stromal cell-synthesized ECM is predominantly fibrillar with a microporous architecture and predominantly constituted of collagens [11]. On the other hand, glandular epithelial cells secrete ECM that is rich in laminins, non-fibrillar collagen IV and sulfated proteoglycans that constitute a sheet-like nanoporous superstructure, known as the basement membrane (BM) (figure 1(A)) [12]. Early biochemical studies conducted by Vaheri and co-workers have shown that tumor cells secrete characteristic ECM mixtures: irrespective of their tissue of origin, mesenchymal-like cancer lines tend to secrete collagen-rich ECM, similar to untransformed stromal cells, whereas epithelioid cancer cells secrete laminin-rich ECM [13]. In fact, early pathological studies indicate that cancers can broadly be identified based on the ECM associated with them. For example, neural tumors and sarcomas in pediatric patients can be distinguished from each other by their secretion of laminin-collagen IV-fibronectin pattern and collagen (except collagen V) pattern, respectively [14].
With the advent of sophisticated omic technologies, ECM and associated gene products have been actively included within transcript signatures reported to be cognate to specific tumors (figure 1(B)). For example, Yuzhalin and co-workers propose that a set of ECM proteins including collagens like COL1A1, COL10A1, and COL11A1, and proteoglycans such as aggrecan and biglycan are elevated across alimentary cancers as well as breast and lung cancers [15]. Signatures based on transcription of ECM genes have also been proposed to prognose the fate of non-small cell lung cancers [16]. Notably, tumor ECM is synthesized not just by tumor epithelia but also by associated stromal cells such as fibroblasts and macrophages [17]. Such untransformed stromal cells are recruited to the tumor through morphogenetic cues such as epidermal growth factor and transforming growth factor-β. Furthermore, analyses carried out on triple-negative breast cancer xenografts and isogenic invasion-diverse cancer lines show distinct ECM mixtures secreted by stromal and cancer cells within tumors formed by them [18].
The deposition of a dense fibrillar ECM around a growing tumor, known as a desmoplastic reaction, is one of the notable characteristics of cancers such as pancreatic ductal adenocarcinoma and invasive ductal carcinoma of breast, and has been used as a histopathological cue to prognose the disease long before cancer-related changes in ECM composition, chemistry and rheology were understood [19]. Desmoplastic changes are the result of collagen deposition and signify a more aggressive progression [20]. Collagen patterning and fibrillogenesis are also sensitive to other ECM constituents: sulphated proteoglycans such as decorin and biglycan are known to bind to collagen using their protein cores, whereas their hydrophilic glycosaminoglycan (GAG) chains alter the kinetics and geometry of fibrillogenesis [21, 22]. Chondroitin and dermatan sulfate polymers may also show self-association, which provides insights into how different levels of such GAGs could alter their miscibility with polymerizing collagen fibrils, leading to heterogeneously patterned and constituted ECM across spatial scales [23].
2.2. Degradation
Cancer cells are intimately involved in the enzymatic degradation of protein and glycan constituents of the ECM [11]. ECM degradation creates spaces for cells to migrate; in addition, the degraded components of ECM also may potentiate invasion (figure 1(B)). Although matrix metalloproteinases (MMPs), represent the most well studied family of secreted ECM proteases, the ability to degrade matrix extends to other family of proteases as well. For example, cysteine proteases such as cathepsins are involved in the degradation of BM proteins like nidogen, collagen IV and tenascin as well as stromal proteins such as fibronectin [24]. Serine and aspartate proteases not only degrade ECM, some of its members are also involved in activating MMPs [25]. Proteases are secreted both by cancer cells as well as cancer-associated stromal fibroblasts. Their effect on cancer progression is highly context-specific: whereas proteases have been shown to spur progression, there may be distinct ways through which proteases may also impede invasion and metastasis [26]. In addition to proteins, the degradation of glycan components of ECM is mediated by glycosidases. The heparan sulfate cleaving endoglycosidase heparanase is frequently overexpressed in tumors wherein its heightened canonical activity results in the release of chemotactic factors sequestered within the ECM resulting in greater cancer invasion [27].
An increasing number of papers implicate protease-independent ECM modifying mechanisms for invasion of cancer cells. Such processes involve the deformation of BM mediated through the proliferative or migrative force of cancer cells: cancer invadopodia, transient and cyclically extended cellular protrusions act both as locally concentrated hotspots for protease activity as well as ECM-deforming pore-forming agents [28]. Not just cancer cells, even cancer-associated fibroblasts deform BM in a metalloprotease-agnostic manner by exerting contractile forces on the BM molecules and altering their physical properties [29]. These observations suggest how the tandem interplay of chemical and mechanical contributions to ECM remodeling is a norm rather than an exception in the context of tumor microenvironmental dynamics.
2.3. Modification
ECM is subject to extensive biochemical alterations by the cells of the tumor microenvironment (figure 1(B)). These could include dysregulation of otherwise physiological posttranslational modifications that are essential to their secretion and assembly. One of the best-known examples is the hydroxylation of procollagen mediated by lysyl hydroxylases and the crosslinking of collagen fibrils through lysyl oxidases: such processes are crucial to the assembly of stromal collagen ECM during tissue homeostasis [11]. However, an overexpression of such enzymes in cancers leads to stiffening of tumor ECM exacerbating the proliferation and migration of cancer cells (the role of lysyl oxidases in cancer has been extensively reviewed in [30]).
In fact, cancer cells regulate the sulfation of GAG chains of proteoglycans both through upregulation of sulfating enzymes and through downregulation of desulfating counterparts, resulting in altered ECM and a tumor microenvironment promoting invasion and metastasis [31, 32]. Another important matrix constituent, hyaluronic acid, a GAG is subject to changes in size because of the interplay between its biosynthetic enzyme hyaluronic acid synthase and its degrading enzyme hyaluronidase [33]. As a result, high molecular weight hyaluronic acid (HMWHA) can be broken down into low molecular weight hyaluronic acid (LMWHA): whereas HMWHA is inhibitive to cancer cell migration, LMWHA promotes it. The sculpting of the ECM by transformed and associated cells of the tumor microenvironment results in topological alterations that have been showcased using methods such as second harmonic generation microscopy (SHG). In fact, SHG imaging of ECM alterations have been used to exhibit the histopathological distinctions in untransformed tissues, benign- and malignant-tumors of the ovaries as well as to generate collagen signatures that can prognose progression of specific cancers (please see a detailed treatment of the various methodologies used to study the biological, chemical and physical characteristics of the ECM in the context of the tumor microenvironment in box 1) [34].
| Box 1 | |
| An investigation of the contributions of the ECM to tumor heterogeneity can be achieved through techniques that | |
| interrogate the biochemical, architectural, and mechanical behaviors of ECM. Here, we briefly summarize | |
| a set of techniques based on each of the above three properties: | |
| Biochemical: | |
| Picrosirius red staining: | Staining histological sections with picrosirius red reveals the presence of fibrillar collagen networks in tissues. Sections treated with the dye, when exposed to polarised light display collagen bundles in different colours—green, red, or yellow—based on the orientation of collagen bundles [35]. |
| Raman spectroscopy: | Based on Raman scattering, wherein photons incident on an ECM sample scatter inelastically depending on the interaction between the incident photons and molecules of different vibrational modes, shifts in the energy of incident photons can be used to infer the composition of the ECM within tissues in terms of relative concentration of different classes of biomolecules. Advantages of this method involves non-destructive sample analysis as well as lack of intense sample preparation steps [36]. |
| Mass spectrometry imaging (MSI): | Mass spectrometry is an analytical technique that can be used to identify specific molecules based on their mass-charge ratio. MSI has the added advantage of being able to identify spatial distributions of different molecules in a biological specimen as opposed to simply detecting their presence from a homogenous sample mixture [37]. |
| Laser capture microdissection coupled mass spectrometry (LCM-MS) | The analytical advantages of mass spectrometry are enhanced and better applied to flash-frozen and formalin-fixed spatial sections by combining it with laser capture microdissection. Herein, identification of regions of interest (such as multicellular patches) can be achieved through hematoxylin and eosin (H&E) staining and in case of unfixed specimen, through fluorescence, removed from the surrounding cells and subjected to MS-based biochemical characterization [38]. |
| Nuclear magnetic resonance spectroscopy: | NMR spectroscopy detects magnetic spin properties of atomic nuclei, which can be used to further derive spatial conformation of atoms in a molecule. Solid-state NMR spectroscopy can be used to identify variations in water content within tissues and hydrogel, in turn shedding light on spatial heterogeneities in expression of GAGs such as hyaluronan [39]. |
| Architectural: | |
| Second harmonic generation imaging (SHGI): | The technique is based on an optical effect in which two photons with the same frequency interact with a non-linear material resulting in the generation of a new photon with combined frequencies of the initial two photons. Biological specimen assessed using SHGI require a specific molecular orientation (non-centrosymmetric structures such as collagen, cytoskeletal elements) capable of producing SHG signals so that it can capture the variations in the specimen. A major advantage of this techniques is that it does not require exogenous probes to identify specific molecular targets in the sample, obviating the use of fluorophores that could contribute to photobleaching or phototoxic effects [34]. |
| Reflectance confocal microscopy (RCM): | RCM uses near-infrared low power lasers emitting monochromatic coherent light to scan tissues and biomaterials. Reflected light from the focused area of sample passes through a gating pinhole to the detector to yield grayscale images based on relative refractive indices of different components of the sample. Sample sections made of components with higher refractive index appears brighter and vice versa [40]. |
| Electron microscopy (EM): | Electron microscopes use a beam of accelerated electrons as an illumination source. A beam of electrons is raster scanned across a sample and the secondary or back-scattered signals from the sample are detected yielding high-resolution images of the sample. Since the wavelength of electrons are folds smaller than that of visible light, it can be used to obtain images of smaller structures with better resolution. Scanning electron microscopy has been used successfully to gather information on geometrical and biochemical properties of ECM surface topographies [34, 41]. |
| Mechanical: | |
| Shear wave elastography (SWE): | This technique uses a focused ultrasound beam to create a 'push' from the acoustic radiation force/acoustic radiofrequency force impulse deep in the analyzed tissue. This generates transversely oriented shear waves that move through the surrounding tissue at velocities that are a function of the tissue stiffness and can be used to estimate spatial differences in tumor ECM content of collagen and elastin [42]. |
| Atomic force microscopy (AFM): | Atomic force microscopy can be used to study subtle spatial variations in mechanical properties of matrix surfaces such as their elastic modulus and adhesion force. Interactions between the AFM cantilever tip and sample yields FD (force-distance) curves, which can be used to calculate Young's modulus and adhesion force at multiple given points on scaffold samples [43]. |
| Resonant acoustic rheometry (RAR): | A recently developed technique for the quantitative characterization of mechanical properties of soft materials, RAR employs focused ultrasound pulses to perturb hydrogel surfaces and detects resonant modes of surface waves, which inform spatial differences in viscoelasticity driven by differential compositions of ECMs [44]. |
| Optical or magnetic tweezers : | Optical tweezers utilize beams of light that can be used to hold and manipulate molecules or cells. Magnetic tweezers utilize magnetic fields that are employed to exert forces on probes that are tagged to proteins or protein networks [45]. |
2.4. Physical-mechanical consequences of ECM remodeling by cells
Unlike elastic structures which deform upon stress impingement and relax to their original state upon stress release, biological structures dissipate a proportion of energy that has been exerted to deform them. Therefore, the mechanical behavior of tissues and tumors can be characterized as being viscoelastic, meaning they show properties of both viscous and elastic materials, typically by deforming initially like an elastic material followed by a viscous material-like time-dependent mechanical response and energy dissipation [46]. Viscoelastic materials can be characterized quantitatively using both a storage modulus (which can be measured as the ratio of the elastic stress to strain, indicating in effect, the capacity of a material to store energy), and a loss modulus (which measures the energy lost per cycle of sinusoidal deformation) [47].
To a considerable extent, geometries of arrangement and interaction between elements constituting materials influence their mechanical properties. This is evident especially for polymeric materials, such as the ECM making them one of the predominant determinants of tissue- or tumor-mechanical behaviors [46]. For instance, the presence of loose polymeric ends within their network, the concurrent presence of weaker polymeric networks overlain on existent covalently linked networks, or the conversion of long chain polymers into short chains with lesser entanglement and connections predispose to a viscoelastic behavior, wherein the deformation is transient and is known as non-viscoplastic type of viscoelasticity [48]. In contrast, the presence within the network of interspersed weak crosslinks or even entirely constituting the network, allows for transient breaking and rejoining of polymers to form new network architectures (leading to residual deformations) and is called viscoplastic viscoelasticity [49, 50].
Despite some recent progress in deciphering the effect of mechanical changes in ECM on cell phenotype, driven mainly by our ability to tune the storage and loss moduli independently of each other, (see next section), how cells alter such mechanical properties endogenously is relatively unclear. That tumor ECM is stiffer in nature (and shows elevated storage modulus) can be explained by a greater degree of covalent crosslinking mediated by remodeling enzymes that are secreted by cancer cells as well as their associated stromal cells as described in the previous section [51]. On the other hand, the concurrent presence, within collagen fibrillar architectures of weaker polymeric networks brought about by aggregative sulphated proteoglycans may also alter the loss moduli of such tumor matrix microenvironments [23]. Synthesis of a collagen-rich ECM by cancer cells and their associated fibroblasts to replace the non-fibrillar BM ECM potentially alters the nonlinearity of tumor microenvironmental elastic response [47]. The viscoplastic nature of fibrillar collagen I matrices is used to advantage by migrating cancer cells that reorient the fibers parallel to their egress leading to the tumor associated collagen signatures that have been described for cancer migration [52].
3. The effect of extracellular matrix on cancer cell phenotype
One of the earliest demonstrations of the importance of the ECM in determining gene expression in cells came from pioneering studies on untransformed mammary epithelia. When cultured on BM, the cells secreted the milk protein β-casein. However, when embedded in collagen I-scaffolds, milk protein was not expressed [53]. Subsequent studies showed that the BM (but not collagen) organized the mammary cells into a quiescent hollow multicellular cluster that not just resembled acini seen in mammary glands but mimicked them physiologically through milk production [53]. More recent studies show that laminin-5, an important constituent of the BM, signals through nitric oxide (NO) and HoxD10 to contribute to the homeostatic mammary structure [54]. NO signaling is silenced in cancer cells leading to the upregulation of MMP-9, which degrades the BM, and facilitates proliferation- and migration-positive signal transduction in an ERK- and PI 3-kinase-dependent manner [55].
3.1. Biochemical, architectural, and mechanical effects of the ECM on cancer cell behavior
Like their non-malignant counterparts, cancer cells show distinct behaviors in different ECM milieu (figure 1(C)). Such milieu may differ broadly in three ways: through different compositional chemistries of ECMs, through variations in their architectures (fiber arrangements), and through altered mechanical-rheological behaviors. Although differences in each of these categories are concurrent with, and consequential to, changes in other two, most investigations have focused on demonstrating the consequences of variations of one category on cancer cell phenotype.
A recent study in high grade serous ovarian cancer rigorously demonstrates that substrata of different ECM molecules or their combinations (including, but not limited to collagen I, IV and VI, laminin, and fibronectin) modulate the response of the cancer cells to chemotherapy as well as apoptotic cues in a FAK- and β1 integrin-pMLC-YAP signaling-dependent manner [56]. Recent studies show that behaviors such as migration and chemoresistance are sensitive to the nanospacing of the ECM ligands that engage their cognate receptors on the cell surface, suggesting that such plasticity is linked to dynamical changes in signaling landscapes [57]. Ligand density may have profound effects on decisions made by cancer cells to switch between dormant and activated states. Elegant experiments using hydrogels with tunable ligand concentrations and mechanical behaviors of matrices indicate that increased ligand densities and a degradable ECM encourage activation of dormant cancer cells, whereas increased ECM degradability by itself results in restricted survival and cellular dormancy [58, 59]. Similar results have also been observed for switches between growth and dormancy for ER+ breast cancer cells [60]. Such approaches explore the contributions of ligand density to cancer cell phenotype by tuning the former and observing variations in the latter; however, there are fewer studies that explore endogenous densities of bound ligands within tumor-like microenvironments. Efforts in this direction have focused on measuring adhesion with cell substrata to estimate concentrations of ECM ligands that engage cells causing them to latch on to the ECM surface [61]. Even so, measuring adhesion within 3D ECM microenvironments is challenging. Mooney's group has used Forster resonance energy transfer signals between fluorescein-painted cells embedded in Rhodamine-conjugated RGD-alginate gels to calculate bound ligand densities [62]. A second approach has used fluorescently tagged integrin domain constructs that can measure specific integrin ligand densities within collagen-gelatin hybrid scaffolds using multiphoton microscopy [61].
Recent investigations demonstrate how altering the ECM architecture may modulate cancer cell traits. Studies using reconstituted in vitro collagen I scaffolds show that as fiber diameter is increased, the invasiveness of breast cancer cells, MDA-MB-231 which employ mesenchymal migration and MCF-7, which employ amoeboidal migration, increases [63]. On the other hand, collagen fiber alignment has been shown to regulate not just migration, but also MMP secretion and invadopodia kinetics [64]. The presence of dermatan sulfates in collagen I scaffolds alters their alignment properties (as detected using second harmonic imaging) leading to changes in network-like behaviors of the aggressive SKOV3 ovarian cancer cells [21]. Macroscale architectural differences in tissues may deeply influence cancer progression: Paul and co-workers show how the disordered blood vessel topography of the caudal vascular plexus in zebrafish predispose colonization of intravasated breast and mammary cancer cells relative to the linear topographic arrangement of a less preferred organotypic site: the brain [65]. Investigating organ-specific differences in ECM compositions that may regulate vascular topographies is likely to provide rich insights into architectural modulation of cancer organotropism.
In addition to architecture, the mechanical properties of ECM have shown to intimately regulate cell behavior in both physiological and pathological contexts [46]. The state of ECM crosslinking as mediated by cells of the tumor microenvironment has contextual effects on transductive cues guiding cancer progression. When stromal ECM constituents, such as fibrillar collagens are crosslinked, they stiffen and induce integrin clustering and migratory signaling cognate to focal adhesion formation and PI3K activity [66]. Activation of FAK/Src pathway by a stiffer stromal ECM in an integrin signaling dependent manner rewires the signaling to drive cancer cell proliferation in primary colorectal and breast tumors [51]. The effect of changes in storage modulus on cell spreading and proliferation have shown that softer substrata are inhibitive to such processes. In addition to compositional complexity, stiffness of ECM too tunes phenotypes such as proliferation, migration, progenitory potentials and sensitivity to drugs in ovarian and breast cancers [21, 67]. In the context of ovarian cancer, stiffness of the ECM microenvironment has been observed to increase cell migration in a FAK-dependent manner and disaggregation of spheroids constituted from the aggressive SKOV3 cell lines [68]. However, some of these studies have typically been conducted with covalently crosslinked elastic substrata such as polydimethylsiloxane and polyacrylamides, whereas ECM is mechanically viscoelastic and viscoplastic.
Chaudhuri and co-workers have sought to examine the changes in behaviors of transformed and untransformed cells grown on covalently (elastic) and ionically (viscoplastic) cross-linked alginate gels: they observed that the cell spreading on soft viscoelastic gels exceeds that on elastic gels with similar storage moduli [69]. In one study comparing the response of normal and cancer cells, primary hepatocytes and hepatocellular carcinoma cells show opposite behaviors when cultured on soft viscoelastic substrata: whereas hepatocytes spread to a lower extent (relative to their spreading on elastic substrata), cancer cells spread faster [70]. This has been attributed to differential rates of binding kinetics between cell surface ligands and substrata, but should ideally be tested using isogenic lines of benign and malignant cells. Analytical approaches combined with experiments have further shown that an intermediate viscosity in softer gels engenders spreading of cells, whereas at higher stiffnesses, cell behavior is agnostic to variations in loss moduli [71].
3.2. Effect of ECM on migrating tumor populations
As tumor cells migrate through ECM, the latter acts as a mechanical barrier to discern differences in physical properties of cells such as their size, shape, and rigidity (figure 1(D)). For example, a more crosslinked BM tends to restrict invadopodia formation and impedes migration [72]. Dissemination of cancer cells depends also on the plasticity of cellular populations to switch between various modes of dissemination. Migration constitutes a spectrum, whose extremes consist of collective cell migration and dispersed single-cell migration [73]. Collective cell invasion involves ensembles of cells moving as 'leaders' and 'followers' and has been proposed to require greater cell–cell adhesion and matrix degradation as requisites for migration. However, even mesenchymal cells, with decreased expression of the epithelial markers such as E-cadherin are known to migrate collectively [74]. This is consistent with recent studies that show that despite low cadherin expression, tumor cells of the invasive lobular carcinoma of breast invade in a collective manner: Ilina and co-workers have proposed a crucial role for the ECM as a confining environment that in the context of cell density, regulates jamming-unjamming transition irrespective of E-cadherin expression [75]. On the other hand, when cell adhesion is minimal and pore sizes between stromal ECM fibers are narrow, a dispersed 'mesenchymal' mode of migration is evidenced. Such a process is typically associated with the ability of cells to actively adhere and de-adhere from ECM. Another variant, amoeboid dispersed migration typically eschews high levels of cell–ECM and cell–cell adhesion [73]. The effects of the ECM as a physical confining agent have profound effects on cellular behavior within spheroidal ensembles. Delarue and co-workers have dissected multiscalar mechanisms of regulation of cell proliferation in confined colorectal-, breast-, and sarcoma cancer spheroids. The compressive stress generated by the confinement decreases cellular volume at the spheroidal core, followed by the expression of the proliferation inhibitor p27Kip1, and finally a cell cycle arrest [76].
The different behaviors of migrating cancer cells can be assimilated into a single framework in the form of a phase diagram, wherein spheroidal cores resemble solid phase, streams of connected migrating cells resemble liquid phase, whereas dispersed mesenchymal cells represent gaseous phases. These could be primarily understood to be a function of the cells to attach to one another but also the dynamics of confinement provided by the ECM surrounding them. Kang and co-workers propose that unjamming transition explains the gas-like dispersion seen for cancer cells in sparsely distributed stromal-like environments (low density collagen) but liquid-like dispersion in dense counterparts (high density collagen) follows a jamming transition [77].
Chaudhuri and others propose a framework wherein the biochemical, architectural, and mechanical properties of the ECM can be integrated together to assess how it influences cancer cell migration. The confining ability of the peritumor ECM is not just a function of the fiber lengths and pore sizes (a function of the polymerization process of ECM macromolecules) and the balance between diffusive degradative (proteolytic and glycosidic) and anti-degradative biochemical cues, but also the mechanical properties of the ECM [47]. If the ECM fibers are sufficiently plastic, their smaller pores can be remodelled even in a protease-independent fashion using invadopodia resulting in tumor cell migration through wider pores [72]. The greater propensity for LMWHA to allow cancer cell migration could be driven by their greater degree of viscoplasticity driven by weaker linkages between smaller polymers along with greater porosity. More elastic matrices (rendered through covalent cross linking of hyaluronic acid polymers and glycol-heparin) on the other hand, have been found to be inhibitive to the growth and migration of glioblastoma spheroids.
4. Phenotypic plasticity and building a case for ECM-driven heterogeneity
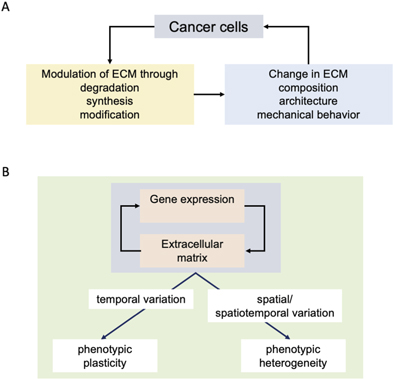
Understanding the reciprocal effects of cancer cells and ECM as described in the previous sections (figure 2(A)) is essential towards arriving at the origin of ITH. The change in phenotypic traits in response to differing environments is known as phenotypic plasticity. Plasticity is exhibited by multicellular organisms in response to their macroenvironments and by cells, tissues, and organs in response to variations in parameters of their microenvironments [78]. Plasticity is measured and compared through the metric of reaction norm, which represents the array of phenotypes or the change in phenotypic traits exhibited by a single genotype upon variations in its environmental parameters (figure 2(B)) [79].
Figure 2. Locating links between ECM and intratumoral heterogeneity. (A) Aberrant genomic and epigenetic changes associated with cancer results in transformation of the tumor microenvironment through degradation of existent ECM (for e.g., through proteases), synthesis of new ECM proteins (e.g., desmoplastic reaction) and enzymatic modifications (such as cross-linking of collagen). Such changes lead to alteration in the chemical composition (and hence ligand presentation), the architecture (parameters such as porosity) and mechanical behavior (viscoelastic/viscoplastic transitions). These changes in turn feedback on cancer cell behaviors as well as can exert influence on mechanisms regulating the stability of cancer cell genomes. (B) In physiologically functioning tissues, the extracellular matrix (ECM, specifically the basement membrane for epithelial tissues and organs) serves to steer gene expression of parenchymal cells away from proliferation, migration and production of ECM modulators: this dynamical and reciprocal relationship between gene expression and the ECM results in homoeostatic tissue architectures. In cancer, these interactions are subverted and replaced with multiscale reciprocal processes described in figures 1(B) and (C). Temporal variations in such reciprocal oncogenic interactions results in plasticity of phenotypic traits associated with cancer cells. On the other hand, spatial or spatiotemporal variations in such interactions drive phenotypic heterogeneity, wherein multiple different tumor cells phenotype coexist within the same tumor population.
Download figure:
Standard image High-resolution imagePlasticity and heterogeneity are conceptually connected: an agent (be it a cell, an organism, or their populations) will exhibit phenotypic differences in response to temporally varying environmental cues: however, if the cues are quantitatively variable across space for an agent population, a wide reaction norm can translate to a spatially varied response, i.e., phenotypic heterogeneity (figure 2). If such cues bring about variation in motility, leading to a continuous reorganization within the population (with constantly changing neighbors), such heterogeneities cascade, given that phenotypes within cellular populations feedback on gene expression through signaling. The result is a further amplification in the phenotypic diversity (and heterogeneity) within a cell population. Such mechanisms of phenotype diversification are commonly observed in developing tissues within embryos and have been proposed by Salazar-Ciudad and colleagues to have been instrumental to the rapid evolution of multicellular embryonic architectures [80, 81].
Given the multiple modes and scales, described in the previous section, by which cancer cells and ECM affect each other, even slight variations in combinatorial deployments of such interactions can lead to prolific ITH. For example, in a cell population with variable stiffnesses, cells which are soft enough can deform and pass easily through narrow porous gaps of a fibrillar ECM. A recent study by Asadullah and co-workers demonstrates that the invasive front of a tumor within an ECM scaffold is enriched with stem-like cells that are softer when compared to the uninvaded cells in the core of the centrifugally growing population [82]. Heterogeneity of ECM architecture can also drive specific invasive behaviors such as collective migration as demonstrated by Zhu and co-workers using funnel-like setups filled with laminin-rich matrices [83].
The presence of cells with more progenitory capabilities at the invasive edge of tumoroids has also been observed in another recent study, which has been among the earliest reports on ITH based on differences between cells in surface levels of a glycan moiety: α2,6-linked sialic acids (2,6-Sial). Pally and co-workers were able to observe distinct levels of the glycan linkage on the surface of breast cancer cells in culture and in vivo, using lectin flow cytometry and quantitative glycan mass spectrometry. Cells expressing moderate levels of 2,6-Sial adhered stronger to ECM than high 2,6-Sial cells [84]. This property allowed the former to sort out and migrate to the invasive front. In consonance with Asadulla et al, these invasive cells also exhibited the capacity to give rise to cells with disparate levels of 2,6-Sial. An important aspect of this study is the demonstration of the role played by the ECM: the migration seen for the progenitory moderate 2,6-Sial expressing cells was of a dispersed mesenchymal type and could be understood as the outcome of the confinement dynamics mediated by the fibrillar porous ECM exterior to, and a jammed tumoroid core interior to, the invasive front respectively [84].
Han and co-workers have also showcased the role of ECM confinement dynamics in potentiating mechanobiological heterogeneities within multicellular breast tumoroids [85]. Using cell line- and patient-derived tumoroids within a composite ECM environment of laminin-rich BM and collagen ECM, they observe a gradient in cell volume and fluid content: the core represents smaller and less fluidized cells, whereas the invasive front consists of larger and softer cells. This fluidization is enhanced by gap junction-based fluid exchange between cells at the front. Abrogation of the ECM confinement as well as use of the gap junction inhibitors impairs such spatial heterogeneities.
A theory of ECM-driven heterogeneity incorporates spatial diversification not just in cellular phenotypes but also in terms of properties associated with the ECM. Recent studies employing high-throughput proteomics approaches are beginning to reveal stunning variegation in ECM characteristics within tumors as well as between different locales of metastasized cancer [86]. Evidence in the form of novel heterogeneous patterns of collagens has been observed using MALDI-imaging by Angel and colleagues on breast cancer tissue microarrays [37].
MALDI imaging has also led to the demonstration of heterogeneity in collagen proline hydroxylation within lung adenocarcinoma [87]. Such alterations result not just in spatial asymmetry of ligand presentations, but also could mediate spatial variations in physicochemical properties of the matrix. A recent study by Dasgupta and co-workers used helical nano propellers manoeuvrable through magnetic fields to observe that an ECM layer protruding to characteristic length scales beyond tumoroid-like clusters of the invasive breast cancer cells MDA-MB-231 (but not untransformed breast epithelia cultured in the same microenvironment) shows high levels of negative charge. The zone of charge was found to be congruent with the presence of a specific negatively charged sugar linkage, α2,3-sialic acids, which are generally known to be conjugated to proteins and lipids on the surface of cells. The zone was also coincident with a fibrillar form of ECM, that could be distinguished ultrastructurally from the non-fibrillar laminin-rich BM scaffold, indicating that cancer cells were responsible for the secretion of sialic acid-containing negatively charged fibrillar matrix [41]. The study serves to showcase the degree of complexity in heterogeneity brought through chemical modifications with the ECM, where intensity and spatial length scales of charge distributions could sculpt tumoroid rheology.
In addition to spatial variations in biological and chemical properties, the mechanical behaviors of tumors may show differences from one locale to another. For example, Insana and colleagues show site-specific variation in elastic and loss moduli within breast tumor microenvironments using ultrasonic elastography [88]. Shear wave dispersion ultrasound vibrometry is amenable to delineation of spatial variance in mechanical parameters and has been used to elicit differences in viscoelastic properties within malignant and benign breast tumors [89]. Liu and colleagues have tuned the stiffness of collagen-alginate hybrid hydrogels to match the elastic parameters of the core and periphery of tumors to show that the cancer cells within the softer core and the stiffer periphery have distinct transcriptomic signatures, including that for the expression of specific ECM genes [90].
Finally, rather than relative differences in properties of ECM, do absolute values drive cellular heterogeneity? Research by Discher and colleagues reveals an association between genomic variations and stiffness of the tissue of origin of the tumor (and by extension that of the stromal ECM). An increased stiffness due to crosslinking results in architectural changes such as lower porosity—migration through such narrow pores results in enhanced DNA damage and genetic variation among cancer cells [91].
The positive feedback on phenotype driven by cancer cells and their surrounding ECM contributes significantly to phenotypic heterogeneity within the tumor microenvironment. Conceptually, such a framework paves the way for distinct heterogeneities across spatial scales: not just would two neighboring cells acquire individual expression states because of differential mutation signatures or epigenetic cues driven through signaling, by segregating distinct cell subpopulations within tumors, the ECM may also ensure phenotypic heterogeneities between them. Such a surmise is supported by simulation studies that incorporate evolutionary dynamics within spheroid-like discrete populations of rapidly dividing cells. Under high mutation rates (mimicking the genomically unstable cancer cells) genetically heterogeneous variants naturally emerge and cooperate with each other as a stratagem towards maximizing fitness [92].
An ECM-driven mechanism for ITH is consistent with the framework proposed by Hausser and Alon that distinguishes between 'specialist' and 'generalist' tumors [93]. The latter tends to evolve multiple cellular sub-niches each of which is geared towards being able to cope with specific strategies for survival. This could shed mechanistic insight into how experimentalists are increasingly observing diverse modes of migration playing out simultaneously within tumors [77, 94]. Computational studies indicate the role of matrix adhesion in being able to achieve such multimodal invasion phenomena. Consistent with an ECM-driven diversification of multiple cancer phenotypes, research on the dissemination of ovarian cancers reveals distinct spheroidal populations within the peritoneum: one with the ability to quickly adhere and spread on peritoneal surfaces, and the other, with the tendency for long-term peritoneal survival through impaired attachment to the peritoneum. Such spheroidal morphologies can be distinguished by specific ECM expression: the first is rich in fibronectin, whereas the second is protected by a coat of BM proteins such as laminin and collagen IV. Such morphologies coexist within malignant ascites of patients and showcase mesoscale heterogeneity in transitory cancer niches [95].
5. Concluding thoughts and challenges
Despite the strong correlations between ECM and ITH as showcased through multiple examples, we are still far from a sound theory of ECM-driven cell state diversification. This is partly because our understanding of the biophysical and biochemical properties of ECM macromolecules is limited to a small set of molecular exemplars. The situation is worse when it comes to our understanding of how mixtures of ECM macromolecules behave biophysically, based on an emerging set of literature that the mechanical behaviors of even simple ECM scaffolds is far more complex than what was thought earlier [23]. Finally, given that the ECM present within the tumor microenvironment is wrought at least in part by tumor cells, the genomic instability inherent to the latter is likely to result in changes in structure and expression of ECM conjugates with wide ranging consequences on the properties of the matrix they will constitute. A study by Izzi and colleagues observes an astounding frequency of copy number alterations and mutations within the matrisomal genes with deep consequences not just on the structure of proteins but their expression as well [96]. An appreciation of such limitations has spurred the emergence of promising programs such as the matrisome project to painstakingly document the rich diversity of ECM dynamics both in tissues and tumors [16].
More frequent use of approaches combining imaging with mass spectroscopy would lead to a better understanding of the spatial resolution in heterogeneity of the tumor ECM. For example, the distinctions in behaviors of cells within the core and at the boundary of tumor spheroids have so far been investigated in terms of their motility, proliferation, signaling, glycan expression etc. A thorough spatiotemporal examination of synthesized ECM or the expressions of mediators of their modification and lysis within tumor populations would add to the mechanistic understanding of intra-niche heterogeneities.
Our understanding of how, and to what spatial extent, cancer cells sense variations in mechanical behaviors of ECM is still limited. Studies that concurrently interrogate ECM microarchitecture and mechanical properties are likely to shed light on how single cells and collective populations 'read' changes in their local and distant ECM microenvironments in order to optimize their invasion [97]. Combining such investigations with spatial analyses of protein expression is likely to deepen our understanding of the feedbacks that exist between the tumor (epi)genome and physical properties of the surrounding ECM.
Investigations into the rules of spatial patterning of heterogeneous tumor populations has grave translational implications: they influence the size and number of biopsies that need to be taken to understand the phenotypic capacities of a malignant tumor. Studies across cancers provide strong evidence for the ability of ECM to regulate response to chemotherapy. In the light of our thesis above that ECM is also responsible for engendering phenotypic heterogeneity, we look to future studies that investigate whether ECM-driven molecular mechanisms hold the key to establish modulatory correlations between chemoresistance and heterogeneity, allowing one to coevolve with the other as cancers progress. A recent paper by Beerenwinkel's group discusses how cell–cell interactions and cellular patterning can influence the evolution of tumors: through rapid clonal expansion within non-spatialized tumors (such as in chronic myeloid leukemia), through progressive diversification within isolated islands of tumor buds (such as in colorectal cancer), through extensive phylogenetic diversification and branching (such as in glandular tumors), and lastly drift-driven evolution at discernible boundaries of large tumors such as myomas [98]. The demonstration by several groups that ECM may play crucial roles in cell dispersal creating divergent patterns within single tumors [99–101] suggests that the coexistence of diverse evolved phenotype within the same tumor driven by the matrix microenvironment may well be a norm in the coming days.
Acknowledgments
We would like to thank Satyarthi Mishra and Mallar Banerjee for their careful reading of the manuscript and helpful comments. The work which forms the basis of the review is supported by the Wellcome Trust/DBT India Alliance Fellowship/Grant [Grant Number IA/I/17/2/ 503312] awarded to R Bhat. In addition, R Bhat was supported by funds from the Department of Biotechnology, India [BT/909 PR26526/GET/119/92/2017] during the time of writing the review. R Bhat also benefited from discussions with members of the 'Cellular agency in multicellular development and cancer' cluster that is part of the Agency, Directionality and Function cohort program, funded by the John Templeton Foundation. D Pally and S Goutham are supported by Senior Research Fellowship (SRF) from the Ministry of Education, India.
Data availability statement
No new data were created or analysed in this study.