Abstract
Calcium phosphate was formed on nickel-free high-nitrogen stainless steel (HNS) by chemical solution deposition. The calcium phosphate deposition was enhanced by glutamic acid covalently immobilized on the surface of HNS with trisuccinimidyl citrate as a linker. X-ray diffraction patterns and Fourier transform infrared spectra showed that the material deposited on glutamic acid-immobilized HNS within 24 h was low-crystallinity calcium-deficient carbonate-containing hydroxyapatite (HAp). The biological activity of the resulting HAp-coated HNS was investigated by using a human osteoblast-like MG-63 cell culture. The HAp-coated HNS stimulated the alkaline-phosphate activity of the MG-63 culture after 7 days. Therefore, HAp-coated HNS is suitable for orthopedic devices and soft tissue adhesion materials.
Export citation and abstract BibTeX RIS
1. Introduction
Biometals are the oldest biomaterials, and have been the subject of extensive research [1]. Currently, SUS316L and cobalt–chromium alloy are extensively used in clinical applications because of their good mechanical properties, high wear resistance and low cost. These biometals contain high levels of nickel to allow the fabrication of thin or complex structures. However, when they are implanted in the body, Ni ions are eluted from the biometal surface, which may induce an inflammatory response and an allergic reaction [2, 3]. Therefore, Ni-free biometals are attracting considerable interest due to their superior biocompatibility.
We have reported that Ni-free high-nitrogen stainless steel (HNS) can be prepared by N2-gas pressurized electroslag remelting (P-ESR) process [4–6]. HNS has excellent strength, corrosion resistance and wear resistance compared with SUS316L [4–7]. Furthermore, we have successfully immobilized vascular endothelial growth factor (VEGF) on the HNS using trisuccinimidyl citrate (TSC) as a linker [8–10]. The VEGF-immobilized HNS significantly enhanced the growth of endothelial cells in culture medium compared with the original HNS (Org-HNS) and VEGF-immobilized SUS316L [11]. Therefore, modifying the surface of NHS with bioactive molecules is an effective method for adding biological functionality.
Hydroxyapatite (HAp) is a form of calcium phosphate (CaP) and is the main inorganic component of bone and tooth. In addition, HAp is one of the most effective bioactive materials for enhancing osteogenesis and osseointegration [12, 13] and can be employed for orthopedic applications in combination with various metals [14].
HAp has been formed on metals and polymers using various techniques [15, 16], such as dip coating [17, 18], electrophoretic deposition [19, 20], the alternate soaking process [21–24], thermal spraying [25, 26], dynamic mixing [27], RF magnetron sputtering [28, 29], pulsed laser ablation [30] and low-voltage deposition [31]. The coating temperature of HNS during HAp formation is important, because the release of N2 from HNS by high-temperature treatment causes the phase change from austenite to ferrite [32, 33]. We have employed chemical solution deposition for forming HAp on HNS at temperatures below 100 °C [34, 35]. We have also established a simple chemical solution deposition method using aqueous solutions of various pH at 90 °C for uniformly depositing highly crystalline HAp-coatings on pure magnesium (Mg) and Mg alloys [36–41]. The corrosion of the surface, where Mg ions were eluted, initiated the rapid nucleation of HAp in aqueous solution [40, 41]. This suggested that the high corrosion resistance of the HNS surface suppresses nucleation.
In this study, we have immobilized glutamic acid on the HNS surface with TSC to promote the nucleation of calcium phosphate by chemical solution deposition. Furthermore, we have investigated the conditions for forming calcium phosphate on the surface through the carboxyl groups. The resulting substrates were characterized by scanning electron microscopy (SEM), x-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and inductively coupled plasma atomic emission spectroscopy (ICP-AES). Additionally, we have evaluated the biological activity of each substrate by using human osteoblast-like MG-63 cells.
2. Materials and methods
2.1. Materials
We used HNS disks of 10 mm diameter and 1 mm thickness (E.S.Q Ltd, Japan). Dimethyl sulfoxide (DMSO), 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), 10% formalin neutral buffer solution (pH 7.4), L-glutamic acid, potassium dihydrogen phosphate (KH2PO4) and 4',6-diamidino-2-phenylindole (DAPI) were purchased from Wako Pure Chemical Industries Ltd, Japan. Ethylenediaminetetraacetic acid calcium disodium salt hydrate (Ca-EDTA) was purchased from Sigma-Aldrich Co., USA. Alexa Fluor 488 phalloidin was purchased from Molecular Probes, Inc., USA. Trisuccinimidyl citrate (TSC) was prepared in accordance with a previously reported method [8].
2.2. Preparation and characterization of substrate
2.2.1. Preparation of HNS
An HNS ingot was prepared by P-ESR, which produced a Fe-23Cr-1Mo-1N (wt%) alloy. Discs 10 mm in diameter and 1 mm in thickness were cut from the alloy. They were mirror-polished with 240 and 400 grit sandpapers and a polishing pad (Unifi Manufacturing Inc., USA) and then with 9, 3 and 1 μm diamond suspension (Buehler Ltd, USA). The discs were sonicated for 15 min in acetone three times to remove any extraneous matter from the surface.
2.2.2. Immobilization of glutamic acid on HNS
To immobilize glutamic acid on the surface of the HNS, TSC-immobilized HNS (TSC-i-HNS) was prepared. The HNS substrate was irradiated with UV light at 185 and 254 nm (Miyata Elevam Inc., Japan) for 60 min to remove organic residues and increase the number of hydroxyl groups on the HNS surface. The pre-treated surface was placed in a 20 mM TSC/DMSO solution at room temperature for 15 min, rinsed with DMSO and HFIP, and then dried in a stream of nitrogen gas. The discs were then immersed in HFIP for 15 min and dried under vacuum for 12 h.
The TSC-i-HNS substrate was incubated with glutamic acid (100 μg ml−1 glutamic acid in 10 mM phosphate buffered saline (PBS), (pH 7.4) at 4 °C for 3 h, rinsed with ultrapure water (Nihon Millipore K.K., Japan), and dried in a vacuum to produce the immobilized glutamic acid substrate (Glu-i-HNS).
2.2.3. CaP coating of HNS by chemical solution deposition
An aqueous solution of 0.25 M Ca-EDTA and 0.25 M KH2PO4 was prepared, and the pH was adjusted to 8.1 using 1 N NaOH solution. The Org-HNS and the Glu-i-HNS substrates were immersed in the solution at 90 °C for 3, 6, 12 and 24 h, rinsed in ultrapure water, and dried in a vacuum. The pH of the solutions was not altered by the treatment.
2.2.4. Characterization of the CaP-coated HNS surface
The water contact angles for HNS after UV irradiation and treatment with TSC or glutamic acid immobilization were measured with a contact angle meter (DM800, Kyowa Interface Science Co., Ltd, Japan). Furthermore, each substrate was analyzed by x-ray photoelectron spectroscopy (XPS; VG Theta Probe, Thermo Fisher Scientific Inc., USA) with a monochromatic source of Al Kα radiation at 1486.6 eV. The peak positions were adjusted in reference to the C 1s peak derived from TSC at 284.5 eV.
The surface of each CaP-coated HNS substrate was analyzed by SEM (JSM-5600, JEOL Co., Ltd, Japan), XRD (RINT-Ultima III, Rigaku Co., Ltd, Japan), and FTIR spectrometry (FTIR-8400S, Shimadzu Co., Ltd, Japan).
2.2.5. Determination of Ca and P content of deposited CaP
CaP-coated HNS was immersed in 10 mM citric acid solution at room temperature for 24 h to dissolve the deposited CaP. The amounts of Ca and P extracted from the CaP were quantified by ICP-AES (SPS1700HVR, Seiko Instruments Inc., Japan).
2.3. Biological evaluation of the substrates
2.3.1. Cell culture
Human osteoblast-like MG-63 cells (Riken, Japan) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 2 mM L-glutamine, at 37 °C and in 5% CO2 until the cells reached confluence. The medium was changed several times a week. The cells were trypsinized and re-suspended in fresh media. The procedure was repeated up to passage 6.
2.3.2. Adhesion and proliferation of MG-63 cells on the substrates
Each substrate was put into a 48-well plate (Thermo Fisher Scientific Inc., USA), and MG-63 cells were seeded at a density of 1.8 × 104 cells cm−2. The culture medium was added (500 μl per well) and changed every 3 days. The cells were incubated at 37 °C and in 5% CO2, and the number of MG-63 cells at day 1 and 7 was determined using Cell Counting Kit-8 (Wako Pure Chemical Industries Ltd, Japan) according to the manufacturer's instructions. The MG-63 cells were incubated in the cell counting kit solution with water soluble tetrazolium salt for 3 h, and the released formazan dye was detected by its absorbance at 450 nm using a microplate reader.
2.3.3. Morphology of MG-63 cells
The distribution and morphology of the MG-63 cells on the CaP-coated HNS surface was determined by fixing the cells with 10% formalin neutral buffer solution for 10 min and permeabilizing them in 0.2% Triton-X100 in PBS for 2 min. The cells were then incubated in a PBS solution of Alexa Fluor 488 phalloidin for 60 min and then in a PBS solution of 0.1% DAPI for 10 min in the dark at room temperature. The cells were observed in an inverted fluorescence microscope (IX71, Olympus Co., Japan).
2.3.4. ALP activity of MG-63 cells
After incubation for 7 days, the cells were rinsed with PBS and then incubated in 0.2% Triton-X100 at 4 °C for 60 min. The ALP activity of MG-63 cells on the CaP-coated HNS surface was determined using LabAssayTM ALP (Wako Pure Chemical Industries Ltd, Japan) according to the manufacturer's instructions. The cells were incubated in the ALP assay kit solution including p-nitrophenylphosphate disodium at 37 °C for 30 min, and then the released p-nitrophenol was detected using a microplate reader via optical absorbance at 415 nm.
2.4. Statistical analysis
The results are presented as the mean ± standard deviation (SD). The statistical significance of the results was analyzed using the f-test and t-test with Microsoft Office Excel 2003. Differences were considered significant for p < 0.05.
3. Results and discussion
3.1. Immobilization of glutamic acid on HNS using TSC
The surface of HNS is covered with an oxide layer, which does not readily react with organic molecules. UV irradiation was used to introduce hydroxyl groups on the HNS surface; this treatment is also effective for removing residues adsorbed on the metal surface [42–44]. We have previously reported that UV irradiation of an HNS surface for 60 min effectively removes organic residues and introduces hydroxyl groups [11]. We also demonstrated that one active TSC ester group covalently reacts with the HNS hydroxyl groups after the UV-irradiated HNS is immersed in TSC/DMSO solution for 15 min, meaning that the immobilized TSC on the HNS surface still has two active ester groups which can react with biomolecules containing primary amino groups.
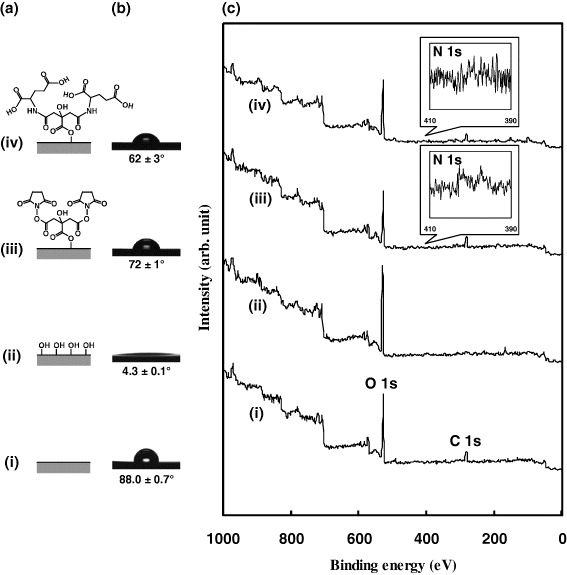
Carboxyl groups were introduced onto the resulting TSC-i-HNS surface by covalently immobilizing glutamic acid on the surface of TSC-i-HNS through the reaction of the residual active ester groups of the immobilized TSC and the amino group of glutamic acid. We predicted that the carboxyl groups of the glutamic acid would interact with the Ca ions in the treatment solution, which would lead to nucleation of calcium phosphate [45]. After the immobilization of glutamic acid on the TSC-i-HNS surface, the HNS surface properties were analyzed through contact angle measurement and XPS. Figure 1(b) shows that the water contact angle of Org-HNS was 88.0 ± 0.7°. The angle decreased to 4.3 ± 0.1° after UV irradiation for 60 min, indicating that HNS had acquired a superhydrophilic surface. After the UV-irradiated HNS was immersed in TSC/DMSO solution for 15 min, the water contact angle increased to 72 ± 1°, indicating that TSC was introduced on the surface of HNS by the reaction between the TSC active ester groups and the HNS hydroxyl groups. Furthermore, when glutamic acid was immobilized on TSC-i-HNS, the water contact angle was 62 ± 3°. Glutamic acid is more hydrophilic than TSC; therefore, the decrease in the water contact angle indicated that glutamic acid was covalently immobilized on the HNS surface by the reaction between the amino groups and the residual active ester groups of the immobilized TSC.
Figure 1. Characterization of Glu-i-HNS. (a) Schematic, (b) water contact angle and (c) XPS spectra of each substrate in a series of immobilization processes: (i) Org-HNS, (ii) UV-irradiated HNS, (iii) TSC-i-HNS and (iv) Glu-i-HNS.
Download figure:
Standard imageFigure 1(c) shows the XPS spectra for the different surfaces. Sharp C 1s and O 1s peaks were observed for the Org-HNS surface (figure 1(c)(i)). After the substrate was irradiated with UV for 60 min the C 1s peak disappeared and the O 1s peak remained (figure 1(c)(ii)). After the UV-irradiated HNS was immersed in TSC/DMSO solution for 15 min, C 1s and N 1s peaks were observed (figure 1(c)(iii)), indicating that TSC was covalently immobilized on the HNS surface. A slight decrease in the O 1s peak intensity was consistent with the formation of ester bonds between the TSC active ester groups and the HNS hydroxyl groups. Figure 1(c)(iv) shows that the O 1s, N 1s and C 1s peaks were also detected on the Glu-i-HNS surface.
3.2. Effect of immobilized glutamic acid on CaP deposition
Figure 2(a) shows the SEM images of calcium phosphate formed on the substrates, Org-HNS and Glu-i-HNS, after chemical solution deposition at pH 8.1 for 3 h. A significant difference in the coatings was observed; a small amount of CaP was formed on Org-HNS, whereas the CaP on Glu-i-HNS was composed of integrated needle-like crystals. Figure 2(b) shows that the Ca content of Glu-i-HNS was much higher than that of Org-HNS.
Figure 2. Effect of the immobilized glutamic acid on calcium phosphate formation. (a) SEM images of calcium phosphate formed on Glu-i-HNS and Org-HNS after chemical solution deposition for 3 h. (b) Amount of calcium incorporated into various coatings. Data are the mean ± SD of three samples, with (*) indicating p < 0.05.
Download figure:
Standard imageTanahashi and Matsuda reported that phosphate or carboxyl groups in self-assembled monolayer on substrate promoted the nucleation of calcium phosphate because of the interaction between the negatively charged functional groups and the Ca ions in a simulated body fluid. Positively charged functional groups, such as amino groups, did not initiate the nucleation because the interaction with HPO42− ions was weak [45]. These results suggest that the interaction between the glutamic acid carboxyl groups and the Ca ions released from Ca-EDTA promoted the nucleation of calcium phosphate on the HNS surface. Our previous studies have shown that the immobilized TSC was gradually liberated by the hydrolysis of the ester bonds at the TSC–HNS interface. However, about 40% of the initial TSC remained on the HNS surface after immersion in the culture medium at 37 °C for 14 days [11]. This demonstrated that the immobilized glutamic acid played an important role in promoting the nucleation of calcium phosphate before it was liberated by hydrolysis at the TSC–HNS interface.
3.3. Characterization of the CaP coating on Glu-i-HNS
Figure 3 shows the XRD patterns of calcium phosphate formed on Glu-i-HNS after treatment at pH 8.1 for various periods of time. For coating times from 3 to 12 h, various peaks around 26.0° (0 0 2), 28.9° (2 1 0), 31.8° (2 1 1), 32.2° (1 1 2), 32.9° (3 0 0), 34.1° (2 0 2) and 39.8° (3 1 0) were observed, indicating that HAp (PDF no. 00-009-0432) was deposited on the surface of Glu-i-HNS. A weak diffraction peak at 4.7°, which originated from the (0 1 0) plane of octacalcium phosphate (OCP; PDF no. 44-0778) was observed, indicating that the deposited calcium phosphate consisted of HAp and a small amount of OCP. It has been reported that HAp-coatings that contained a small amount of OCP were formed on Mg alloy after a short chemical solution deposition [38]. In the present system, carboxyl groups on Glu-i-HNS interacted with Ca ions released from Ca-EDTA, and then reacted with hydrogen phosphate ions (HPO42−), resulting in the deposition of HAp and a small amount of OCP. After 24 h, the peak at 4.7° disappeared and only the characteristic HAp peaks were observed, indicating that OCP was gradually converted to HAp during the chemical solution deposition [46].
Figure 3. XRD patterns of calcium phosphate formed on Glu-i-HNS after chemical solution deposition for 3, 6, 12 and 24 h.
Download figure:
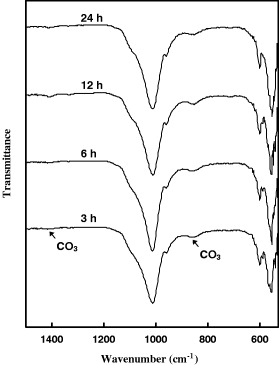
Standard imageFigure 4 shows the FTIR transmittance spectrum of the substrate surface. The characteristic HAp peaks were present; the peak at around 600 cm−1 originated from the vibrational mode of the hydroxyl group [47], and the peaks around 1020, 970, 560 and 520 cm−1 were assigned to the internal modes of the phosphate group. It was assumed that the small peaks around 1430 and 870 cm−1 originated from a small amount of carbonate [48]. Furthermore, determination of the Ca and phosphate ion content showed that the Ca/P ratio of the deposited HAp was 1.34. Theoretically, the stoichiometric Ca/P value of HAp is 1.67. Therefore, the coating consisted of low-crystallinity Ca-deficient carbonate-containing HAp.
Figure 4. FTIR spectra of calcium phosphate formed on Glu-i-HNS after chemical solution deposition for 3, 6, 12 and 24 h.
Download figure:
Standard image3.4. MG-63 cell culture on CaP-coated HNS
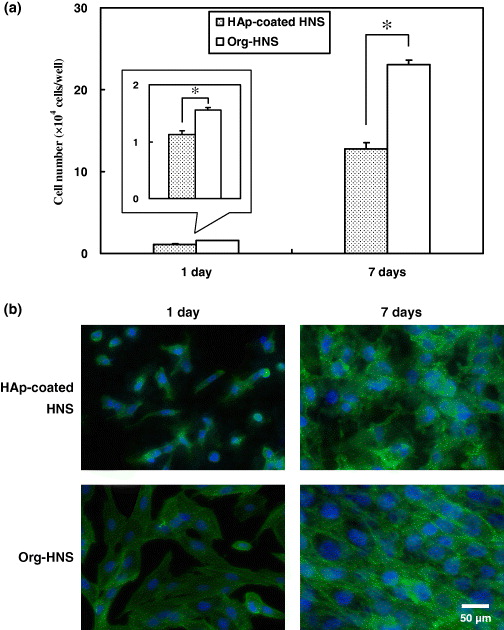
To evaluate the biological activity of HAp-coated HNS, human osteoblast-like MG-63 cells were cultured on HAp-coated HNS and Org-HNS. Figure 5(a) shows that the number of the cells adhered to Org-HNS was significantly higher compared with HAp-coated HNS (p < 0.05) after 1 day. After 7 days, the number of the cells was approximately ten-fold higher than that at 1 day, and the number of cells on Org-HNS was larger than that on HAp-coated HNS (p < 0.05).
Figure 5. Osteoblasts adhered on HAp-coated HNS and Org-HNS. (a) Number and (b) morphology of MG-63 cells attached to the surface after the cells were cultured for 1 and 7 days. Data are the mean ± SD of five samples, with (*) indicating p < 0.05.
Download figure:
Standard imageThe distribution and morphology of the MG-63 cells on each substrate were observed by staining the nuclei and cytoskeletons of the cells after 1 and 7 days. Figure 5(b) shows that the cytoskeletons of the cells spread out on the surface of Org-HNS compared with HAp-coated HNS after 1 day. On HAp-coated HNS, most of the cells were individually dispersed and were small and elongated. After 7 days, the cells became confluent on Org-HNS; however, the cell density was low on HAp-coated HNS.
It was reported that the interaction between a biomaterial and MG-63 cells depends on the topography [49–53], wettability [53, 54] and surface energy and charge [55, 56] of the biomaterial surface. We assumed that the cell adhesion was suppressed by the surface structure of HAp-coated HNS during the initial stage of the culture. The micro-roughness did not contribute to the initial cell adhesion, whereas the mirror-polished surface of Org-HNS induced initial cell adhesion [57]. The low level of initial cell adhesion on HAp-coated HNS meant that the cell proliferation was low after 7 days.
3.5. ALP activity of MG-63 cells cultured on CaP-coated HNS
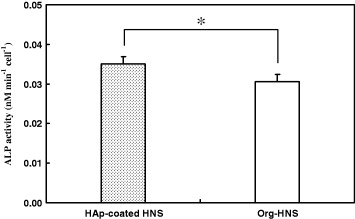
Human osteoblast-like MG-63 cells enhance ALP activity during the early stage of differentiation [58]. Therefore, the ALP activity of the cells was evaluated as an indicator of initial bone differentiation after the cells were cultured for 7 days. Figure 6 shows that the ALP activity (nM min−1 cell−1) was significantly higher on HAp-coated HNS than on Org-HNS (p < 0.05). This result suggests that the HAp deposited on the surface of HNS stimulated the function of the MG-63 cells.
Figure 6. ALP activity of MG-63 cells grown on HAp-coated HNS and Org-HNS after the cells were cultured for 7 days. Data are the mean ± SD of five samples, with (*) indicating p < 0.05.
Download figure:
Standard imageRecently, Nebe et al demonstrated that, after being cultured for 2 days, MG-63 cells that adhered to HAp-coated pure titanium were deeply anchored into the rough surface and had a small ring-like actin shape in contrast to cells adhered to the bare mirror-polished titanium. The ALP gene expression on HAp-coated titanium was significantly increased compared with the cells on bare titanium, even after 2 days [59]. Thus, the HAp-coated surface stimulated the differentiated cell function of MG-63.
Similar to the results of our study, several short-term in vitro assays demonstrated that the coated HAp with the micro-roughened surface suppresses the cell adhesion and proliferation, while stimulating the differentiated cell function [59–61]. On the other hand, it has been reported that the higher surface area from the micro-roughness is one of the most effective factors for accelerating osseointegration of implant material during long-term implantation in the body. In the biological evaluation over 32 weeks using transcortical plug model of adult mongrel dogs, Thomas et al [62] demonstrated that the use of the HAp-coated implant significantly increased mechanical strength at the implant-bone interface because of the direct mineralization onto the roughened surface. In the evaluation over 12 weeks using mature miniature pigs, Wong et al [63] also demonstrated that the roughened surface possessed the significant osseointegration property because of the higher surface coverage by bone. Therefore, HAp-coated HNS is likely to be an effective bioactive material for enhancing osteogenesis and osseointegration.
4. Conclusions
Low-crystallinity Ca-deficient carbonate-containing HAp was formed on a glutamic acid-immobilized HNS surface by chemical solution deposition. The glutamic acid-derived carboxyl groups introduced on HNS effectively promoted the deposition of HAp compared with the Org-HNS surface. Human osteoblast-like MG-63 cell adhesion and proliferation on HAp-coated HNS was low compared with Org-HNS, although the ALP activity of osteoblasts cultured on the HAp-coated HNS was significantly higher than that on Org-HNS. Therefore, HAp-coated HNS is suitable for use as a hard-tissue substitute material and a soft-tissue adhesive material.
Acknowledgments
We thank Ms M Ueno and Ms T Ishizuka of Biomaterials Unit, Nano-Bio Field, National Institute for Materials Science, Japan, for technical support. This work was supported financially in part by the Japan Society for the Promotion of Science (JSPS) through the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program), initiated by the Council for Science and Technology Policy (CSTP), MANA and the World Premier International Research Center (WPI) Initiative on Materials Nanoarchitectonics, MEXT, Japan.