Abstract
A imidazolium-based poly (ionic liquid), poly(1-allyl-3-methyl imidazolium chloride) (P[AMIm]Cl) was combined with the temperature-sensitive polymer poly(N-isopropylacrylamide) (PNIPAM) via random copolymerisation of the monomers [AMIm]Cl and NIPAM. The synthesised copolymer was characterised by nuclear magnetic resonance (NMR), Fourier transform infrared (FTIR) spectroscopy, and scanning electron microscope (SEM). Then its stimuli-responsive properties were studied at different electric field strengths and temperatures when it was dispersed in silicone oil. It was found that the colloidal copolymer particles were electro-responsive, showing excellent electrorheological (ER) effect. It was interesting that the ER effect of the copolymer was temperature-dependent. As the temperature increased from 5 °C to 50 °C, the dynamic yield stress of the suspension was found to increase with temperature. The temperature dependence of both yield stress and current density became stronger as the temperature was higher than 30 °C. The significant temperature-dependence of the suspension was attributed to the PNIPAM segment in the copolymer which could be swelled by silicone oil at higher temperature.
Export citation and abstract BibTeX RIS
1. Introduction
Smart materials are those whose one or more properties can be significantly altered by external stimuli, such as stress, temperature, pH, and electric or magnetic fields, supporting their considerable potential applications for controllable or switchable systems [1–5]. Electrorheological (ER) fluid, as a representative member of the class of smart materials, is able to respond to an external electric field and show reversible phase transition from liquid state to solid or solid-like states [6, 7]. The electric-field-controllable phase transition makes ER fluids attractive in many industrial applications, such as braking systems, damping systems, tactile display or feedback systems, and other intelligent control devices [8–11].
The ER fluids mainly consist of electric-field-polarisable particles from nanometres to microns as the dispersed phase with insulating liquids as the continuous phase. Properties of the dispersed particles including chemical, physical structures, and morphologies are critical factors that significantly influence the ER effects [12–15]. To date, various inorganic and organic materials have been studied as the dispersed phase of ER fluids. It is found that inorganics with high dielectric constant or polarisability, such as TiO2, Ti-O-containing compounds and one or two-dimensional particles, usually have a high ER effect even up to hundreds of kPa [16–20]: however, inorganics with much higher density than carrier liquid readily settle in a static state and exhibit significant friction on devices. The application of polymeric ER materials is able to avoid the above issues of inorganics because of the low density and soft nature of polymeric materials. Semi-conducting polymers [21–23] and polyelectrolytes [24] are the most attractive categories of polymeric ER materials, however, such ER materials sometimes generate a high current density due to the directional movement of electrons or ions toward electrodes.
Recently, poly(ionic liquid)s (PILs) were developed as a new class of polyelectrolytes that offered well-defined morphologies, excellent mechanical and rheological properties, and some unique properties of ionic liquids (ILs). PILs or ILs-containing materials have also caused concern among researchers interested in ER materials since Yin et al first reported the excellent ER effect of microwave-synthesised PILs leading to a new direction for the application of PILs [25–27]; however, the working temperature of the ER fluid of PILs was narrow due to the low glass transition temperature (Tg) of PILs. To address this issue, cross-linked and core–shell-structured PILs were developed [13, 28, 29]. In fact, mechanism and structural influences of the ER effect generated by PILs require further study. As a new smart polymer, its multiple effects when combined with other substances are of great interest to researchers in related disciplines.
In the present study, an alkenyl-functionalised IL was copolymerised with N-isopropylacrylamide (NIPAM) to study the electro-responsive ER effect of the copolymer P(IL-co-NIPAM). Poly(N-isopropylacrylamide) (PNIPAM) is able to show temperature-dependent phase separation in water with a lower critical solution temperature (LCST) of about 32 °C, and is widely used for design of thermoresponsive actuators [30–34]. In previous studies, PILs and PNIPAM were combined to fine tune the stimuli-responsive phase separation behaviour and assembled structures of PNIPAM [35], or to develop dual- or multi-responsive smart gels not only sensitive to temperature but also to ionic strength and/or electric field [36, 37]. Currently, Li et al[ 38] used NIPAM copolymerised with [2-(methacryloyloxy) ethyl trimethylammonium] [bis(trifluoromethanesulphonyl) amide] ([MTMA][TFSI]) as a model of soft colloids to study its electro-responsive ER effects. The shear stress curves of the soft copolymer-based ER fluid showed a typical characteristic of pseudo-plastic fluids, which was rather different from that of common ER fluids. In addition, the good mobility of [TFSI]¯ resulted in large leakage current when the electric field exceeded a critical value. Thus, there were still issues needing to be resolved in this special ER material, for example, how to restrict the electrophoretic behaviour of the mobile counter ions in PILs, and how the temperature influences the ER effect of the copolymer. Temperature effect on ER performance has been carefully concerned and studied because this issue has to be considered on the way to practical application for ER fluids. It was proved that for hydrous ER materials, the ER effect decreases with temperature because of the evaporation of water at high temperature [39, 40]. While for the unhydrous ER materials, ER effect will increase with temperature because of the similar trend in dielectric properties (i.e. dielectric constant of the ER fluid) [41]. For the polymers with low glass transition temperatures, the temperature effect on ER performance might be more complex.
Herein, in our study, an IL monomer 1-allyl-3-methyl imidazolium chloride ([AMIm]Cl) was selected as the comonomer with NIPAM based on the relative low degree of dissociation and high glass transition temperature of the Cl¯□containing PILs than those of PILs with organic counter ions. Initially, the combination of PIL with PNIPAM was not done out of concern for the temperature sensitivity of PNIPAM but its limitation of ion leakage from PIL, however, it was found that the ER effect of the copolymer in silicone oil had a strong dependence on temperature, which broadens the accommodative range of ER effect or viscosity of the ER fluid. To understand this phenomenon, we analysed the ER and dielectric performances of the copolymer-based ER fluid at different temperatures. Pure PNIPAM and PIL were prepared and compared with the copolymer. The dual-controllability of the ER fluid by both electric field and temperature promotes its applications in dual-field-controlled devices with fine step controls.
2. Experimental
2.1. Materials
N-isopropylacrylamide (NIPAM) (TCI, 98%) was recrystallised in a mixture of hexane and toluene (volume ratio of hexane to toluene = 5:1). 2, 2'-azobis(2-methylpropionitrile) (TCI, 98%) was recrystallised in methanol. The ionic liquid (IL) 1-allyl-3-methyl imidazolium chloride ([AMIm]Cl) was purchased from Lanzhou Greenchem ILs and used directly. Other chemicals including divinylbenzene (DVB), sodium dodecylsulfate (SDS) (Sigma Aldrich, 99%), dehydrated tetrahydrofuran (THF) (Macklin, 99%), N,N-dimethylformamide (DMF) (TCI, 99%), and dimethyl silicone oil (KF-96, 100 cSt, Shin-Etsu Chemical Co., Ltd) were used as received without further purification.
2.2. Synthesis of poly(NIPAM-co-[AMIm]Cl) (PNIPAM-[AMIm]Cl)
NIPAM of 2.96 g (26 mmol), [AMIm]Cl of 0.41 g (2.6 mmol), and AIBN of 0.205 g were added to 10 ml THF in a 100 ml one-necked, round-bottomed flask. The solution was magnetically stirred under N2 purging to remove oxygen. Thereafter, the flask was placed in an oil bath and heated at 65 °C for 12 h. The obtained product was purified by several re-precipitation cycles in cold diethyl ether. Then, the solid product was dried under vacuum for 5 h at 50 °C [42]. The dried copolymer was characterised by 1H NMR (Avance III 500 Bruker A&T Center BNU 500 MHz) as shown in figure 1. The two monomers were also individually polymerised for comparative study. P[AMIm]Cl: 1.18 g [AMIm]Cl and 1.3 g DVB were added in 4.00 ml DMF in a 100 ml one-necked, round-bottomed flask. After removing oxygen by purging N2, the system was heated at 70 °C under mechanical agitation with a speed of 180 rpm. After 12 h, the product was wahsed with ethanol and de-ionized water for several times, then freeze-dried for 24 h. PNIPAM was synthesised using the similar process for the copolymer without adding the IL monomer.
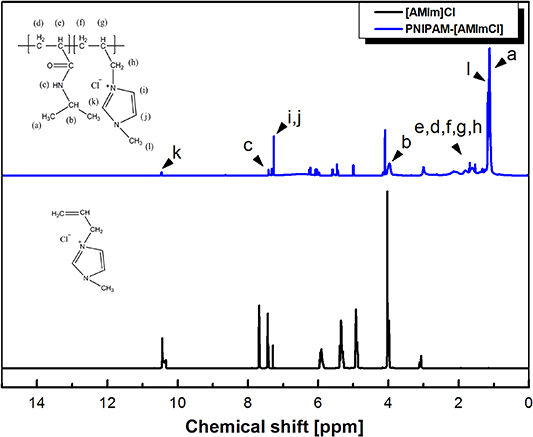
Figure 1. 1H NMR spectra of monomer [AMIm]Cl and the copolymer PNIPAM-[AMIm]Cl.
Download figure:
Standard image High-resolution imageP(NIPAM-[AMIm]Cl). 1H NMR (500 MHz, CDCl3, δH, ppm): 10.46 (1H, NCHN), 7.41 (1H, NHCO), 7.32 (1H, NCHCHN), 7.26 (1H, NCHCHN), 3.97 (1H, CH(CH3)2N), 1.70 (1H, CH2CHCH2), 1.68 (2H, CH2CHCH2), 1.61 (2H, CHCH2CH), 1.52 (1H, CHCH2CH), 1.32 (2H, CHCH2N), 1.17 (3H, N(CH)2CH3), 1.12 (6H, CH(CH3)2).
2.3. Preparation of PNIPAM-[AMIm]Cl suspensions
The synthesised solid PNIPAM-[AMIm]Cl was milled and sieved (325 mesh) to form uniform powders with particle sizes in the range of 5 to 20 μm. An oil suspension, i.e. electrorheological (ER) fluid, was prepared by mixing the copolymer particles with dimethyl silicone oil by mechanical stirring and ultrasonication. The concentration of the ER fluid is 20 wt.%.
2.4. Characterisation and measurement
1H nuclear magnetic resonance (1H NMR) measurement of the sample was conducted at room temperature using a Bruker Avance III 500 NMR spectrometer (500 MHz). CDCl3 was used as a solvent.
The rheological properties of the suspensions were measured using a rotational rheometer (MCR 502, Anton Paar, Austria) with a cylindrical cone-plate geometry (CC17E, gap distance: 0.71 mm) and a DC high-voltage generator. The dielectric spectra of the ER fluid were detected using a broadband dielectric spectrometer (Concept 80, Novocontrol) under AC electric field conditions with frequencies ranging from 0.01 to 107 Hz at different temperatures. A bias electrical potential of 1 V was applied during the measurement to avoid formation of fibrous structures in the suspension.
3. Results and discussion
Figure 2(a) shows SEM image of the synthesised copolymer PNIPAM-[AMIm]Cl. The milled polymer particles are irregular in shape with smooth fracture surfaces. The particles are micron-sized with diameters of between 4 to 18 μm. Figure 2(b) shows the TG curve of the copolymer PNIPAM-[AMIm]Cl measured from 20 °C to 700 °C in an argon atmosphere at a heating rate of 10 °C min−1. In the temperature range of 20 °C to 100 °C, there is only 4% weight loss which is attributed to the absorbed water. Then a weight loss of about 13% appears between 200 °C to 300 °C and another main weight loss of nearly 80% from 300 °C to 500 °C follows. These two weight losses are related to the degradation of the two polymeric segments of P[AMIm]Cl and PNIPAM, respectively.
Figure 2. SEM (a) and TG analysis curve of the copolymer PNIPAM-[AMIm]Cl.
Download figure:
Standard image High-resolution image3.1. ER characteristics
The thermo-sensitivity of PNIPAM in water required the investigation of the temperature dependence of the copolymer PNIPAM-[AMIm]Cl in silicone oil: the leakage current density at different temperatures was also identified. Figure 3 shows the flow curves of the ER fluid measured at 20 °C and 50 °C, respectively, in which figures 3(a)–(b) are the shear stress curves, and figures 3(c)–(d) are the shear viscosity curves. Without stimulus from an applied external electric
Figure 3. Shear stress vs. shear rate at different electric field strengths of the ER fluid measured at 20 °C (a) and 50 °C (b); shear viscosity vs. shear rate at different electric field strengths of the ER fluid measured at 20 °C (c) and 50 °C (d)
Download figure:
Standard image High-resolution imagefield, the ER fluid is non-Newtonian with shear thinning observed at both 20 °C and 50 °C. The shear viscosity at 50 °C is slightly higher than that at 20 °C in the low-shear-rate region, however, this changes as the shear rate exceeds 100 s−1( the reason for this will be explained later). When an electric field is applied, Bingham-plastic behaviour is observed with an electric-field-dependent yield stress. As the electric field strength increases, the yield stress increases. The shear stress at 50 °C is significantly higher than that at 20 °C when measured at the same applied electric field strength: the temperature has a remarkable positive effect on the ER performance of the PNIPAM-[AMIm]Cl-based ER fluid.
To demonstrate the temperature-dependence of the ER performance clearly, flow curves of the PNIPAM-[AMIm]Cl-based ER fluid were measured at different temperatures ranging from 5 °C to 50 °C. The result is shown in figure S1 available online at stacks.iop.org/SMS/29/124001/mmedia. At 5 °C, yield stresses of the ER fluid are very weak at about 1 to 10 Pa as the electric field strength increases from 0.5 to 4.0 kV mm−1. The zero-field shear stress is a typical Newtonian fluid flow curve rather than a non-Newtonian fluid as shown by the ER fluid measured at other higher temperatures. When the temperature is above 10 °C, relatively stable and electric-field-dependent shear stress curves are observed. In addition, it seems that as the shear rate increases, the shear stress undergoes a slight decrease until the shear rate reaches a critical value, which could be explained as the balance between break-up and reformation of the chain structures by the suspended copolymer particles [43]. To describe the flow behaviour of the ER fluid in detail, a modified Bingham model called Cho-Choi-Jhon (CCJ) with six parameters is adopted to fit the shear stress curves [44]:

where τy is the dynamic yield stress that can be obtained by extrapolating the shear stress curves to the zero shear rate direction, η∞ represents the viscosity at an infinite high shear rate, t2 and t3 are time constants, α and β are both between 0 and 1 and are related to the change of shear stress in low and high-shear-rate regimes, respectively. The solid lines in figure 3 and figure S1 are obtained from fitting by equation (1), which are able to describe the shear stress curves over the whole shear rate range.
To compare the dynamic yield stresses of the ER fluid at different temperatures, the fitted values of τy are plotted in figure 4(a) and S2, respectively, as a function of temperature and electric field strength (E). As shown in figure 4(a) clearly, the dynamic yield stress increases with temperature in the measured temperature range. There is an inflection point near 30 °C, beyond which the slope of the curve becomes larger. It means when the temperature is higher than 30 °C, the temperature dependence of τy becomes stronger. In figure S2, the dynamic yield stress increases following an increment in the electric field strength. Generally, the dependence of the yield stress on the electric field strength follows a power-law relationship as follows:

Figure 4. Dynamic yield stresses of the ER fluid as a function of temperature measured at different electric field strengths (a); the power-law fitting parameter m( square) in equation (2) and current density I( circle) of the ER fluid as a function of temperature (b).
Download figure:
Standard image High-resolution imageIn figure S2, the solid lines are fitted by a best-fit method using equation (2). It seems that the slope of the fitting line (m) is also related to temperature. Therefore, the fitting parameter m is plotted as a function of temperature in figure 4(b). There is a significant change in the value of m when the temperature is increased from 5 °C to 50 °C. In the low temperature region, m increases with temperature; however, it reaches a maximum at about 30 °C, then decreases as the temperature continues to rise. This result indicates that polarisation of the copolymer particles at an electric field is enhanced by increasing temperature, but there is a critical temperature above which the facilitating effect of the rising temperature diminishes.
The current density (I) in the ER fluid when explored in an electric field is also detected and shown in figure 4(b). It increases slightly with temperature when the temperature is below 30 °C. As the temperature increases, the current density increases rapidly to much higher values, which is similar with the phenomenon that is shown by the dynamic yield stress. Even though, the value of current density remains at an acceptably low level (μA cm−2) compared with other ER fluids. The same critical temperature of 30 °C for parameter m and current density I implies that high-temperature-induced current leakage might be the main reason for the decrease in m.
The temperature effect on ER properties has been reported in a previous study. It has been confirmed that, within an appropriate temperature range, the ER response could be improved by increasing temperature because of the facilitated polarisation at higher temperatures [45]. Yin et al[ 46] reported that for a carbonaceous-polyaniline-nanotubes (CPN)-based ER fluid, the yield stress increased as the operating temperature was increased. Similar results were also found in TiO2- and the recently reported PIL-based ER fluids [28, 47] however, in these studies, the increment in yield stress or shear stress with temperature is significantly lower than that found in our study. To observe the difference more clearly, a relative increment in yield stress (Δτr) with temperature is defined as follows:
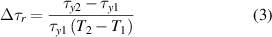
where τy1 and τy2 are the yield stresses at temperatures T1 and T2, respectively. For the aforementioned TiO2 ER fluid, Δτr is 0.009 1 °C−1( E = 3 kV mm−1) when the temperature is increased from 20 °C to 50 °C. It is even lower for the CPN and pure PILs ER fluids, whereas it is 0.116 1 °C−1( E = 3 kV mm−1) for the copolymer PNIPAM-[AMIm]Cl-based ER fluid when the temperature increases from 20 °C to 50 °C. The relative increment in yield stress of this PNIPAM-[AMIm]Cl-based ER is much higher than that of previously reported ER fluids. This means the ER effect of the copolymer PNIPAM-[AMIm]Cl is more sensitive to temperature. Fortunately, the influence of temperature is towards a positive intensification of the ER effect.
Rotational rheological measurements at a constant shear rate (1 s−1) with the application of a square voltage pulse (50 s) were used to observe the response sensitivity of the ER fluid of PNIPAM-[AMIm]Cl to electric field. In figure 5, it is also found that the electric-field-responsibility of the ER fluid depends on temperature. At the same electric field, the shear stress measured at 50 °C is significantly higher than that measured at 20 °C. In addition, the shear stress changes more rapidly to a stable value after the electric field is switched on or switched off. The obvious distinction on both shear stress value and response time between the two temperature situations can be attributed to the temperature-sensitive properties of the PNIPAM segment. As the temperature exceeds some critical value, the swelling of PNIPAM segment leads to an increase in the particle volume which weakens the binding of the polymer skeleton to the anion. The effect facilitates the polarisation of the copolymer particle which is aroused by the migration of anions in the particle, and enhances the interaction between particles [48].
Figure 5. Shear stress of the ER fluid of PNIPAM-[AMIm]Cl at 20 °C and 50 °C and a fixed shear rate of 1 s−1. A square pulsed electric field was applied at 50 s intervals.
Download figure:
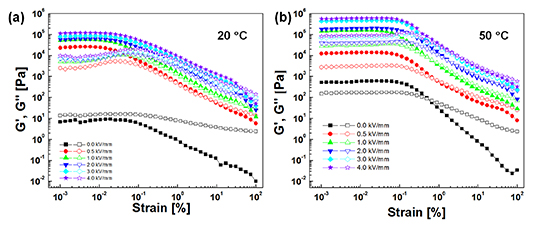
Standard image High-resolution imageDynamic oscillation testing is an important tool used when examining the viscoelastic characteristics of the PNIPAM-[AMIm]Cl ER fluid. At first, a strain sweep test was investigated at a fixed angular frequency of 6.28 rad s−1 to determine the linear viscoelastic region (γLVE), as shown in figure 6. In the low-strain region, the storage modulus G'( the formation of energy storage in the sample during the shear process) is always higher than that of the loss modulus G'' (the formation of energy dissipated during the shear process) [49]. In addition, the values of G' and G'' are independent within the region under strain (the so-called linear viscoelastic region) [50]. In this region, elasticity predominates (as opposed to viscosity); the chain structures are formed under the applied electric field and remain stable. Notably, as the temperature increases from 20 °C to 50 °C, the linear viscoelastic region broadens because the increasing temperature not only causes the volumetric expansion of the particles, but also facilitates the stronger polarisation forces between the particles by the migration of counter ions within the particles [51]. As the strain amplitude increased, going beyond the LVE region, both G' and G'' decrease gradually due to an irreversible change in the chain structures caused by the oscillatory shear deformation. In addition, the value of G'' exceeds that of G', showing that the structures begin to be destroyed over a certain range of deformation, and the elasticity of the ER fluid disappears at a particular strain amplitude.
Figure 6. Strain sweeps of the PNIPAM-[AMIm]Cl ER fluid at 20 °C (a) and 50 °C (b) (angular frequency: 6.28 rad s−1).
Download figure:
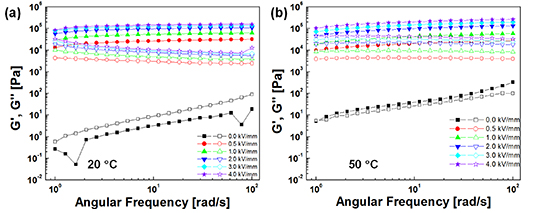
Standard image High-resolution imageThe dynamic moduli as a function of the angular frequency at 0.01% strain in the linear viscoelastic region were measured and the results are shown in figure 7. In the absence of an electric field, both G' and G'' are dependent on the angular frequency: however, after the application of the electric field, both G' and G'' increase with the increase of electric field strength, so G' is always greater than G'', showing the predominance of elastic behaviour in the structure of PNIPAM-[AMIm]Cl ER fluids. Over the entire frequency range, G' remains stable, showing that the ER fluids exhibit behaviour typical of cross-linked rubber. Additionally, similar to the temperature sweep, it shows that at zero field, G' < G'' at 20 °C showing liquid-like behaviour. When the temperature is 50 °C, G' > G'' showing solid-like behaviour, thus verifying the accuracy of the temperature-scan data.
Figure 7. Frequency sweeps of the PNIPAM-[AMIm]Cl ER fluid at 20 °C (a) and 50 °C (b) (strain amplitude: 0.01%).
Download figure:
Standard image High-resolution image3.2. The reason for temperature-dependent ER effect
To determine further the reason for dramatic increase in ER effect with temperature, we polymerised the monomer [AMIm]Cl containing DVB as a cross-linking agent, and also prepared pure PNIAM as another comparison sample. Figure 8 shows that, under an external electric field, the ER fluid of P[AMIm]Cl exhibits similar shear stress values at both 20 and 50 °C. The difference in shear stress at zero field may be caused by the dispersion state of the particles. In figure S3, for the PNIPAM-based ER fluid, as the electric field increases from 0.5 to 2 kV mm−1, there is no change in shear stress, indicating no ER effect in this ER fluid. Comparing the zero and on-field shear stress curves, the increment of shear stress in the low shear rate region is due to the pressure of the pin (connected to the power supply) on the rotating bob. Thus we can conclude that PNIPMA itself has no ER effect, the ER response of the copolymer is originated from the PIL part. The interesting point is the addition of PNIPAM gives the copolymer significant temperature-dependent ER properties.
Figure 8. Shear stress vs. shear rate curves of the ER fluids (20 wt%) based on P[AMIm]Cl.
Download figure:
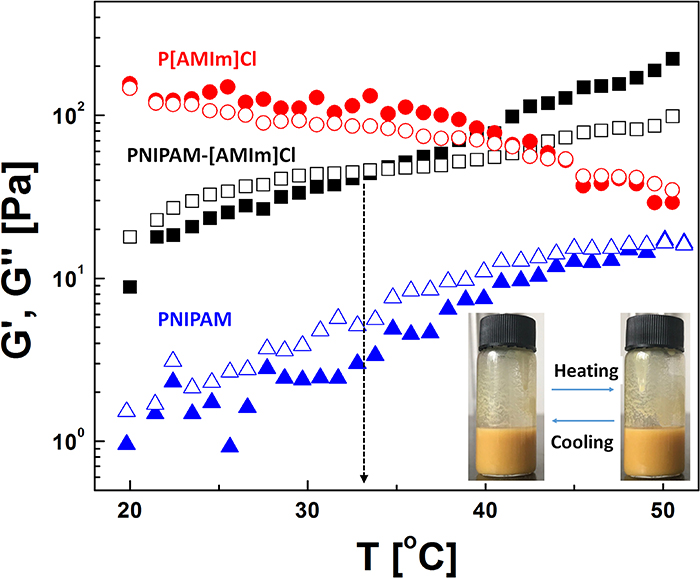
Standard image High-resolution imageThen, dynamic temperature sweeps and calorimetric analyses were conducted on these polymer-based ER fluids. As mentioned, PNIPAM is a temperature-sensitive polymer with an LCST of about 32 °C when dispersed in water, however, in this study, the copolymer particles are dispersed in silicone oil, of which the temperature effect has not been considered. To visualise the temperature dependence of the ER fluid, we also conducted dynamic temperature sweeps for the ER fluid, and for the suspension of P[AMIm]Cl and PNIPAM in silicone oil with the same mass fraction for sake of comparison. As shown in figure 9, both storage modulus (G') and loss modulus (G'') of the PNIPAM-[AMIm]Cl ER fluid increase with temperature. There is an intersection of G' and G'' plots near 32 °C, before which G'' exceeds G' indicating the liquid-like nature of the ER fluid, and after which G' exceeds G'' implying that elastic interaction is dominant in the ER fluid. It seems that the polymer chains are swollen by silicone oil at higher temperature, which enhances the volume fraction and then the interaction between polymeric particles. In the temperature sweep curve of the PNIPAM suspension, a similar trend is observed wherein both G' and G'' increase gradually with temperature; however, the values of G' and G'' for the PNIPAM suspension are lower than those of the PNIPAM-[AMIm]Cl ER fluid. By contrast, the suspension of pure P[AMIm]Cl shows slight decrease in G' and G'' with increasing temperature. This implies that the swelling of the copolymer mainly occurs in the PNIPAM segment rather than in the P[AMIm]Cl part. Thus, the temperature effect for the pure PNIPAM or copolymer PNIPAM-[AMIm]Cl in silicone oil is very different from that of the PNIPAM hydrogel, in which G' and G'' decrease or increase suddenly when the temperature is higher than LCST because of the phase transition occurring in the PNIPAM hydrogel [52, 53]. Similar phenomenon was also observed in the aqueous suspension of PNIPAM-[AMIm]Cl (20 wt.%) as shown in figure S4.
Figure 9. Dynamic temperature sweeps of the polymer/oil suspension in the temperature range of 20 °C–50 °C at fixed frequency of 6.28 rad s−1 and strain of 0.1%. Inset is the optical image of PNIPAM-[AMIm]Cl/oil suspension at 20 °C and 50 °C.
Download figure:
Standard image High-resolution imageTo confirm the predicted swelling of PNIAPM in silicone oil, the heating capacity of suspensions containing different polymers PNIPAM-[AMIm]Cl, PNIPAM, and P[AMIm]Cl were respectively measured using a differential scanning calorimeter (DSC) in the temperature range of 10 to 60 °C. As shown in figure 10, broad endothermic peaks are observed in the Cp curves of PNIPAM/silicone oil and PNIPAM-[AMIm]Cl/silicone oil suspensions. There is no endothermic effect in the Cp curve of P[AMIm]Cl/silicone oil suspension. This confirms that the endothermic peak in PNIPAM-[AMIm]Cl/silicone oil is induced by the PNIPAM segment, however, the Cp curves of both the PNIPAM/silicone oil and PNIPAM-[AMIm]Cl/silicone oil are very different from that of aqueous suspension of PNIPAM, which shows a narrow endothermic peak because of the hydrophilic-hydrophobic transition at the temperature of LCST. In addition, according to the optical image of the PNIPAM-[AMIm]Cl/silicone oil suspension (inset, figure 9), it is found that the suspension is non-transparent and brown whether at lower (25 °C) or higher (50 °C) temperature, therefore, we predict that the endothermic effects in both PNIPAM/silicone oil and PNIPAM-[AMIm]Cl/silicone oil suspensions are attributed to the interaction between silicone oil and the PNIPAM chain, such as evinced by the swelling of the PNIPAM chain in silicone oil with increasing temperature.
Figure 10. Upscan heat capacity curves of the ER suspension (heating rate: 5 K min−1).
Download figure:
Standard image High-resolution image3.3. Dielectric properties
ER effect is generally considered to be aroused by the polarization induced electrostatic force between particles, the value of which is related to the dielectric properties of both the solid particles and the dispersion liquid. Therefore, dielectric analysis is a critical method to understand the ER effect of ER fluids. It has proved that a large dielectric strength and a dielectric relaxation in a proper range (102 ∼ 105 Hz) are necessary for a good ER effect. From the study of dielectric spectra of ER fluids with different volume fractions or at different temperatures, one can achieve the information about the dielectric parameters [12], phase parameters [54], and even activation energy [13] for dielectric relaxation.
In this study, dielectric properties of the PNIPAM-[AMIm]Cl ER fluid were measured to correlate dielectric characteristics of the ER fluid with its rheological performance under different electric and temperature field conditions. To clarify the dielectric characteristics, the polarisation effect was investigated using two significant parameters: the dielectric constant ( ') and dielectric loss factor (
') and dielectric loss factor ( ''). Figure 11 shows the dielectric constant and dielectric loss factor as a function of frequency. The Havriliak–Negami equation is used to analysis the dielectric spectra of the ER fluid [55]:
''). Figure 11 shows the dielectric constant and dielectric loss factor as a function of frequency. The Havriliak–Negami equation is used to analysis the dielectric spectra of the ER fluid [55]:
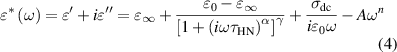
Figure 11. Dielectric spectra  '( a) and
'( a) and  ''( b) of the PNIPAM-[AMIm]Cl ER fluid at different temperatures.
''( b) of the PNIPAM-[AMIm]Cl ER fluid at different temperatures.
Download figure:
Standard image High-resolution imagewhere α and γ are the shape factors both in the range of 0 ∼ 1,  0 is the dielectric constant when the frequency is close to 0 and
0 is the dielectric constant when the frequency is close to 0 and  ∞ is that at a high-frequency limit. Δ
∞ is that at a high-frequency limit. Δ □ =
□ =  0 −
0 −  ∞ is the dielectric strength also considered achievable polarisability of ER fluids closely related the magnitude of the interparticle interaction [27, 56], σ is the DC-conductivity, and τHN represents the relaxation time expressed in terms of as the reciprocal of the frequency (fmax) where the maximum dielectric loss appears (i.e. τHN = 1/(2πfmax).
∞ is the dielectric strength also considered achievable polarisability of ER fluids closely related the magnitude of the interparticle interaction [27, 56], σ is the DC-conductivity, and τHN represents the relaxation time expressed in terms of as the reciprocal of the frequency (fmax) where the maximum dielectric loss appears (i.e. τHN = 1/(2πfmax).
Parameters in equation (4) are listed in table 1. For the PNIPAM-[AMIm]Cl ER fluid, as the temperature increases, Δ also increases, indicating the enhancement of interparticle strength. The relaxation time of the ER fluid shows the opposite tendency and decreases as the temperature increases, which means the response of the particles becomes faster with the increasing of temperature. This shows that, as the temperature increases, the polarisability (Δ
also increases, indicating the enhancement of interparticle strength. The relaxation time of the ER fluid shows the opposite tendency and decreases as the temperature increases, which means the response of the particles becomes faster with the increasing of temperature. This shows that, as the temperature increases, the polarisability (Δ ) of the particles increases and the relaxation time (τHN) decreases, resulting in better ER performance. Therefore, the analysis of dielectric spectra can confirm the results obtained from a comparison of their shear stress and storage modulus at different temperatures.
) of the particles increases and the relaxation time (τHN) decreases, resulting in better ER performance. Therefore, the analysis of dielectric spectra can confirm the results obtained from a comparison of their shear stress and storage modulus at different temperatures.
Table 1. The optimal values of the parameters in equation (4).
| Temperature (°C) | Δ
| α | γ | σdc | τHN( s) |
|---|---|---|---|---|---|
| 20 | 3.23 | 0.86 | 0.66 | 0.02 | 8.13 |
| 25 | 3.59 | 0.83 | 0.70 | 0.02 | 2.86 |
| 30 | 3.65 | 0.82 | 0.71 | 0.03 | 0.92 |
| 35 | 3.52 | 0.85 | 0.64 | 0.03 | 0.30 |
| 40 | 2.77 | 0.89 | 0.69 | 0.25 | 0.08 |
| 45 | 2.44 | 0.99 | 0.51 | 1.12 | 0.02 |
In figure 11, it is clear that the relaxation peaks of the ER fluid at different temperatures all locate in the low frequency range referring to the interfacial polarisation, which is caused by the dissociation of ion pair and the local diffusion of dissociated ions. Herein, the cationic ion [AMIm]+ covalently bonded on the polymer backbone is almost immovable; therefore, the interfacial polarisation is only associated with the movement of Cl-, however, the relaxation peak in this work is not in the above mentioned proper range (102–105Hz). To understand this, we calculated the activation energy (Ea) via fitting the experimental data by the Arrhenius equation:

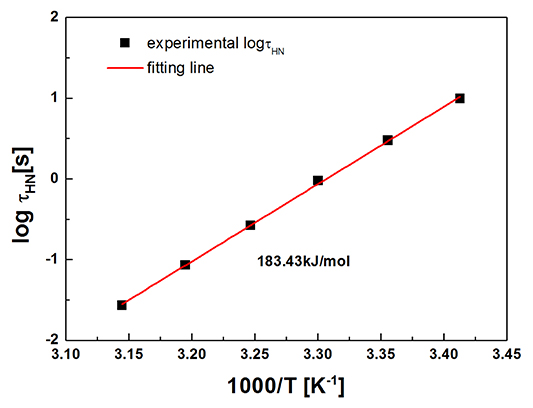
where R is the molar gas constant, T is the absolute temperature. The result is shown in figure 12 . Herein, Ea is related to the dynamics of ions in the interfacial polarization process. It is calculated to be 183.43 kJ mol−1, which is much higher than that presented by the PILs with organic counter ions. The high activation energy may be the reason for the low current density of the PNIPAM-[AMIm]Cl ER fluid under electric field as well. Nevertheless, temperature-induced swelling of the backbone provides larger space for the ions to move through, which enhances the ER effect of the ER fluid.
Figure 12. Temperature dependence of the relaxation time (τHN) of the PNIPAM-[AMIm]Cl ER fluid. The solid line is fitted by the Arrhenius equation.
Download figure:
Standard image High-resolution image4. Conclusions
A copolymer of PNIPAM-[AMIm]Cl was successfully synthesised, which combined the thermal sensitive segment and ionic liquid segment. The copolymer in silicone oil exhibited a significant temperature-dependence of its ER effect. As the temperature was increased from 5 °C to 50 °C, the
ER effect of the ER fluid increased. To explore the reason for this temperature-enhanced ER effect, temperature sweep and calorimetric analyses were conducted on the copolymer PNIPAM-[AMIm]Cl and pure polymers (PNIPAM and P[AMIm]Cl). These indicated that the higher shear stress at elevated temperature was attributed to the swelling of the PNIAM segment in the copolymer, which provided larger space for the mobile ions to pass through and resulted in stronger polarisability. Even though activation energy of the dielectric relaxation of the PNIPAM-[AMIm]Cl is in a relative high level, temperature-induced swelling of the PNIPAM segment makes the ER effect of the copolymer can be adjusted in a broad range by electric field and temperature. It is worth noting that the temperature range concerned in this study is narrow because high temperature could result in current leakage in ER fluid. For high temperature applications, proper chemical crosslinking should be employed in further study.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21872118, 51703193 and 21403186), Colleges and Universities Science and Technology Research Project of Hebei Province (No. QN2018107). One of the authors (HJC) was supported by National Research Foundation of Korea (2018R1A4A1025169).