Abstract
The long-term shape-recovery behavior of shape memory polymers has often been shown to be dependent on the length of time the material has been stored in the secondary shape. Typically, recovery performance and shape fixity will decrease with increased time in the secondary shape. In medical materials, a shelf-life is crucial to establish as it sets the upper threshold for device performance in a clinical setting, and a reduction in shape recovery would limit the development of SMP medical devices. Here, we present a two-year study of strain recovery, strain fixity, and shape recovery kinetics for passively and actively actuated SMPs intended for vascular devices. While kinetic experiments using immersion DMA indicate slight material relaxation and a decrease in the time to recovery, these changes are not found for bulk recovery experiments. The results indicate that a two-year shelf-life for these SMPs is very reasonable, as there is no change in the recovery kinetics, strain recovery, or strain fixity associated with this aging time. Further, a thermal accelerated aging test is presented for more rapid testing of the shape memory behavior of these SMPs and is compared with the real time aging results, indicating that this test is a reasonable indicator of the two-year behavior.
Export citation and abstract BibTeX RIS
Introduction
Shape memory polymers (SMPs) are a class of smart material that has gained attention in medical device development because of their ability to be delivered in a compressed, less invasive shape before undergoing shape recovery to the original, larger configuration. For many implantable medical devices, this less invasive delivery reduces trauma for the patient, which in turn reduces cost, recovery time, and surgical complications while simultaneously improving outcomes for both the patients and physicians. One of the limitations for any medical device is determining the shelf-life and the performance limitations over the lifespan of the product. For stimuli-responsive materials, such as SMPs, this is due to both the material degradation as well as the aging-related changes in device performance. Here we present the two-year study of porous polyurethane SMPs that were examined in both real-time and accelerated aging studies to determine the changes in shape fixity and shape recovery kinetics and how these relate to device performance for implantable vascular devices.
Accelerated aging of biomaterials has been traditionally performed using either solvent or thermal methods. The use of solvents and solutions for aging is one method of evaluating the performance of the material once it has been implanted, allowing for predictions of toxicological concerns and changes in material properties. Thermal methods have also been used for this, but they are much less accurate performance indicators. For evaluating the shelf-life and packaging of devices, however, thermal aging has successfully predicted long-term performance [1]. For various polymer systems, changes include mechanical property migration, thermal transitions, and even color of the material. For example, poly ether ether ketone and some polyethylene species display increases in elastic moduli and decreases in strain to failure [2–8]. By contrast, medical grade silicone elastomeric cylinders did not display altered mechanical properties over the course of four months of accelerated age testing for the equivalent of three years [9]. However, in many of these cases the temperature used for accelerated aging was found to be more important than the duration of the test, at least out to approximately 12–36 months [6]. While factors such as strain, solvent, temperature, and environmental conditions can be tailored, a key focus of medical devices is long-term storage, which can be limited to thermal aging in a controlled environment [9–11].
As mentioned previously, aging of materials is associated with a number of property changes, and some polyurethane systems have demonstrated spectroscopic indicators of these changes in addition to loss of physical properties [12–18]. Acceleration of porous, segmented polyurethane aging was shown to decrease hydrogen bonding as a result of rearrangement of the hard segments. The mobility of the soft segment allowed for relaxation of the material and a decreased concentration of physical crosslinks after aging [13]. Aging of polyurethane foams has been previously characterized through the use of x-ray diffraction on the hard segment in thermoplastic segments. Accelerated aging was found to have an impact on hard segments, but it may not necessarily affect the overall foam if the hard segment is not altered [16]. In certain thermoplastic polyurethanes foams, the diffusion of moisture into the polymer bulk will result in diminished thermo-mechanical properties. This is presumably due to the hydrolysis of the polymer chain at elevated temperatures in the presence of moisture [17]. The use of differential scanning calorimetry (DSC) has further been used to investigate the aging of several species of polyurethane foams, such as polyether urethanes and polyester urethanes [18]. A separate study of thermoplastic polyurethanes demonstrated an increase in the glass transition temperature (Tg) as measured by DSC over the course of aging, which is discussed further below. The Tg increased from −2.3 °C to 5.0 °C, and significant surface cracking was found using scanning electron microscopy (SEM) [19]. This increase in Tg was found in composites as well [20].
The impact of moisture and age as they relate to the mechanical properties of thermoplastic polyurethanes was examined by Boubakri et al who found that aging will be largely dependent on the time of exposure to moisture and that structural changes can be identified using SEM. It was found that failure analysis of mechanically tested samples denoted the extent of aging and could be used for determining the impact of conditions on the polymer integrity [21]. For polyurethane foams aged in a humid moisture and elevated heat (90 °C), surface analysis using Fourier transform infrared spectroscopy (FTIR) and SEM have demonstrated chemical and morphological changes [22].
Shape memory polymers have been proposed for a variety of medical devices due to their ability to change shape, length, or structure with the application of external stimuli [23–27]. PLA and PCL blends have been used to control the degradation of SMPs, with PCL undergoing scission of chain end groups that maintain the overall chain molecular weight (surface erosion) while the PLA undergoes chain scission (bulk degradation) [24]. The aging of shape memory polyurethane polymer foams has been investigated, with materials tested out to six months displaying a reduction in strain fixity under no load (no constraints). The materials also displayed a reduction in the total shape recovery achieved (reduction in strain recovery), although no recovery kinetic data was presented [28].
While strain has been shown to be a good method of aging materials, cyclic testing of SMPs at a temperature sufficiently above Tg (with the polymer allowed to equilibrate) demonstrated consistent strain recoveries of 85%–95%. In composite materials, this is limited by strains greater than 100% that result in delamination or fracture of fibers as well as by strains induced at temperatures below Tg. In these conditions, SMPs behave similarly to polymers that are not stimuli-responsive [29].
It has been found that in thermoset SMPs, the physical aging will result in relaxed networks that cause a delay in the onset of shape recovery [30]. Other authors have found that the rate of recovery is increased, with shape recovery achieved faster with aged samples but with reduced recoverable strain [31].
Theory: accelerated aging
The basis of accelerated thermal aging is the Arrhenius equation (1), which can be further simplified to the accelerated aging factor (AAF) equation (2)


For the AAF, Q10 is defined as the assumed aging factor, which is dependent on how aggressively the user anticipates the experiment to be relative to real-time aging. Typical values are 1.5 or 2.0 [32]. Lower values of Q10 are indicative of longer experimental times or a more realistic analysis of the effect of higher temperature on a system.
Some acceleration methods include humidity as a test parameter. While these tests will include fixed percentages of humidity, or thresholds that are not exceeded, the temperature profiles are maintained in accordance with the Van't Hoff Q10 rule [33]. In some medical materials, particularly in latex-based devices and products, humidity plays a large role in the shelf- and performance-lifespan, with humidity levels of approximately 65% shortening the lifespan of latex in burst-pressure testing from 5 yr to 3 yr at 20% humidity, both at room temperature [33]. For moisture-sensitive SMPs, excess humidity may result in polymer plasticization and shape recovery during accelerated aging, which would remove material functionality in the medical device. For this reason, humidity can be removed from testing protocols provided that packaging and storage of the final medical device takes this into account. Device shipping conditions should also be considered as the devices may be exposed to temperatures up to 52 °C during transport to the treatment facility [32].
Q10 = 2 has been suggested as an appropriate model for accelerated aging protocols. While these methods are simplistic, their application is appropriate when they are used reasonably and responsibly, according to some authors [35, 36]. For the use of AAF, several assumptions are in place, including constant activation energy (reasonable over a small range of temperatures used in medical device aging), a constant reactant concentration (reasonable for a low concentration of reactive species being used), and a gamma factor of 1 where appropriate (suitable for testing below 60 °C). Previous researchers suggest the aging temperature should be below any major thermal transition temperature and that the aging temperature should be kept below 60 °C unless a higher temperature is demonstrated to be appropriate, due to non-linear changes in the actual behavior that would otherwise be modeled by the AAF as linear above this temperature threshold [35, 36]. It is also suggested that a real-time component accompany the accelerated aging test.
For SMPs used in medical devices, one of two approaches is typically applied to initiate the shape change: passive actuation or active actuation [37, 38]. Passive actuation refers to shape recovery that will occur as the material comes to thermal equilibrium within the body [37, 38]. SMPs that utilize this will have a Tg that is sufficiently low for either only body heat to drive shape recovery or for a combination of body heat and moisture to lower the Tg and drive shape recovery [37–39].
Active actuation refers to shape recovery that requires an external stimulus to be applied directly by the user, typically at temperatures above body temperature [37, 38]. Solvent actuation in the presence of water allows for shape recovery at body temperature without the need for additional heating, a passive actuation [38–40]. Therefore, the simplest utilization of SMPs is passive actuation, but this provides a distinct limit for accelerated aging, as surpassing the upper threshold may result in the shape recovery of the material. Here we present a comparison of accelerated and real-time aging of actively and passively actuated SMPs, designed to examine the effects of long-term storage on the shape recovery, shape fixity, and kinetics of recovery.
Materials and methods
Materials
N,N,N',N'-tetrakis(2-hydroxypropyl)ethylenediamine (HPED, 99%, Sigma Aldrich), triethanolamine (TEA, 98%, Alfa Aesar), hexamethylene diisocyanate (98%, HDI, TCI America) and 2, 2, 4-trimethyl hexamethylene diisocyanate (98% TMHDI, TCI America, a mixture of 2, 2, 4 and 2, 4, 4 monomers) were used as monomers without further modification. Isopropyl alcohol (IPA, Sigma Aldrich) and reverse osmosis (RO) H2O were used for cleaning.
Synthesis
SMP foams were synthesized using a standard two-step method: isocyanate premix and alcohol premix syntheses. The isocyanate premix was made approximately 48 h prior to foaming, by mixing stoichiometric amounts of alcohols with HDI to achieve an OH/NCO ratio of approximately 0.35. The premix was then cured in an oven at 50 °C for 36 h and cooled to room temperature. At this time, the alcohol premix was made, comprised of the remaining alcohols, surfactants (8% wt., Dabco DC5943 and Dabco DC197, Air Products), tin catalyst (1% wt., Dabco T131, Air Products), amine catalyst (2% wt., Dabco BL-22, Air Products) and blowing agents (Enovate 245fa Blowing Agent, Honeywell Inc.) [41, 42]. The two premixes were mixed together using a high shear mixer and cured in an oven at 90 °C for 20 min. Foams were then allowed to cure at room temperature overnight, followed by qualitative analysis of pore homogeneity. Foams were cleaned using alternating sonication in RO water and IPA and were then dried overnight at 50 °C in a vacuum oven (30 in Hg) [38, 39, 41, 42].
This synthetic process was performed for both HDI- and TMHDI-based SMPs, denoted in the following text as HDI or TMHDI. The alcohol ratio (for 100% of hydroxl groups) was 60% HPED and 40% TEA, adjusted slightly during the isocyanate premix synthesis to control viscosity with the remaining measure of alcohols added with the alcohol premix [41, 42].
Fourier transform infrared spectroscopy
FTIR was used to confirm the polyurethane synthesis and to determine any aging effects due to accelerated or real-time aging. Samples were mechanically compressed and scanned using a Bruker ALPHA FTIR-ATR (Bruker, Billerica, MA) and were analyzed using OPUS software to determine peak identification, as well as for baseline and atmospheric corrections. Spectra were compared to previously reported spectra, as well as between original and aged samples [42].
Differential scanning calorimetry
DSC was performed with a Q200 TA DSC (TA Instruments, New Castle, DE). Samples of approximately 4.0 mg were submerged in 50 °C RO water for five minutes, manually dried, and sealed in TZero pans at room temperature and then were placed into the testing cell. Samples were examined using the following protocol: equilibration for 5 min at −40 °C, followed by heating to 120 °C at 10 °C min−1. The half-height transition is the reported Tg value. A peak associated with the freezing of water was found to occur with insufficiently dried samples.
Shape recovery
Shape memory was determined for samples through volume recovery experiments, which are reported as both volume recovery and strain recovery. Samples were cut into 2.5 mm diameter cylinders approximately 1 cm in length and strung on wire before being placed in a SC150-42 stent crimper (Machine Solutions, Inc., Flagstaff, AZ), heated to 100 °C and compressed radially before cooling to room temperature. The samples were stored in a sealed box with desiccant overnight and tested the next day. Volume recovery testing was performed in an RO water bath heated to 37 °C for HDI samples and 50 °C for TMHDI samples. Samples were imaged every 30 s for 30 min and were analyzed using Image J software (NIH, Bethesda, MD). Comparisons were made between constrained and unconstrained samples to determine shape fixity and strain recovery, defined by equations (3) and (4) below

Strain fixity (Rf(N)) is determined from the ratio of the fixed shape immediately after fixing and the strain of the fixed shape after it has relaxed at ambient conditions
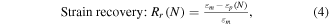

where  is the final strain after equilibration in solvent and
is the final strain after equilibration in solvent and  is the original strain of the material. The swelling ratio, S(N), is obtained using equation (5) from the ratio of the final geometry and the original geometry. The volume expansion ratio was also calculated using equation (6) below [42]
is the original strain of the material. The swelling ratio, S(N), is obtained using equation (5) from the ratio of the final geometry and the original geometry. The volume expansion ratio was also calculated using equation (6) below [42]

Aging: shape recovery
Samples were compressed to the same geometries as those previously discussed (Shape Recovery) and stored either constrained (heat shrink tubing pre-shrunk to the diameter of the compressed SMP) or without constraint over a wire. For accelerated aging tests, these samples were then placed into a vacuum oven heated to 55 °C and held isothermal for 90 days under vacuum (30 in Hg). Real-time samples were placed in a sealed box with desiccant at room temperature. Desiccant was changed monthly, and samples were stored for up to two years; sacrificial samples were tested at day 1, year 1, and year 2 (n = 4 at each time point).
Aging: dynamic mechanical analysis (DMA)
Thermomechanical analysis via DMA was performed with a Q800 TA DMA (TA Instruments, New Castle, DE) on dry, cylindrical samples (Diameter × Length: 2 mm × 4 mm). Foam samples were uncompressed and placed between two compression clamps in the DMA furnace for testing. Storage modulus (E') and loss modulus (E'') were used to calculate the tan δ (E'/E''), the maximum value of which was recorded as the Tg.
For relaxation experiments, samples were compressed to 90% strain at temperatures exceeding the material's Tg and cooled to room temperature [43]. Samples were stored unconstrained in desiccant for up to two years; sacrificial samples were tested at day 0, 1, 3, 15, 21, 28, 35, and year 1 and 2. The testing within the first month was used to determine the necessary time points for shape recovery testing (mentioned above), as well as the limits on the SMP relaxation. Origin software was used for data processing and analysis, with curve fitting used to smooth data and find the maximum values and inflection points.
The samples were tested with two experiments: kinetic relaxation and a thermal sweep. For both experiments, the frequency was 1 Hz. In the thermal sweep experiment, the samples were equilibrated at 0 °C and then heated at a rate of 2 °C min−1 to 75 °C while submerged in RO water. The tan δ peak was recorded as the Tg for each respective sample. For kinetic experiments, samples were submerged in 37 °C water and held isothermally for the duration of the test. Peak tan δ values and the inflection point of E' over time were taken as relaxation points [41]. Origin software was used for curve fitting and data analysis.
Results
Material characterization
SMPs were synthesized in 16 g batches and were cleaned using IPA and water. Spectroscopic signal comparison was also performed using FTIR (figure 1), which confirmed the formation of the polyurethane SMPs. The presence of the carbonyl peak at 1689 cm−1, the urea peak at 1640 cm−1, the skeletal carbon peak at 1253 cm−1, and the amine peaks found at 1137 and 1053 cm−1 were used for confirmation [42, 43].
Figure 1. Starting materials and FTIR spectra for (A) HDI- and (B) TMHDI-SMP foams at 0 days (1 day after synthesis, black line) and 2 yr (red line) real-time aging.
Download figure:
Standard image High-resolution imageThermal measurements were used to confirm that the synthesized compositions matched those used in previous studies (table 1). The increase in the Tg is due to the increased rigidity of the TMHDI molecule compared with the HDI molecule, due to the three methyl groups located at the 2 and 4 positions. The asymmetric/symmetric stretching and bending of the methyl groups are expected to occur at 2970-2950/2880-2860 cm−1 and 1470-1430/1380-1370 cm−1 [43]. The stretching vibrations appear as a set of three peaks for TMHDI, compared with the two peaks for HDI (figure 2). The bending bands, which are weaker, overlap with the urea bands in the 1500–1350 cm−1 region; this is sufficient to confirm the formation of TMHDI-based polyurethanes [43].
Table 1. Thermomechanical properties of HDI and TMHDI porous SMPs over the course of aging (n = 4).
| Isocyanate | Age | Wet Tg (DSC) (°C) | Wet tan δ (°C) |
|---|---|---|---|
| HDI | 1 day (0 yr) | 27.3 | 34.5 |
| year 1 | 26.9 | 33.3 | |
| year 2 | 26.9 | 33.4 | |
| year 1a | 26.9 | 33.6 | |
| year 2a | 26.9 | 33.5 | |
| TMHDI | 1 day (year 0) | 54.2 | 54.1 |
| year 1 | 53.9 | 52.3 | |
| year 2 | 54.2 | 52.5 | |
| year 1a | 53.9 | 52.1 | |
| year 2a | 54.2 | 52.6 |
Figure 2. HDI SMP recovery kinetics of a fitted curve to a representative immersion experiment, displaying normalized storage modulus for 0 yr and 2 yr aged foams (left), and the normalized tan δ for 0 yr and 2 yr aged foams (right), examined at 50 °C. (n = 3).
Download figure:
Standard image High-resolution imageShape recovery: DMA
Relaxation measurements of HDI SMPs examined at 50 °C indicate that for temperature ranges 20 °C above Tg (Tg + 20 °C) (DSC) or 15 °C above Tg (Tg + 15 °C) (DMA) there are no changes in the recovery kinetics. The average values to recovery onset, as determined by the peak tan δ and the inflection point of the storage modulus curves (figure 2), were approximately 22.5 s (E') and 143 s (tan δ). The tan δ was very narrow (full width half maximum (FWHM) of 149 s). Comparatively, at 37 °C, the FWHM was approximately 400 s (0 yr) to 371 section (2 yr) (figure 3).
Figure 3. Relaxation kinetics for HDI SMPs examined at 37 °C. Fitted curves of representative immersion trials are presented. (n = 3).
Download figure:
Standard image High-resolution imageAs expected, the time to the onset of relaxation and maximum tan δ was increased at 37 °C compared with 50 °C. In addition, the onset of recovery was found to be decreased for the 2 yr samples compared with the 0 yr samples (43.47 ± 13.32 s to the modulus inflection value for 2 yr decreased from to 58.11 ± 11.40 s for 0 yr, and 426.61 ± 8.93 s at 0 yr that decreases to 354.62 ± 7.66 at 2 yr for tan δ maximum).
Kinetic experiments and temperature sweeps for the TMHDI materials within the first month of real-time aging confirmed the trend that the material undergoes initial relaxation from the higher Tg to a slightly decreased wet Tg (54.1 °C–52.5 °C for TMHDI SMPs) within 72 h of shape fixation. There is no decrease in Tg after this, and while this does alter the shape recovery kinetics of the bulk samples tested (figure 4), the changes are less distinct compared with the HDI SMPs. The modulus slightly decreased for the 2 yr samples compared the original samples (61.05 s to peak modulus for the 0 yr sample and 47.65 s for the 2 yr). However, the tan δ indicates a longer time to recovery for the aged sample compared with the original (0 yr achieved relaxation in 24.9 s while 2 yr samples achieved relaxation in 50.5 s).
Figure 4. TMHDI kinetic experiments, displaying the fitted curves of normalized storage modulus (E', left) and the tan δ (right) tested at 50 °C. (n = 3).
Download figure:
Standard image High-resolution imageShape recovery: aging data
Shape recovery testing was compared between the accelerated aging samples at 0-year, 1-year and 2-year equivalent times (figure 5). As presented, there is no substantial variation in the recovery behavior between these aged samples at 50 °C; the tests at 37 °C demonstrated a similar behavior.
Figure 5. HDI-SMPs artificially aged to approximately year 1 (A) and year 2 (B) at 50 °C, with the full experiment (A1, B1), and during the initial five minutes of the experiment (A2, B2). (n = 3).
Download figure:
Standard image High-resolution imageReal-time aging samples for HDI samples aged to 2 yr displayed no changes in shape recovery behavior compared with the original sample, and no changes were found between samples that were constrained during the aging compared with those not constrained at 37 °C or 50 °C (figure 6). Recovery kinetic metrics were quantified using equations (3)–(6), and are presented in table 2.
Figure 6. HDI-SMPs aged in real time to approximately 2 yr at 37 °C (A) and 50 °C (B), with the full recovery time (A1, B1) and the initial five minutes of the experiment (A2, B2) displayed. (n = 3).
Download figure:
Standard image High-resolution imageTable 2. Shape recovery kinetics of aged HDI porous SMPs tested at 37 °C (n = 3).
| Isocyanate | Age | Time to 10% diameter (min) | Time to 50% diameter (min) | Time to 100% diameter (min) | Final diameter (mm) |
|---|---|---|---|---|---|
| HDI Control | 1 daya | 0.12 | 0.75 | 3.11 | 2.81 ± 0.07 |
| 1 day | 0.11 | 0.71 | 3.07 | 2.82 ± 0.03 | |
| HDI (constrained) | 2 yra | 0.11 | 0.74 | 3.14 | 2.79 ± 0.10 |
| 2 yr | 0.10 | 0.71 | 3.08 | 2.80 ± 0.09 | |
| HDI (unconstrained) | 2 yra | 0.12 | 0.77 | 3.12 | 2.85 ± 0.13 |
| 2 yr | 0.11 | 0.74 | 3.06 | 2.73 ± 0.08 |
As expected, the time to complete volume recovery was faster for HDI in the 50 °C water bath compared with the 37 °C bath. There also were no distinct changes in the recovery rates comparing the aged and original samples, a trend that also displayed no changes depending on storage conditions. TMHDI samples, when aged to 2 yr and tested at 50 °C, displayed no differences between aged samples, nor were there substantial differences between the constrained and unconstrained samples.
Comparison of the general behavior, including storage relaxation and actuation, as well as final diameter of the material (including swelling), are presented in table 3. Similar swelling ratios were achieved for all tested materials, regardless of age or aging method. Samples that were aged via acceleration and were unconstrained demonstrated slightly reduced strain fixity compared with samples aged in real time.
Table 3. Shape memory properties of HDI porous SMPs over the course of aging at 37 °C testing (n = 3).
| Isocyanate | Age | Strain fixity (%) | Strain recovery (%) | Swelling ratio (%) |
|---|---|---|---|---|
| HDI | 1 day | 99.8 | 100 | 12.4 |
| HDI (constrained) | 2 yr | 99.9 | 100 | 11.6 |
| 2 yra | 99.8 | 100 | 12.0 | |
| HDI (unconstrained) | 2 yr | 99.0 | 100 | 14.0 |
| 2 yra | 99.7 | 100 | 9.2 |
Discussion
FTIR comparisons of spectra (figure 1) indicate a slight shoulder formation at approximately 970 cm−1, indicative of slight oxidation of the amine to a N-oxide. N-oxide formation occurs at approximately 970 cm−1 (N–O band vibration), and secondary and tertiary bands were found at approximately 950 cm−1 and 935 cm−1, respectively [44]. This would result in a slightly decreased Tg and decreased time to recovery at temperatures that are approximately plasticized Tg (T ∼ Tg). For temperatures greater than 15 °C above plasticized Tg (15 °C + Tg), as with the HDI tested at 50 °C, the thermal contribution would be more significant than the increased plasticization and hydrophilicity, resulting in similar recovery profiles.
Accelerated aging of the materials at 55 °C did not seem to induce any additional spectroscopic changes, which is somewhat expected, as the materials were aged under vacuum; typical methods for storing medical devices include vacuum-sealed pouches, with desiccant or another method of controlling humidity as a part of the packaging [32–35]. Without the presence of oxygen or a radical source, the elevated temperature would not result in significant reactions, considering that the temperature is still low compared with the thermal decomposition temperature of the polyurethanes (approximately 240 °C) [41]. This would limit the N-oxide formation. If the materials were aged at ambient moisture conditions with elevated temperature, it is expected that some oxidation would occur, resulting in formation of N-oxides.
Hydroxyl group formation has also been associated with aging in polymers, denoted by the presence of broad bands around 3400 cm−1 when examined using FTIR; these bands are attributed to hydroxyl groups, either as a result of oxidation during storage or moisture penetration into the bulk [45]. For the accelerated aging samples examined in this study, no moisture would be present in the environment to penetrate the SMPs and result in the hydroxyl peak formation as a result of moisture or oxygen. The real-time samples, which were stored with desiccant as opposed to vacuum, were still exposed to oxygen and some moisture, although there was no noticeable hydroxyl formation, only the oxide formation. This was an important consideration during the experimental design, as the combination of elevated temperature and presence of moisture could result in the shape recovery of the materials, with the HDI SMP being the most susceptible due to its lower Tg.
One of the major considerations for accelerated testing of stimuli responsive materials in medical devices is the driving mechanisms or forces for the responses. In the case of SMPs, these driving forces can be temperature (as explored here), as well as moisture, solvent, electric field application, magnetic field application, or light. In some types of polymers, these stimuli overlap or can have synergistic effects, such as temperature and moisture. The permanent shape in thermoset polymers is determined by the structure during synthesis, as this is a maximized point of entropy of the material. Deformation results in decreased entropy, which can be fixed through shape programming, but ultimately the material will eventually return to the original shape. The fixed shape geometry is a point of relative entropic maxima, which allows for long-term shape setting, as opposed to the absolute maxima obtained during synthesis [25, 27]. Hydrogen bonding, such as occurs in polyurethane and polyurea formation, is the cause of this relative maximum, and the physical bonds that form help fix the shape. However, in the presence of moisture, the hydrogen bonding is disrupted, the mobility of the polymer chains increases, and the temperature required for the SMP to undergo shape recovery is decreased from the dry Tg. For this reason, there are considerations to be made for accelerated testing of stimuli responsive materials, because while the aging process itself may not impact the performance, the elevated temperature for testing may result in shape recovery and skewed results with regard to shape fixity.
As mentioned previously, urethane and urea bonds can be plasticized, allowing for greater mobility at a lower-than-expected Tg. Amides, esters, and anhydrides, as well as others, have been found to possess depressed Tg's when exposed to moisture. Similar materials possessing stimuli-responsive characteristics, especially thermally driven shape-changing materials such as those presented here, will be limited to an upper thermal limit during accelerated aging. This boundary will not only be due to the accuracy of the acceleration prediction but also due to the device functionality. For shape set (in the secondary, temporary shape) SMPs, this will be a distinct limitation for accelerated testing, which will limit how quickly shelf-life testing can be performed on devices.
Comparison with thermoplastic SMPs indicates that while some relaxation, in addition to thermomechanical property migration, is expected in thermoplastic SMPs, this is not the case for thermoset materials. Lorenzo et al noted that accelerated and real-time aging in thermoplastic polyurethane SMPs results in changes in the micro-hardness, as well as the Tg (measured using DSC) and that this will be a limiting factor for utilization of such materials in medical devices [46].
Choi et al found that in amorphous SMP networks (thermoset SMPs), there is a change in the recovery response of unconstrained materials after aging, with the aged materials displaying a faster response at a higher temperature. However, it was noted that there are optimal storage temperatures for these materials that will limit the relaxation but increase the rate of recovery, which was a goal of the study. While the authors did not examine this, it is also expected that due to the structure of the polymer examined by Choi there are long chains between the crosslinks that will act more similarly to thermoplastic than thermoset polymers in those regions [31].
Variation in recovery kinetics, such as those presented in figures 5 and 6, are similar to those found by Tey et al and Boyle et al [39, 43]. The material response was found to be somewhat variable (still within less than a standard deviation) but not identical between repeated recovery tests using aged materials as well as those immersed in a variety of environmental conditions [39, 43]. For porous materials, the macrostructure contributes to the recovery kinetics and will not be identical between samples, which will greatly influence the behavior between samples. The porous SMPs are produced in a gas-blowing process, relying on the viscosity of the premix, the surfactants and surface tension, and the concentration of blowing agents to define the pore morphologies. Even this is only an approximation, as the pore morphology, size, and size distribution will vary based on the location of the sample taken in reference to the geometry used during synthesis. These differences in the macrostructure result in differences in the recovery rates (within a standard deviation) [43]. As demonstrated in table 3, these differences will result in variances in the recovery kinetics, with the overall trend remaining the same with aging, similar to results with SMPs based on TMHDI and isophorone diisocyanate (IPDI) investigated previously [43].
The 2-year TMHDI tan δ plots display a different trend compared with the other tested samples, which may be the result of similar macrostructure differences. However, based on the obtained data using DMA, it is expected that the E' plot is more representative of the kinetic behavior (figures 3 and 4). Bulk shape recovery, similar to the HDI samples, did not vary with the changes in DMA kinetics.
The shape fixity of greater than 99% has been found with many other SMP systems, such as PLA/PGA, although many polymer systems, such as poly(propylene sebacate), methacrylate-based polymers/copolymers, and polycaprolactone-based systems, may display reduced strain fixity [25, 31, 47–51]. The shape recovery (% strain recovered) is also widely varied. Porous SMPs examined by Tey et al displayed excellent strain fixity (not quantified but stated as total) and 100% strain recovery, implying that for polyurethanes the intermolecular forces are a major factor for both strain fixity and strain recovery [25]. Tobushi examined long term storage of porous SMPs, and found that out to six months without mechanical constraint shape recovery and fixation were approximately 99% and 98%, respectively [53, 54]. As mentioned by Lendlein and Kelch, many SMP systems are polyurethanes, due in part to the biphasic nature of the thermoplastic polymer coupled with the hydrogen bonding that occurs with the urethane linkage [25]. These secondary forces present in polyurethanes allow for tailoring of strain and stress recoveries, with thermoplastic systems possessing decreased strain recovery with increased recoverable strains; these systems are also limited as heating the SMP above its Tg while maintaining a mechanical load will influence the shape recovery kinetics and strain [25, 53, 54]. However, the swelling that is displayed in these porous SMPs is a behavior that will occur in many polymer systems, but it is more noticeable in porous polymer systems [39]. This swelling behavior is useful for vascular devices used for occlusion and embolization, as it allows devices to be delivered in a manner that is in a minimally invasive manner and ultimately expand to fill very large volumes [37–39]. The use of solvents, specifically H2O, allow for the rapid recovery of SMPs that would otherwise retain their secondary shape at body temperature, providing the means to deploy the device, which will further occlude the vessel through swelling at the decreased or plasticized Tg [38–42, 52–54].
Conclusions
This work presents an accelerated aging method and comparison of the shape recovery behavior of polyurethane SMPs synthesized from aliphatic diisocyanates and multi-functional amino alcohols. For the two diisocyanates used, the recovery kinetics of the bulk material did not noticeably change with aging, either in real time or accelerated conditions. Using DMA, a decrease in the time to relaxation of the E' and tan δ representative phase change was found in the aged samples. The cause of this was attributed both to the oxidation of the material, as well as possible moisture presence and the relaxation of the material after compression over the 2 yr period. Overall, it appears that the use of accelerated aging to test thermoset SMPs out to at least two years is possible using temperatures up 55 °C. As discussed here, higher temperatures may result in unwanted shape recovery, as well as additional oxidation, and this is a limiting factor in testing medical-oriented SMPs in device configurations. Despite these limitations, it appears that the presented SMPs have a shelf-life of at least two years based on retention of shape recovery, shape fixity, and structural characteristics.
Acknowledgments
The authors would like to thank Dr Todd L Landsman for discussion of shape memory polymer behavior and their utility in medical devices, and Lauren Harrell for editing the manuscript. We also are appreciative of Dr Jeffery E Raymond's expertise and consultation with using immersion DMA, as well as the Laboratory for Synthetic-Biologic Interactions at Texas A&M University. We gratefully acknowledge our funding sources, including the NASA H Jenkins Fellowship (NNX15AU29H), the National Institutes for Health /National Institute of Biomedical Imaging and Bioengineering Grant R01EB000462. This work was partially funded by the NIH National Institute of Neurological Disorders and Stroke Grant U01-NS089692. The authors would also like to thank the Texas A&M University graduate diversity fellowship and the Department of Biomedical Engineering for funding.







