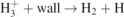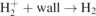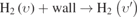Abstract
Complex simulations (e.g. multi-dimensional fluid models) usually prevent the use of very large chemistry models. In this work, the important physicochemical processes in a volume-production high-current negative hydrogen ion source (HCNHIS) are identified for a pressure range of 1–100 Torr using a global model. The particle species include H2(υ = 0–14), H(n = 1–3),  ,
,  , H+, H−, and electrons. The simulation results indicate that for the production of negative hydrogen ions through dissociative attachments, the high vibrational levels of hydrogen molecules are important at low pressures, and the ground state and low vibrational levels of hydrogen molecules play an increasingly important role with increasing pressure. The reactions involving the ground state, low vibrational levels, and neighboring levels of each level tend to dominate the production of vibrational levels and transitions between them in the volume-production HCNHIS. With increasing pressure, the electron energy dissipation shifts from dissociation of H2(υ = 0) and electron excitation of H2(υ = 0) through an indirect mechanism followed by radiative decay (the EV process) to electron excitation of H2(υ = 0) through a resonant mechanism (the eV process). A valuable subset of reactions provided by the simplified model is proposed in the investigated pressure range, which could be incorporated in more complex simulations. The results obtained with the simplified model under different simulation conditions are within an acceptable error margin of the results for the full model, which indicates that the robustness of the simplified model of chemical reactions is guaranteed to some extent.
, H+, H−, and electrons. The simulation results indicate that for the production of negative hydrogen ions through dissociative attachments, the high vibrational levels of hydrogen molecules are important at low pressures, and the ground state and low vibrational levels of hydrogen molecules play an increasingly important role with increasing pressure. The reactions involving the ground state, low vibrational levels, and neighboring levels of each level tend to dominate the production of vibrational levels and transitions between them in the volume-production HCNHIS. With increasing pressure, the electron energy dissipation shifts from dissociation of H2(υ = 0) and electron excitation of H2(υ = 0) through an indirect mechanism followed by radiative decay (the EV process) to electron excitation of H2(υ = 0) through a resonant mechanism (the eV process). A valuable subset of reactions provided by the simplified model is proposed in the investigated pressure range, which could be incorporated in more complex simulations. The results obtained with the simplified model under different simulation conditions are within an acceptable error margin of the results for the full model, which indicates that the robustness of the simplified model of chemical reactions is guaranteed to some extent.
Export citation and abstract BibTeX RIS
1. Introduction
Neutral beam injection (NBI) is one of the most reliable auxiliary heating and current drive systems used in fusion reactors for thermonuclear fusion [1, 2]. An NBI system for a fusion reactor needs to be capable of producing neutral hydrogen or deuterium beams with particle energies up to the MeV range [3]. At MeV energies, the neutralization efficiency for negative hydrogen ions extracted from an ion source is about 60%, whereas for positive ions the neutralization efficiency falls to negligible values at energies above 200 kV [4]. It is therefore necessary to develop an NBI system based on negative hydrogen ion sources (NHIS) instead of positive ion sources in a fusion reactor, due to the higher neutralization efficiency of NHIS at high energies.
Negative hydrogen ions are mainly created either by collisions of energetic hydrogen atoms and positive ions with surface plasma source electrodes based on surface processes [5, 6] or by binary collisions between electrons and hydrogen molecules based on volume processes [7, 8]. In surface processes, the energetic hydrogen atoms and positive ions collide with the surface covered with a low-work-function material (typically cesium), and they are then reflected back as negative hydrogen ions by absorbing electrons from the surface coated with cesium. In volume processes, the negative hydrogen ions are created in the plasma volume through a two-step process. The vibrationally excited hydrogen molecules are firstly produced through electron excitations, and negative hydrogen ions are then produced through electrons attaching to vibrationally excited hydrogen molecules in the process of dissociative attachment. At present, most of the radio-frequency (RF) inductively coupled NHIS are operated with the help of cesium [9–12], because the significantly higher production efficiency of negative hydrogen ions, as compared to that of the two-step volume process, is capable of meeting the requirements of fusion reactors such as ITER. However, the use of cesium may cause problems in maintenance and also unstable source operation, which is an undesirable process in ITER.
In order to guarantee the survivability of negative hydrogen ions, a very low pressure of 0.3 Pa is required inside the RF inductively coupled NHIS [13]. Such a design of low-pressure operation leads to the lack of electrons necessary for dissociative attachment reactions if only the volume process is taken into account. Researchers have investigated alternatives to the present RF inductively coupled NHIS, due to the limitations of the source configuration in terms of volumetric production of negative hydrogen ions. Fantz et al proposed that helicon coupled devices may be suitable alternatives, because very high electron densities can be achieved in helicon sources with rather low RF powers [14]. Subsequently, Corr et al confirmed the initial feasibility of helicon devices as NHIS for the NBI systems of ITER [15]. RF inductively coupled plasma sources have potential advantages over high density wave-heated sources, including simplicity of concept, and no requirement for dc magnetic fields (as required for helicons). Averkin et al proposed a high-current negative hydrogen ion source (HCNHIS) driven by an RF inductive coil [16, 17]. Compared with the previously mentioned RF inductively coupled NHIS, the HCNHIS is a new design based on the volumetric production mechanism of negative hydrogen ions without additional cesium. It consists of a high-pressure (2–65 Torr) radio-frequency discharge (RFD) chamber, a nozzle, and a low-pressure (1–15 mTorr) negative ion production (NIP) chamber. In the RFD chamber, the high densities of the vibrationally excited states of the hydrogen molecules and the low-energy electrons can be produced due to the frequent electron-heavy particle collisions. The hydrogen plasma flow in the RFD chamber is reduced by a series of bypass tubes and enters through a nozzle into the NIP chamber where negative hydrogen ions are mainly created by the dissociative attachment of low-energy electrons to vibrationally excited hydrogen molecules. The HCNHIS is capable of achieving more low-energy electrons in the production of negative hydrogen ions, and has been initially confirmed to achieve a high negative hydrogen ion current (90 A m−2) [17].
Averkin et al developed a global enhanced vibrational kinetic model (GEVKM) with quite comprehensive reaction set to numerically study the RF hydrogen discharge in the HCNHIS [16, 17]. Even if the computational efficiency of the global model is weakly dependent on the large chemistry models, a better understanding and simplification of chemical dynamics of this kind of discharge can achieve the study of plasma transport properties with a self-consistent description of negative hydrogen ion production in more complex simulations (e.g. multi-dimensional fluid models) and benefit further optimization of the HCNHIS. This study aims to unravel the role that chemistry played in the HCNHIS using the global model for negative hydrogen ion sources (GMNHIS) [18]. Fundamental conservation laws used in the formulation of the GMNHIS include mass conservation described via a particle balance for each species, charge conservation reduced to the quasi-neutrality condition, and energy conservation law expressed through power balance. The GMNHIS has been benchmarked against the GEVKM [17] and validated using experimental measurement data obtained in an electron cyclotron resonance discharge [19]. GMNHIS is capable of achieving very high computational efficiency even if more than 1000 chemical reactions are taken into account. In our previous study, a detailed evaluation of chemical reactions contributing to the creation and loss of vibrational levels of hydrogen molecules was performed at low pressures (less than 20 mTorr) according to GMNHIS [20]. It was found that the combined effects of different kinetic processes responsible for forming the vibrational distribution function (VDF) of hydrogen molecules could be decoupled, and a convenient analytical model for the VDF (i.e. a reduced linear model, RLM) was therefore developed. The RLM may not be applicable to the high-pressure discharges such as the HCNHIS. Moreover, a simplified chemical reaction set not only for the VDF but for all particle species is required in complex simulations where the use of large chemistry models may significantly reduce the computational efficiency.
In this study, the reactions contributing to the creation and loss of all particle species, i.e. H2(υ = 0–14), H(n = 1–3),  ,
,  , H+, H−, and electrons, are evaluated in detail using the GMNHIS. We analyze the reaction mechanisms of H2(υ = 0–14), H−, and electrons in the main text, and that of other particle species in the
, H+, H−, and electrons, are evaluated in detail using the GMNHIS. We analyze the reaction mechanisms of H2(υ = 0–14), H−, and electrons in the main text, and that of other particle species in the
2. Description of the model
The GMNHIS is employed to study the importance of different reactions contributing to the creation and loss of each particle species included in the model. A brief description of the GMNHIS as well as improvements and modifications compared with previous hydrogen plasma models are presented here, and the details of the GMNHIS can be found elsewhere [18]. The GMNHIS is also called a zero-dimensional model because it does not solve numerically for the spatial variation of plasma properties, but rather relies upon an analytical solution for the respective profiles. The power is assumed to be deposited uniformly into the plasma bulk. A Maxwellian EEDF is assumed in the GMNHIS. The densities of all particle species and the electron temperature are determined by particle balance equations, a quasi-neutrality condition, and a power balance equation.
GMNHIS is the first to perform quite a comprehensive benchmarking test of this kind for models of NHIS. Benchmarking against other code, i.e. the GEVKM [16, 17], has helped to fix a number of mistakes in the code. The initial chemical reaction data were collected separately for both codes and revealed some inconsistencies in the published literature. Therefore, the chemical reaction data used in the GMNHIS are carefully evaluated. The wall de-excitation rate coefficient of vibrational states, i.e. ![${k}_{s,\mathrm{w}\mathrm{a}\mathrm{l}\mathrm{l}}^{d}={\left[{{\Lambda}}^{2}/{D}_{\mathrm{e}\mathrm{ff},s}+2V\left(2-{\gamma }_{s}\right)/A{v}_{s}{\gamma }_{s}\right]}^{-1}$](https://content.cld.iop.org/journals/0963-0252/30/6/065027/2/psstac02aeieqn5.gif) , used in the GEVKM [16, 17] caused overestimation of the loss of vibrational states at high pressures. Therefore, it was modified in the GMNHIS. For the wall loss of vibrational states, γs
= 1 has been assumed. This indicates that the H2(υ) particles are always de-excited in collisions with the walls. The rate coefficient for the repopulation of vibrational states of H2(υ) molecules flowing from the walls is given as
, used in the GEVKM [16, 17] caused overestimation of the loss of vibrational states at high pressures. Therefore, it was modified in the GMNHIS. For the wall loss of vibrational states, γs
= 1 has been assumed. This indicates that the H2(υ) particles are always de-excited in collisions with the walls. The rate coefficient for the repopulation of vibrational states of H2(υ) molecules flowing from the walls is given as  , where
, where  is the repopulation coefficient of vibrational states of H2 molecules reflected from the walls, and υ' is any higher vibrational level than υ. This diffusion coefficient, Deff,s
in the GMNHIS is different from that in the work of Huh et al [21] where the Knudsen diffusion seems to be double-counted. The second term of the expression for the wall de-excitation rate coefficient of vibrational states, i.e.
is the repopulation coefficient of vibrational states of H2 molecules reflected from the walls, and υ' is any higher vibrational level than υ. This diffusion coefficient, Deff,s
in the GMNHIS is different from that in the work of Huh et al [21] where the Knudsen diffusion seems to be double-counted. The second term of the expression for the wall de-excitation rate coefficient of vibrational states, i.e.  , has been found to have the same form as the Knudsen diffusion when γs
is equal to one. The Knudsen diffusion is therefore not included in diffusion coefficient Deff,s
again.
, has been found to have the same form as the Knudsen diffusion when γs
is equal to one. The Knudsen diffusion is therefore not included in diffusion coefficient Deff,s
again.
In the previous study, the chamber structures are conventional cylindrical or cubic [18, 20]. In this study, the chamber structure is modified based on the HCNHIS-1 [17] where one nozzle (∅ = 0.381 mm) and five bypass tubes (∅ = 0.762 mm) are included. The particle flow rates at the inlet and outlets of nozzle and bypass tubes can be found in the work of Averkin et al [17]. A schematic diagram of HCNHIS (HCNHIS-1) is shown in figure 1, where the driver chamber has diameter D = 1.745 cm and length L = 7.39 cm. The simulations are performed for the driver region with diameter D = 1.745 cm and effective length Leff = 7.684 cm [17]. This effective cylindrical volume accounts for the cylindrical and conical parts of the RFD chamber as shown in figure 1. The absorption power is 590 W and the neutral gas temperature is set to be 1400 K [17]. These parameters are kept constant unless otherwise specified in this paper.
Figure 1. Schematic diagram of HCNHIS.
Download figure:
Standard image High-resolution imageTables 1 and 2 respectively present the chemical reactions excluding and including the vibrationally excited hydrogen molecules. Table 3 presents the surface reactions. The reaction set mainly refers to the previous work [18], and some reactions (i.e. nos. 38 and 39 in table 1 and nos. 1106–10 in table 2) that apply for high-pressure discharges [16, 17] are added into this work. The cross sections of chemical reactions used here are adopted from the recently published studies [22–39]. The rate coefficients of surface reactions refer to the published studies [40–43].
Table 1. Chemical reactions excluding vibrationally excited hydrogen molecules.
| No. | Reaction | Note | Reference |
|---|---|---|---|
| 1 | e + H2 → e + H2 | S | [22] |
| 2 | e + H → e + H | [23] | |
| 3 | e + H2 → 2e + H+ + H | S | [24] |
| 4 |
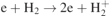
| S | [22] |
| 5 | e + H2 → e + 2H | S | [22] |
| 6–7 |

| S | [24] |
| 8 | e + H2 → H− + H | S | [25] |
| 9 | e + H → 2e + H+ | S | [25] |
| 10–11 |

| S | [24] |
| 12 |

| S | [24] |
| 13–14 |
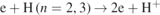
| [24] | |
| 15 |
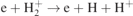
| [25] | |
| 16 |
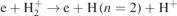
| [24] | |
| 17 |

| [26] | |
| 18 |
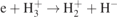
| [27] | |
| 19 |
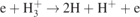
| [24] | |
| 20 |
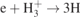
| S | [26] |
| 21 |

| [26] | |
| 22 | e + H− → H + 2e | S | [24] |
| 23 |
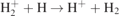
| S | [26] |
| 24 |
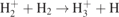
| S | [28] |
| 25 | H+ + H− → 2H | [29] | |
| 26–27 | H+ + H− → H + H(n = 2, 3) | [30] | |
| 28 |
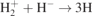
| [29] | |
| 29 |
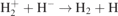
| [30] | |
| 30 |
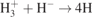
| S | [29] |
| 31 |
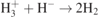
| S | [30] |
| 32 | H− + H → e + H2 | S | [30] |
| 33 | H(n = 3) → H(n = 2)+hν | S | [31] |
| 34–35 |
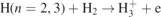
| S | [32] |
| 36–37 | H(n = 2, 3)+H2 → 3H | S | [32] |
| 38 |
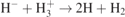
| S | [33] |
| 39 |

| S | [30] |
| 40–46 |

| [22] |
a.
S represents the reactions considered in the simplified model. b.
 .
.
Table 2. Chemical reactions involving vibrationally excited hydrogen molecules.
| No. | Reaction | Full model | Simplified model | Reference |
|---|---|---|---|---|
| 1–210 | e + H2(υ) → e + H2(υ') |
![$\begin{gathered}\upsilon ,{\upsilon }^{\prime }=\left[0,14\right] ;\\ {\upsilon }^{\prime }\ne \upsilon \end{gathered}$](https://content.cld.iop.org/journals/0963-0252/30/6/065027/2/psstac02aeieqn32.gif)
|
![$\begin{gathered}\upsilon =0;{\upsilon }^{\prime }=\left[1,6\right]\\ \upsilon =\left[1,3\right] ;{\upsilon }^{\prime }=\left[\upsilon -1,5\right]\\ \upsilon =\left[4,6\right] ;{\upsilon }^{\prime }=\upsilon {\pm}1\\ \upsilon =7;{\upsilon }^{\prime }=6\end{gathered}$](https://content.cld.iop.org/journals/0963-0252/30/6/065027/2/psstac02aeieqn33.gif)
| [34, 35] |
| 211–435 | e + H2(υ) → e + H2(υ') + hν | υ,υ' = [0, 14] | υ = 0; υ' = [0, 14] | [36] |
| 436–49 |
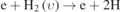
| υ = [1, 14] | υ = 1 | [37] |
| 450–63 |
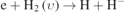
| υ = [1, 14] | υ = [1, 10] | [25] |
| 464–673 |
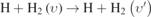
|
![$\begin{gathered}\upsilon ,{\upsilon }^{\prime }=\left[0,14\right] ;\\ {\upsilon }^{\prime }\ne \upsilon \end{gathered}$](https://content.cld.iop.org/journals/0963-0252/30/6/065027/2/psstac02aeieqn37.gif)
|
![$\begin{gathered}\upsilon =0;{\upsilon }^{\prime }=1\\ \upsilon =1;{\upsilon }^{\prime }=0\\ \upsilon =\left[2,5\right] ;{\upsilon }^{\prime }=\upsilon {\pm}1\\ \upsilon =\left[6,7\right] ;{\upsilon }^{\prime }=\upsilon -1\end{gathered}$](https://content.cld.iop.org/journals/0963-0252/30/6/065027/2/psstac02aeieqn38.gif)
| [38] |
| 674–1093 |
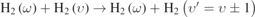
|
![$\begin{gathered}\omega ,\upsilon =\left[0,14\right] ;\\ {\upsilon }^{\prime }=\left[0,14\right]\end{gathered}$](https://content.cld.iop.org/journals/0963-0252/30/6/065027/2/psstac02aeieqn40.gif)
|
![$\begin{gathered}\omega =0;\upsilon =\left[0,14\right] ;\\ {\upsilon }^{\prime }=\upsilon {\pm}1\\ \omega =1;\upsilon =\left[5,10\right] ;\\ {\upsilon }^{\prime }=\upsilon -1\end{gathered}$](https://content.cld.iop.org/journals/0963-0252/30/6/065027/2/psstac02aeieqn41.gif)
| [30] |
| 1094–8 |
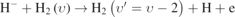
| υ = [2, 6] | υ = 2 | [39] |
| 1099–103 |
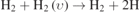
| υ = [10, 14] | υ = [10, 14] | [30] |
a.Specific vibrational levels considered in the full model. b.Specific vibrational levels considered in the simplified model.
3. Results and discussion
3.1. Evaluation of physicochemical processes of basic particle species
The relative contributions of the reactions to the creation and loss of particle species (i.e. H−, electrons,  ,
,  , H+, and H(n = 1–3)) will be studied. For certain NHIS, special attention is usually paid to the production of H−. Therefore, only the relative contributions of different reactions to the creation and loss of H− as well as electrons are shown in the main text, while the detailed discussions on the evaluation of physicochemical processes of
, H+, and H(n = 1–3)) will be studied. For certain NHIS, special attention is usually paid to the production of H−. Therefore, only the relative contributions of different reactions to the creation and loss of H− as well as electrons are shown in the main text, while the detailed discussions on the evaluation of physicochemical processes of  ,
,  , H+, and H(n = 1–3) can be found in the
, H+, and H(n = 1–3) can be found in the
The relative contributions of the reactions to the creation and loss of H− as well as electrons at different pressures are shown in figures 2(a) and (b). Figure 2(a) shows that the dissociative attachments of the ground state and low vibrational levels dominate the creation of H− at relatively high pressures, for example, dissociative attachments of H2(υ = 0) and H2(υ = 1) respectively contribute 58.4% and 26.5% (84.9% in total) at 100 Torr, while the high vibrational levels contribute more at relatively low pressures, for example, dissociative attachments of H2(υ = 6–9) individually contribute 13.4%, 24.0%, 29.9%, and 17.4% (84.7% in total) at 1 Torr. The dissociative attachments of H2(υ = 11–14) contribute very little in the investigated pressure range due to very low densities of H2(υ = 11–14) (see figure 3), and they can therefore be neglected.
Figure 2. Relative contributions of various reactions to the creation and loss of (a) H− ions and (b) electrons at different pressures.
Download figure:
Standard image High-resolution imageFigure 3. VDF at different pressures.
Download figure:
Standard image High-resolution imageThe associative detachment by hydrogen atoms, i.e. H− + H → e + H2 (no. 32 in table 1), dominates the loss of H− with a contribution of 50.5%–87.8% in the investigated pressure range. It is very different from the case in the usual NHIS operated at low pressures [18], where the mutual neutralization of  with H− is the most important channel. This is because there is around a one order of magnitude higher density of hydrogen atoms in the HCNHIS than in the usual NHIS [18]. The contributions of mutual neutralizations of H− with
with H− is the most important channel. This is because there is around a one order of magnitude higher density of hydrogen atoms in the HCNHIS than in the usual NHIS [18]. The contributions of mutual neutralizations of H− with  , i.e.
, i.e.  ,
,  and
and  (nos. 30, 31 and 38 in table 1), show a similar trend with increasing pressure, and the maximum contribution (
(nos. 30, 31 and 38 in table 1), show a similar trend with increasing pressure, and the maximum contribution ( ) is 22.7% at 1 Torr. Electron detachments, i.e. e + H− → H + 2e and
) is 22.7% at 1 Torr. Electron detachments, i.e. e + H− → H + 2e and  (no. 22 in table 1 and no. 1094 in table 2), are respectively responsible for the loss of H− at relatively low and high pressures, for example, reaction e + H− → H + 2e contributes 10.4% at 1 Torr. Electron detachments of H2(υ > 2) (nos. 1095–1098 in table 2) can be neglected due to their small contributions in the investigated pressure range.
(no. 22 in table 1 and no. 1094 in table 2), are respectively responsible for the loss of H− at relatively low and high pressures, for example, reaction e + H− → H + 2e contributes 10.4% at 1 Torr. Electron detachments of H2(υ > 2) (nos. 1095–1098 in table 2) can be neglected due to their small contributions in the investigated pressure range.
Figure 2(b) shows that in addition to the ionization of H2, i.e.  (no. 4 in table 1), with the minimum contribution of 26.2% at 100 Torr and the maximum contribution of 42.8% at 1 Torr, the collisions between neutral species H(n = 2) and H2, i.e.
(no. 4 in table 1), with the minimum contribution of 26.2% at 100 Torr and the maximum contribution of 42.8% at 1 Torr, the collisions between neutral species H(n = 2) and H2, i.e.  (no. 34 in table 1), significantly affect the creation of electrons.
(no. 34 in table 1), significantly affect the creation of electrons.  plays a decreasingly important role as pressure increases, with the contribution being from 42.3% at 1 Torr to 22.0% at 100 Torr, due to the very rapidly decreasing density of H(n = 2) (see figure 8). The associative detachment by hydrogen atoms, i.e. H− + H → e + H2 (no. 32 in table 1), plays a more important role in the creation of electrons at higher pressure due to the increasing density of hydrogen atoms (see figure 8), and becomes the most important source of electrons with a contribution of 47.6% at 100 Torr. The ionization of hydrogen atoms, i.e. e + H → 2e + H+ (no. 9 in table 1), only applies at relatively low pressures with a contribution of 8.1% at 1 Torr. The electron detachment, i.e.
plays a decreasingly important role as pressure increases, with the contribution being from 42.3% at 1 Torr to 22.0% at 100 Torr, due to the very rapidly decreasing density of H(n = 2) (see figure 8). The associative detachment by hydrogen atoms, i.e. H− + H → e + H2 (no. 32 in table 1), plays a more important role in the creation of electrons at higher pressure due to the increasing density of hydrogen atoms (see figure 8), and becomes the most important source of electrons with a contribution of 47.6% at 100 Torr. The ionization of hydrogen atoms, i.e. e + H → 2e + H+ (no. 9 in table 1), only applies at relatively low pressures with a contribution of 8.1% at 1 Torr. The electron detachment, i.e.  (no. 1094 in table 2), contributes very little to the creation of electrons.
(no. 1094 in table 2), contributes very little to the creation of electrons.
The electrons escaping the discharge through the walls are the most important loss channel, and the contribution increases with decreasing pressure from 38.6% at 100 Torr to 92.4% at 1 Torr. As mentioned in figure 2(a), the dissociative attachments of the vibrational levels mainly contribute to the creation of H−. Even if they contribute less to the loss of electrons, the dissociative attachments of H2(υ = 0) and H2(υ = 1) still play important roles at relatively high pressures, with respective maximum contributions of 31.7% and 14.4% at 100 Torr. The recombination of  and electrons, i.e.
and electrons, i.e.  (no. 20 in table 1), contributes very little to the loss of electrons.
(no. 20 in table 1), contributes very little to the loss of electrons.
3.2. The VDF and its creation and loss mechanisms
Figure 3 shows the VDF at different pressures. For the lowest pressure of 1 Torr, the VDF is relatively well represented by a non-Boltzmann distribution with a plateau for υ = 3–9. As the pressure increases, the densities of higher vibrational levels decrease very rapidly and a single temperature Boltzmann distribution is observed. The density of H2 in the ground state, i.e. H2(υ = 0), surpasses by six orders of magnitude the density of H2(υ = 10) even at relatively low pressures, such as 1 Torr. That is the reason why higher vibrational levels (υ > 10) hardly contribute to the creation of H− ions and the lower vibrational levels contribute more at higher pressures, as shown in figure 2(a). Unlike the usual NHIS operated at low pressures [20], the density difference between lower and higher levels is larger in the HCNHIS due to more frequent collisions between H2(υ) molecules in the higher pressure range.
Even though the relative contributions of the reactions to the creation and loss of all vibrational levels are evaluated, only the cases of H2(υ = 1), H2(υ = 4) and H2(υ = 7) marked with green circles in figure 3 are shown in figures 4(a)–(c). The electron excitations of H2(υ = 0–14) molecules mainly include two kinds of process: one is through a resonant mechanism (called the eV process, e + H2(υ) → e + H2(υ'), nos. 1–210 in table 2) and the other is through an indirect mechanism followed by radiative decay (called the EV process, e + H2(υ) → e + H2(υ') + hν, nos. 211–435 in table 2). The eV process usually only contributes to the creation and loss of relatively low vibrational levels, such as H2(υ < 4).
Figure 4. Relative contributions of various reactions to the creation and loss of (a) H2(υ = 1), (b) H2(υ = 4), and (c) H2(υ = 7) at different pressures.
Download figure:
Standard image High-resolution imageFor the low pressures in the usual NHIS, only the electron excitations of ground-state molecules H2(υ = 0) to levels H2(υ = 1–14) apply, and other electron excitations between different vibrational levels H2(υ = 1–14) can be neglected, due to the density of H2(υ = 0) being far higher than that of H2(υ = 1–14) [20]. For the high pressures in the HCNHIS, it is still available for electron excitations through the indirect mechanism (EV process), but not for those through the resonant mechanism (eV process). This is because the low-energy electrons (<4 eV) facilitate the eV process, while the high-energy electrons (>12 eV) facilitate the EV process. The electron temperatures are lower than 3 eV (see figure 6) in the HCNHIS. Moreover, the densities of low vibrational levels are higher and are closer to the density of H2(υ = 0) (see figure 3) than that in the usual NHIS operated at low pressures.
As shown in figure 4(a), H2(υ = 0) is the ground state and the neighboring level of H2(υ = 1) for the eV process, e + H2(υ = 0) → e + H2(υ' = 1). This reaction contributes to the creation of H2(υ = 1) increasing from 32.4% to 82.5% as pressure decreases from 100 Torr to 1 Torr. H2(υ = 2) is also the neighboring level of H2(υ = 1) for the eV process, e + H2(υ = 2) → e + H2(υ' = 1), but this reaction contributes to the creation of H2(υ = 1) with the maximum value of 3.3% at 7.75 Torr, due to the density of H2(υ = 2) being lower than that of H2(υ = 0), as shown in figure 3. The transitions through eV processes between neighboring levels have larger reaction cross sections, and therefore the contributions of other levels to the creation of H2(υ = 1) through the eV processes can be neglected in the investigated pressure range. Figure 4(b) shows that the eV process, e + H2(υ = 3) → e + H2(υ' = 4), contributes most to the creation of H2(υ = 4) in all eV processes with a maximum of 23.7% at 7.75 Torr. Even if H2(υ = 5) is also the neighboring level of H2(υ = 4), the density of H2(υ = 3) is higher than that of H2(υ = 5). Therefore, the eV process, e + H2(υ = 5) → e + H2(υ' = 4), contributes less to the creation of H2(υ = 4) than e + H2(υ = 3) → e + H2(υ' = 4). Note that the contribution of the eV process, i.e. e + H2(υ = 2) → e + H2(υ' = 4), is almost larger than the eV process of the neighboring level H2(υ = 5) in the investigated pressure range, due to the density of H2(υ = 2) being higher than that of H2(υ = 5) (see figure 3). Similarly, the contributions of the eV processes of H2(υ = 0) and H2(υ = 1) to the creation of H2(υ = 4) are non-negligible, especially at higher pressures. Figure 4(c) shows that the eV processes are almost not important any more to the creation of H2(υ = 7) except for the process, e + H2(υ = 6) → e + H2(υ' = 7), with the maximum contribution of 8.1% at 1 Torr. Therefore, the eV transitions between levels H2(υ = 1–7) closer to each other apply in this work, which has been noted in table 2.
The vibrational–translational relaxation of H2(υ = 0–14) in collisions with hydrogen atoms (mono-quantum and multi-quantum Vt processes,  , nos. 464–673 in table 2) and with hydrogen molecules H2(υ = 0–14) (mono-quantum VT process,
, nos. 464–673 in table 2) and with hydrogen molecules H2(υ = 0–14) (mono-quantum VT process,  , nos. 674–1093 in table 2) can be neglected in the usual NHIS operated at low pressures [20]. For the high-pressure range of 1–100 Torr here, it is still available for the multi-quantum Vt process, but not for mono-quantum Vt and VT processes. As shown in figures 4(a)–(c), the mono-quantum Vt processes,
, nos. 674–1093 in table 2) can be neglected in the usual NHIS operated at low pressures [20]. For the high-pressure range of 1–100 Torr here, it is still available for the multi-quantum Vt process, but not for mono-quantum Vt and VT processes. As shown in figures 4(a)–(c), the mono-quantum Vt processes,  ,
,  , and
, and  respectively contribute to the loss of H2(υ = 1), H2(υ = 4), and H2(υ = 7) with maximums of 21.6% at 7.75 Torr, 14.3% at 7.75 Torr, and 6.4% at 1 Torr. They play a decreasingly important role in the loss of vibrational levels with increasing level. Similar to the eV transitions, the mono-quantum Vt transitions between neighboring levels of H2(υ = 1–7) apply in this work, which has been noted in table 2.
respectively contribute to the loss of H2(υ = 1), H2(υ = 4), and H2(υ = 7) with maximums of 21.6% at 7.75 Torr, 14.3% at 7.75 Torr, and 6.4% at 1 Torr. They play a decreasingly important role in the loss of vibrational levels with increasing level. Similar to the eV transitions, the mono-quantum Vt transitions between neighboring levels of H2(υ = 1–7) apply in this work, which has been noted in table 2.
Compared with mono-quantum Vt processes, the mono-quantum VT processes play a more important role in the creation and loss of different vibrational levels. Figures 4(a)–(c) show that the mono-quantum VT process,  ,
,  , and
, and  almost dominate the loss of H2(υ = 1), H2(υ = 4), and H2(υ = 7) at higher pressures and play an increasingly important role with increasing level, with maximum contributions of 74.3%, 83.2%, and 84.1% at 100 Torr. As the vibrational level increases to higher ones, such as H2(υ = 13) (not shown here), the effect of the mono-quantum VT process on the loss of vibrational level is weakened, due to the lower density of higher vibrational levels (see figure 3). The reversed processes,
almost dominate the loss of H2(υ = 1), H2(υ = 4), and H2(υ = 7) at higher pressures and play an increasingly important role with increasing level, with maximum contributions of 74.3%, 83.2%, and 84.1% at 100 Torr. As the vibrational level increases to higher ones, such as H2(υ = 13) (not shown here), the effect of the mono-quantum VT process on the loss of vibrational level is weakened, due to the lower density of higher vibrational levels (see figure 3). The reversed processes,  ,
,  , and
, and  , play a decreasingly important role in the creation of H2(υ = 1), H2(υ = 4), and H2(υ = 7), with maximums of 48.8%, 30.8%, and 16.8% at 100 Torr as level increases, especially at high pressures. However, the contribution of transitions from higher levels,
, play a decreasingly important role in the creation of H2(υ = 1), H2(υ = 4), and H2(υ = 7), with maximums of 48.8%, 30.8%, and 16.8% at 100 Torr as level increases, especially at high pressures. However, the contribution of transitions from higher levels,  ,
,  , and
, and  , to the creation of H2(υ = 1), H2(υ = 4), and H2(υ = 7), increases, with maximums of 10.2% at 40.6 Torr, 28.5% at 40.6 Torr, and 61.1% at 100 Torr as the level increases. In total, the mono-quantum VT transitions through H2(υ = 0–14) in collisions with ground-state molecules H2(υ = 0) and H2(υ = 5–10) in collisions with H2(υ = 1) are required to be taken into account in this work, which has also been noted in table 2.
, to the creation of H2(υ = 1), H2(υ = 4), and H2(υ = 7), increases, with maximums of 10.2% at 40.6 Torr, 28.5% at 40.6 Torr, and 61.1% at 100 Torr as the level increases. In total, the mono-quantum VT transitions through H2(υ = 0–14) in collisions with ground-state molecules H2(υ = 0) and H2(υ = 5–10) in collisions with H2(υ = 1) are required to be taken into account in this work, which has also been noted in table 2.
The combined effects of different kinetic processes responsible for forming the VDF were decoupled in the usual NHIS operated at low pressures, where an analytical solution of VDF at low pressures was obtained [20]. Even if the analytical solution of VDF may not be applicable to the high-pressure cases in the HCNHIS, the kinetic processes are still significantly simplified. The reactions with blue rectangles in figure 4 highlight the most important reactions. The sum effect of some reactions mentioned above still applies and cannot be ignored. It is found that with increasing vibrational level, the VT transitions mentioned above dominate over the eV and EV processes and become the most important sources of the higher vibrational levels. This is very different from the case in the usual NHIS operated at low pressures [20], where the EV process is the most important channel for the creation of higher levels. With increasing pressure and level, the vibrational levels lost on the wall play a decreasingly important role, and the VT transitions through collisions with ground-state molecules H2(υ = 0) become the most important loss channel.
3.3. Evaluation of electron energy dissipation
The reactions with very small contributions to the creation and loss of particle species and to electron energy loss will be removed in the simplified model. Therefore, except for the evaluation of the contributions of reactions to the creation and loss of particle species, the energy losses caused by the collisions between electrons and neutral species also need to be evaluated.
The normalized power dissipation is shown in figure 5. Both the sum of electronic excitations of H2(υ = 0), i.e.  (nos. 1099–1105 in table 2), and the sum of EV processes of H2(υ = 1–14), i.e. e + H2(υ = 1–14) → e + H2(υ' = 0–14) + hν, (nos. 226–435 in table 2), contribute less than 4% to the energy loss in the investigated pressure range, and therefore they can be ignored in the simplified model. The dissociation of H2(υ = 0), e + H2 → e + 2H (no. 5 in table 1) and EV processes of H2(υ = 0), i.e. e + H2(υ = 0) → e + H2(υ' = 0–14) + hν (nos. 211–225 in table 2), contribute significantly to the energy loss at relatively low pressures where the higher electron temperature facilitates these processes. Their maximums are respectively 40.4% and 23.5% at 1 Torr. However, the eV processes of H2(υ = 0) to H2(υ = 1–3), i.e. e + H2(υ = 0) → e + H2(υ' = 1–3) (nos. 1–3 in table 2), contribute significantly to the energy loss at relatively high pressures, with a maximum of up to 77.6% at 100 Torr. The elastic collision of H2(υ = 0) also contributes more at relatively high pressures. The energy loss caused by the surface reactions only applies at relatively low pressures with a maximum of 8.5% at 1 Torr, and the eV processes of H2(υ = 1–7) to H2(υ') (υ' refers to table 2) contribute to the energy loss with a maximum of 4.8% at 7.75 Torr. Therefore, the reactions contributing very little to the creation and loss of particle species also play a small role in the electron energy dissipation.
(nos. 1099–1105 in table 2), and the sum of EV processes of H2(υ = 1–14), i.e. e + H2(υ = 1–14) → e + H2(υ' = 0–14) + hν, (nos. 226–435 in table 2), contribute less than 4% to the energy loss in the investigated pressure range, and therefore they can be ignored in the simplified model. The dissociation of H2(υ = 0), e + H2 → e + 2H (no. 5 in table 1) and EV processes of H2(υ = 0), i.e. e + H2(υ = 0) → e + H2(υ' = 0–14) + hν (nos. 211–225 in table 2), contribute significantly to the energy loss at relatively low pressures where the higher electron temperature facilitates these processes. Their maximums are respectively 40.4% and 23.5% at 1 Torr. However, the eV processes of H2(υ = 0) to H2(υ = 1–3), i.e. e + H2(υ = 0) → e + H2(υ' = 1–3) (nos. 1–3 in table 2), contribute significantly to the energy loss at relatively high pressures, with a maximum of up to 77.6% at 100 Torr. The elastic collision of H2(υ = 0) also contributes more at relatively high pressures. The energy loss caused by the surface reactions only applies at relatively low pressures with a maximum of 8.5% at 1 Torr, and the eV processes of H2(υ = 1–7) to H2(υ') (υ' refers to table 2) contribute to the energy loss with a maximum of 4.8% at 7.75 Torr. Therefore, the reactions contributing very little to the creation and loss of particle species also play a small role in the electron energy dissipation.
Figure 5. Normalized power dissipation at different pressures.
Download figure:
Standard image High-resolution image3.4. The simplified model and its accuracy and robustness
The chemical reactions are significantly simplified through evaluating their contributions to the creation and loss of different species. In order to obtain the generalized simplification, if only the reactions that apply for certain pressures in the investigated pressure rangeare included in the simplified model noted in tables 1 and 2, the number of chemical reactions is reduced from 1149 to 128. The chemical complexity of negative hydrogen plasmas is therefore reduced by a factor of nine using the simplified model. The accuracy of the simplified model needs to be validated by the full model. The electron temperature as a function of pressure obtained using the full model and the simplified model is shown in figure 6. Excellent agreement in electron temperature is achieved between the models in the investigated pressure range. The electron temperature decreases from 2.75 eV to 1.12 eV as the pressure increases from 1 Torr to 100 Torr.
Figure 6. Electron temperature as a function of pressure with the full model and the simplified model. The number of reactions for the full model and the simplified model are respectively 1149 and 128.
Download figure:
Standard image High-resolution imageThe densities of charged particle species and H(n = 1–3) atoms obtained in the simulations with the two models as a function of pressure are respectively shown in figures 7 and 8. The two models are in good qualitative and quantitative agreement for the densities of these particle species in the investigated pressure range, except for the slight difference at relatively low pressures (less than around 8 Torr) where the simplified model predicts a slightly higher value. The small difference in the electron density at relatively low pressures is caused by the collecting effects of ignoring the energy loss of electronic excitation of H2(υ = 0) (nos. 1099–105 in table 2) and the EV process of H2(υ = 1–14) (nos. 226–435 in table 2) in the simplified model, which might result in the differences in the other particle densities.
Figure 7. Charged particle density as a function of pressure with the full model (solid line with symbol) and the simplified model (dashed line). The number of reactions for the full model and the simplified model are respectively 1149 and 128.
Download figure:
Standard image High-resolution imageFigure 8. H2(n = 1–3) density as a function of pressure with the full model (solid line with symbol) and the simplified model (dashed line). The number of reactions for the full model and the simplified model are respectively 1149 and 128.
Download figure:
Standard image High-resolution imageFigure 9 shows the densities of H2(υ = 0–14) as a function of pressure obtained with the two models. The two models show good agreement for levels H2(υ < 3) and H2(υ > 10) in the investigated pressure range, and slight differences for H2(3 ⩽ υ ⩽ 10) at different pressures. The small differences for H2(3 ⩽ υ ⩽ 10) are caused by the collecting effects of ignoring parts of the eV processes and Vt processes. In general, figures 6–9 validate the accuracy of the simplified model. To further determine the scope of application of the simplified model, the robustness of the simplified model is studied by changing the discharge conditions in the following.
Figure 9. H2(υ = 0–14) density as a function of pressure with the full model (solid line with symbol) and the simplified model (dashed line). The number of reactions for the full model and the simplified model are respectively 1149 and 128.
Download figure:
Standard image High-resolution imageThe robustness of the proposed simplified model is evaluated by comparing the simulation results of the simplified model against those of the full model for different diameters and lengths of the driver chamber in the HCNHIS. The error caused by the use of the simplified model is quantified using the root mean squared error (RMSE). The RMSE is expressed as
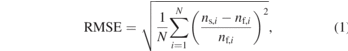
where nf,i and ns,i are the densities of the ith species obtained with the full model and the simplified model, respectively. N is the total number of particles.
Figures 10(a) and (b) show the RMSE under different diameters and lengths of the driver chamber, respectively. The diameters are 0.8725, 1.745, and 3.49 cm for constant length L = 7.39 cm, and the lengths are 5, 7.39, and 10 cm for constant diameter D = 1.745 cm. The RMSE generally decreases with increasing diameter and length. The RMSE is roughly less than 0.2 (20%), which indicates that the simplified model is capable of capturing the main chemical processes even if the chamber size is varied.
Figure 10. Accuracy of the simplified model of chemical reactions under (a) different diameters of driver chamber, (b) different lengths of driver chamber, (c) different absorption powers, and (d) different gas temperatures.
Download figure:
Standard image High-resolution imageThe absorption power affects the electron density and thus the densities of other particle species. Figure 10(c) shows the effect of absorption power on the RMSE as a function of pressure. The absorption powers are 390, 490, 590, 690, and 790 W. The results indicate that lower power decreases the RMSE, and the maximum of RMSE is less than 0.2. For the investigated pressure range, the neutral gas temperature is not kept at room temperature, which has been obtained by the simulation and experiment [16, 17, 44]. The effect of neutral gas temperature on the RMSE is therefore evaluated as a function of pressure as shown in figure 10(d), due to its effect on densities of particle species. The neutral gas temperatures are 1200, 1300, 1400, 1500, and 1600 K. The RMSE increases with decreasing gas temperature and the maximum RMSE is also less than 0.2. For all cases, the RMSE caused by using the simplified model is roughly kept lower than 0.2.
3.5. Validation of the model
In this section, the electron density, electron temperature, and hydrogen atom density predicted by the model are compared with prior experiments [45, 46] to validate our simulations using the GMNHIS and the established H2 reaction set. The experimental data are obtained in RF inductively coupled H2 plasmas [45, 46]. The inductively coupled discharge is ignited in a cylindrical stainless-steel vacuum chamber with four ports used for optical emission spectroscopy (OES) and other diagnostics. The top port contains a dielectric quartz window separating the plasma and a five-turn antenna coil for inductive coupling. An RF power with a frequency of 13.56 MHz is applied and connected to the coil via a P-type inductive matching network. The plasma is confined below the quartz window by a grounded electrode made of stainless steel. The electrode with a diameter of 10 cm is cooled by water. The axial distance between the electrode and the dielectric quartz window is 3 cm. In the model, the ion and neutral temperatures are assumed to be 300 K. The power transfer efficiency, which is defined as the ratio of power absorbed by the plasma over the input power, is assumed to be 70%. The recombination coefficient of hydrogen atoms on the wall is assumed to be 0.05 for the mix of quartz and metal walls [47].
Figures 11(a) and (b) show the electron temperature and electron density as a function of input power. The gas pressure is 15 Pa. The simulations are in very reasonable agreement with Langmuir probe experiments [45]. As shown in figure 11(a), the electron temperature predicted by the model is almost independent of input power, while the experimental data slightly increase with increasing input power. This could be attributed to the fixed neutral temperature assumed in the model. The neutral temperature increases with increasing power in H2 discharges revealed by previous simulations [48] and experiments [44]. The increased neutral temperature with power can lead to the increase in the electron temperature in the experiments. The model-predicted electron density follows its dependence on the power in the experiments [45], as shown in figure 11(b). The simulations and experiments achieve very good qualitative agreement, and they show that the electron density increases with increasing input power. The simulation results are generally larger than the experimental results. This could be due to the power transfer efficiency assumed in the simulations being higher than that in the experiments [49], which leads to overestimation of the electron density.
Figure 11. Electron temperature (a) and density (b) as a function of input power at 15 Pa. The experimental data used for comparison here are taken from the work [45].
Download figure:
Standard image High-resolution imageFigures 12(a) and (b) show the hydrogen atom densities as functions of input power and pressure, respectively. The hydrogen atom densities predicted by our model are in qualitative agreement with the OES experiments [46]. Both simulations and experiments show that hydrogen atom densities increase with increasing input power at 10 Pa, and with increasing pressure at 300 W. The increasing rates tend to be moderated with increasing power and pressure in the experiments, which could be due to the neutral heating effects. As stated earlier, the neutral temperature is assumed to be constant in the simulations. Note that the simulation results are generally smaller than in the experiments. The recombination coefficient of hydrogen atoms on the walls is not accurately known, and it can significantly affect the main properties of the hydrogen plasmas [48]. When increasing the coefficient, hydrogen atom density decreases clearly [48]. Therefore, the lower density of hydrogen atoms in the simulations than in the experiments could be due to a larger recombination coefficient of hydrogen atoms on the walls assumed in the model. In addition, the assumption of volume averaged plasma properties of the model itself can cause the difference of magnitude in the plasma parameters between simulations and experiments.
Figure 12. Hydrogen atom densities as functions of input power (a) at 10 Pa and pressure (b) at 300 W. The experimental data used for comparison here are taken from the work [46].
Download figure:
Standard image High-resolution image4. Conclusion
The GMNHIS [18] is employed to identify the main physicochemical processes in a volume-production HCNHIS. The contributions of different chemical reactions to the creation and loss of each particle species are evaluated under a pressure range of 1–100 Torr. The simulation results show that the main creation source of negative hydrogen ions through dissociative attachment shifts from high vibrational levels to ground state and low vibrational levels of hydrogen molecules with increasing pressure. The production of vibrational levels and transitions between them are determined by the reactions involving the ground state, low vibrational levels and neighboring levels of hydrogen molecules in the HCNHIS. For the electron excitation of H2(υ = 0–14) through a resonant mechanism (eV process) and the vibrational–translational relaxation of H2(υ = 0–14) in collisions with hydrogen atoms (Vt process), only the transitions between the neighboring levels of H2(υ ⩽ 7) are responsible for their creation and loss, due to the high density of low levels in the investigated pressure range. For the electron excitation of H2(υ = 0–14) through an indirect mechanism followed by radiative decay (EV process), only the electron excitations from H2(υ = 0) to H2(υ' = 1–14) play an important role in the creation of H2(υ' = 1–14). In addition, only the vibrational–translational relaxation of H2(υ = 0–14) in collisions with H2(υ = 0) and that of H2(υ = 5–10) in collisions with H2(υ = 1) contribute significantly to the creation and loss of vibrational levels. With increasing pressure, the main electron energy dissipation shifts from dissociation of H2 (e + H2 → e + 2H) and EV process (e + H2(υ = 0) → e + H2(υ = 0–14) + hν) to eV process (e + H2(υ = 0) → e + H2(υ = 1–3)).
A simplified model of chemical reactions is achieved to describe the discharge of the HCNHIS under a pressure range of 1–100 Torr. Even if some reactions only apply for some pressures in the investigated pressure range, they are also included in the simplified model to obtain the generalized simplification. In that case, the chemical complexity of negative hydrogen plasmas is still reduced by a factor of nine (i.e. the number of chemical reactions reduced from 1149 to 128) using the simplified model. The error introduced with the simplified model is quantified by the RMSE in terms of the results obtained with the full model. The simplified model is capable of capturing the main physicochemical processes of the HCNHIS with an accuracy of 0.1–0.15 in the investigated pressure range. The accuracy for different discharge geometries, input powers, and gas temperatures still remains roughly better than 0.2, which indicates the good robustness of the simplified model under the investigated discharge conditions.
In conclusion, insights into the physicochemical processes in the HCNHIS allow better understanding of the reaction mechanisms of all particle species and a significant reduction in the number of reactions. A valuable and generalized subset of reactions is provided for the investigated pressure range, and a more simplified subset of reactions could be achieved for a certain pressure according to the detailed evaluation results of the importance of chemical reactions. The simplified model can be incorporated into complex simulations, where computational cost prevents the use of the chemistry models with large numbers of reactions. Robust simplification allows researchers to obtain reliable results, even if the model is extended to a wider range of discharge conditions.
The model is validated by comparing the simulated electron density, electron temperature, and hydrogen atom density with experimental data obtained in an RF inductively coupled hydrogen discharge [45, 46]. In general, qualitative agreement has been achieved between the simulations and experiments, and the discrepancies observed in these comparisons are reasonably explained based on the assumptions in the GMNHIS. The further validation of the model with experimental data in terms of electron characteristics and negative hydrogen ion production in the HCNHIS is underway.
In future work, a two-dimensional fluid model will be developed to self-consistently study the production and transport of negative hydrogen ions in the HCNHIS. Analysis and simplification of complex plasma chemistry for HCNHIS reported here are prerequisites for developing a self-consistent multi-dimensional model to better understand and optimize the conditions of production and extraction of negative hydrogen ions, ultimately aiding in developing alternatives to the present RF NHIS to obtain optimized negative ion beams for prospective fusion reactors.
Acknowledgments
This work was sponsored by the National Key R&D Program of China (No. 2017YFE0300106), Fundamental Research Funds for the Central Universities (No. 2232020D-40), Shanghai Sailing Program (No. 20YF1401300), and the National Natural Science Foundation of China (No. 12075049). The first author would like to thank Dr. Averkin for offering helpful suggestions about the modification of the model based on the chamber structure of HCNHIS.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
: Appendix. Evaluation of physicochemical processesof positive ions and hydrogen atoms
The relative contributions of the reactions to the creation and loss of positive ions ( ,
,  and H+) at different pressures are shown in figure 13. The collisions of H2 with
and H+) at different pressures are shown in figure 13. The collisions of H2 with  , i.e.
, i.e.  (no. 24 in table 1) and H(n = 2), i.e.
(no. 24 in table 1) and H(n = 2), i.e.  (no. 34 in table 1), almost dominate the creation of
(no. 34 in table 1), almost dominate the creation of  in the investigated pressure range. The collisions of H2 and H(n = 3), i.e.
in the investigated pressure range. The collisions of H2 and H(n = 3), i.e.  (no. 35 in table 1), as well as the reaction of the charge exchange, i.e.
(no. 35 in table 1), as well as the reaction of the charge exchange, i.e.  (no. 39 in table 1), contribute a little to the creation of
(no. 39 in table 1), contribute a little to the creation of  , but they are largely responsible for the loss of H(n = 3) (see figure 14) and H+ at relatively high pressures. For example, the former contributes around 48.3% and the latter contributes 96.5% at 100 Torr.
, but they are largely responsible for the loss of H(n = 3) (see figure 14) and H+ at relatively high pressures. For example, the former contributes around 48.3% and the latter contributes 96.5% at 100 Torr.  ions are mainly lost on the wall in the investigated pressure ranges. The recombination of
ions are mainly lost on the wall in the investigated pressure ranges. The recombination of  and electrons, i.e.
and electrons, i.e.  (no. 20 in table 1), only applies for the reduction of
(no. 20 in table 1), only applies for the reduction of  at higher pressure, contributing 10.0% at 100 Torr. The dissociative recombination of
at higher pressure, contributing 10.0% at 100 Torr. The dissociative recombination of  and electrons, i.e.
and electrons, i.e.  (no. 21 in table 1), contributes very little, not only to the loss of
(no. 21 in table 1), contributes very little, not only to the loss of  but to the loss of electrons as well as to the creation of hydrogen atoms (not shown in figure 14) and H2 (not shown); reaction no. 21 can therefore be neglected. The recombination of
but to the loss of electrons as well as to the creation of hydrogen atoms (not shown in figure 14) and H2 (not shown); reaction no. 21 can therefore be neglected. The recombination of  and H−, i.e.
and H−, i.e.  (no. 30 in table 1), contributes significantly to the loss of H− with decreasing pressure (see figure 2(a)), but contributes very little to the loss of
(no. 30 in table 1), contributes significantly to the loss of H− with decreasing pressure (see figure 2(a)), but contributes very little to the loss of  .
.
Figure 13. Relative contributions of various reactions to the creation and loss of  ,
,  , and H+ (separated using blue dashed lines) at different pressures.
, and H+ (separated using blue dashed lines) at different pressures.
Download figure:
Standard image High-resolution imageFigure 14. Relative contributions of various reactions to the creation and loss of H(n = 1–3) (separated using blue dashed lines) at different pressures.
Download figure:
Standard image High-resolution imageThe ionization of H2, i.e.  (no. 4 in table 1), is the only source of
(no. 4 in table 1), is the only source of  considered in this work. The collisions of H2 with
considered in this work. The collisions of H2 with  , i.e.
, i.e.  (no. 24 in table 1), mainly contribute to the loss of
(no. 24 in table 1), mainly contribute to the loss of  . Even if the charge exchange, i.e.
. Even if the charge exchange, i.e.  (no. 23 in table 1), contributes very little to the loss of
(no. 23 in table 1), contributes very little to the loss of  , it contributes more to the creation of H+ at relatively low pressures, up to 13.2% at 1 Torr. The ionization of hydrogen atoms, i.e. e + H → 2e + H+ (no. 9 in table 1), significantly contributes to the creation of H+. The dissociative ionization of H2, i.e. e + H2 → 2e + H+ + H (no. 3 in table 1), plays an increasingly important role in the creation of H+ with increasing pressure, up to 56.5% at 100 Torr. The ionization of H(n = 2,3), i.e.
, it contributes more to the creation of H+ at relatively low pressures, up to 13.2% at 1 Torr. The ionization of hydrogen atoms, i.e. e + H → 2e + H+ (no. 9 in table 1), significantly contributes to the creation of H+. The dissociative ionization of H2, i.e. e + H2 → 2e + H+ + H (no. 3 in table 1), plays an increasingly important role in the creation of H+ with increasing pressure, up to 56.5% at 100 Torr. The ionization of H(n = 2,3), i.e.  (nos. 13 and 14 in table 1), and dissociative excitation, i.e.
(nos. 13 and 14 in table 1), and dissociative excitation, i.e.  (no. 15 in table 1), contribute very little, not only to the creation of H+ but also to the creation and loss of other species involved in these reactions, and thus they can be neglected. The charge exchange, i.e.
(no. 15 in table 1), contribute very little, not only to the creation of H+ but also to the creation and loss of other species involved in these reactions, and thus they can be neglected. The charge exchange, i.e.  (no. 39 in table 1) mainly contributes to the loss of H+ at relatively high pressures, while H+ lost on the wall plays an increasingly important role in the loss of H+ with decreasing pressure, contributing almost 100% at 1 Torr.
(no. 39 in table 1) mainly contributes to the loss of H+ at relatively high pressures, while H+ lost on the wall plays an increasingly important role in the loss of H+ with decreasing pressure, contributing almost 100% at 1 Torr.
Figure 14 shows the relative contributions of the reactions to the creation and loss of H(n = 1–3). The dissociations of ground-state molecules H2(υ = 0), i.e. e + H2 → e + 2H (No. 5 in table 1), and H2(υ = 1), i.e.  (no. 436 in table 2), mainly contribute to the creation of hydrogen atoms, and in particular the contribution of the former increases from 77.5% to 93.1% as pressure increases from 1 Torr to 100 Torr. The hydrogen atoms lost to the wall mainly deplete the hydrogen atoms. The excitation of hydrogen atoms, i.e.
(no. 436 in table 2), mainly contribute to the creation of hydrogen atoms, and in particular the contribution of the former increases from 77.5% to 93.1% as pressure increases from 1 Torr to 100 Torr. The hydrogen atoms lost to the wall mainly deplete the hydrogen atoms. The excitation of hydrogen atoms, i.e.  (no. 10 in table 1), and the dissociative excitation of H2, i.e.
(no. 10 in table 1), and the dissociative excitation of H2, i.e.  (no. 6 in table 1), mainly contribute to the creation of H(n = 2) in the investigated pressure range, while the radiative decay of H(n = 3) (no. 33 in table 1) is only responsible for the relatively low pressures with a maximum contribution of 15.3% at 1 Torr. The collisions of H(n = 2) and H2, i.e.
(no. 6 in table 1), mainly contribute to the creation of H(n = 2) in the investigated pressure range, while the radiative decay of H(n = 3) (no. 33 in table 1) is only responsible for the relatively low pressures with a maximum contribution of 15.3% at 1 Torr. The collisions of H(n = 2) and H2, i.e.  and H(n = 2)+H2 → 3H (nos. 34 and 36 in table 1), play the same important role in the loss of H(n = 2) in the investigated pressure range. The excitation of H(n = 2), i.e.
and H(n = 2)+H2 → 3H (nos. 34 and 36 in table 1), play the same important role in the loss of H(n = 2) in the investigated pressure range. The excitation of H(n = 2), i.e.  (no. 12 in table 1), only applies for the relatively low pressures, with a maximum contribution of 12.3% at 1 Torr.
(no. 12 in table 1), only applies for the relatively low pressures, with a maximum contribution of 12.3% at 1 Torr.
The excitation of hydrogen atoms significantly contributes to the creation of H(n = 3), i.e.  (no. 11 in table 1), in the investigated pressure range, while the excitation of H(n = 2) (no. 12 in table 1) and the dissociative excitation of H2(υ = 0) (no. 7 in table 1) respectively contribute more to the creation of H(n = 3) at relatively low and high pressures with maximums of 63.5% at 1 Torr and 22.1% at 100 Torr. The collisions of H(n = 3) and H2, i.e.
(no. 11 in table 1), in the investigated pressure range, while the excitation of H(n = 2) (no. 12 in table 1) and the dissociative excitation of H2(υ = 0) (no. 7 in table 1) respectively contribute more to the creation of H(n = 3) at relatively low and high pressures with maximums of 63.5% at 1 Torr and 22.1% at 100 Torr. The collisions of H(n = 3) and H2, i.e.  and H(n = 3)+H2 → 3H (nos. 35 and 37 in table 1), play the same important role in the loss of H(n = 3) in the investigated pressure range, and the contribution of the radiative decay of H(n = 3), i.e. H(n = 3) → H(n = 2)+hν (no. 33 in table 1), rapidly increases with decreasing pressure, being up to 79.1% at 1 Torr.
and H(n = 3)+H2 → 3H (nos. 35 and 37 in table 1), play the same important role in the loss of H(n = 3) in the investigated pressure range, and the contribution of the radiative decay of H(n = 3), i.e. H(n = 3) → H(n = 2)+hν (no. 33 in table 1), rapidly increases with decreasing pressure, being up to 79.1% at 1 Torr.
For the basic reactions excluding the vibrational levels of molecular hydrogen,the simulation results indicate that most of the reactions, i.e. positive ion-electron dissociative excitation and dissociative recombination as well as positive ion-negative ion mutual neutralization, included in the previous modeling of the HCNHIS contribute very little, and they can be therefore ignored.