Abstract
Objective: Storage at temperatures as low as −80 °C and below (cryopreservation) is considered a method for long-term preservation of cells and tissues, and especially blood vessel segments, which are to be used for clinical operations such as transplantation. However, the freezing and thawing processes themselves can induce injuries to the cells and tissue by damaging the structure and consequently functionality of the cryopreserved tissue. In addition, the level of damage is dependent on the rate of cooling and warming used during the freezing-thawing process. Current methods for monitoring the viability and integrity of cells and tissues after going through the freezing-thawing cycle are usually invasive and destructive to the cells and tissues. Therefore, employing monitoring methods which are not destructive to the cryopreserved tissues, such as bioimpedance measurement techniques, is necessary. In this study we aimed to design a bioimpedance measurement setup to detect changes in venous segments after freezing-thawing cycles in a noninvasive manner. Approach: A bioimpedance spectroscopy measurement technique with a two-electrode setup was employed to monitor ovine jugular vein segments after each cycle during a process of seven freezing-thawing cycles. Main results: The results demonstrated changes in the impedance spectra of the measured venous segments after each freezing-thawing cycle. Significance: This indicates that bioimpedance spectroscopy has the potential to be developed into a novel method for non-invasive and non-destructive monitoring of the viability of complex tissue after cryopreservation.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
1.1. Cryopreservation
In order to preserve the viability and structural integrity of living cells and tissues for a long period, they are stored under very low (cryogenic) temperatures where all the chemical, biological and physical reactions in living cells would be suspended or dramatically reduced. This process is called cryopreservation (Karlsson and Toner 1996, Pegg 2007, Jang et al 2017). Cryopreservation is usually performed at −80 °C and below as traces of unfrozen solution can still be found in tissue above this temperature, and consequently the optimal duration of cryopreservation would play an important role in the prevention of any kind of damage to the cells (Mazur 1984). However, if the cryopreservation is performed at −130 °C and below then all biological activities, including biochemical reactions that can lead to cell death, would stop. Furthermore, at −196 °C (liquid nitrogen temperature) there would not be enough thermal energy to drive any type of biochemical process (Mazur 1984, Gao and Critser 2000, Woods et al 2016). Under cryogenic temperatures, background ionizing radiation (which causes photophysical reactions) could be the only cause of damage to tissues, and this is not considerable (Woods et al 2016). Thus, in temperatures lower than −130 °C the duration of cryopreservation does not play a very important role in the viability of the cells, so the cryopreservation period can range from short (Ku et al 1992, Müller-Schweinitzer 1994a) to undefined periods (Elmore et al 1991, Bujan et al 2000).
The advantage of cryopreserved cells and tissues is their constant availability for both research and clinical application, where their quality can be tested for suitability for transplantation purposes (Ibars et al 2016, Jang et al 2017). However, experimental findings have shown that the cryopreservation process can be damaging to the cells (Mazur 1963, Gao and Critser 2000). One of the methods for cryopreservation of cells and tissues is the freezing-thawing procedure (Karlsson and Toner 1996). Although storage under cryogenic temperatures is beneficial for long-term preservation of cells and tissues, the freezing and thawing processes can be detrimental to their viability (Karlsson and Toner 1996, Bia et al 2006, Müller-Schweinitzer et al 2007). However, the challenge is not only to withstand storage at high subzero temperatures but also to withstand the transition phases and to traverse the temperature range of −15°C to −60°C. By the time the tissue reaches the temperature of −15 °C, ice crystals are already formed in the extracellular matrix but the cell membrane blocks the growth of ice in the intracellular matrix. Consequently, water has started flowing out of the cells osmotically, due to its higher chemical potential in comparison to the water in the extracellular matrix. Therefore, the temperature zone in which both ice crystallization in the extracellular matrix and loss of intracellular water happen is considered a lethal zone for cells, especially when they need to traverse it twice (once during freezing and once during thawing) (Mazur 1963, Gao and Critser 2000, Woods et al 2016).
Both cooling (freezing) and warming (thawing) rates play important roles in determining the viability of cells and tissue (Pegg 2007) and should optimally result in the recovery and survival of cell and tissue structure (Karlsson and Toner 1996). The rate of temperature change controls the transport of water out of the cells during freezing and into the cells during thawing, and thus indirectly influences the concentration of the solution surrounding the cells (Mazur 1963, Pegg 2007). At low temperatures, permeability of the cell membrane to water is higher than to the corresponding solute, which makes the cell membrane semipermeable (Lynch and Diller 1981, Karlsson and Toner 1996). In other words, formation of intracellular ice is influenced by cooling rate and what happens to that ice is controlled by warming rate (Pegg 2007). Freezing rates that are too rapid or too slow can both cause damage to the cells, however the damage mechanisms are different (Gao and Critser 2000).
On the other hand, if cells are cooled too rapidly (figure 1) the intracellular water volume remains almost the same, as water does not have time to leave the cells fast enough by osmosis to maintain equilibrium (Karlsson and Toner 1996). In this way, icy crystals would be formed inside the cell (which is called 'Intracellular Ice Formation' or IIF), which can be lethal to the cells (Mazur 1963, Mazur et al 1972, Gao and Critser 2000). Therefore, damage to the cells during rapid cooling is due to IIF or osmotic rupture (Karlsson and Toner 1996).
Figure 1. Physical changes in the cells under different cooling rates: slow and rapid.
Download figure:
Standard image High-resolution imageChanges in temperature and concentrations of intra- and extracellular solutes damage the lipid-protein complexes in the cell membrane (Lovelock 1953, Gao and Critser 2000), causing leakage and also changes in membrane permeability (Pegg 2007, Müller-Schweinitzer 2009). Therefore, an optimal cooling rate (which would be high enough to minimize the solution effect and low enough to avoid IIF) is necessary to maintain the viability and integrity of the cells and their membranes (Mazur 1963, Mazur et al 1972, Gao and Critser 2000).
Warming or thawing can be as challenging for the survival of the cells as the cooling or freezing process (Mazur 1984, Gao and Critser 2000). Studies have shown that maximum thermal stress to the cells occurs during the warming or thawing process (Zhang et al 2005, Müller-Schweinitzer 2009) as small intracellular ice crystals show different behaviors when they are treated by slow or rapid warming (Pegg 2007). Therefore, the extent to which the warming rate would affect the tissue under cryopreservation actually depends on whether the prior rate of cooling has caused cellular dehydration or intracellular ice formation (Gao and Critser 2000, Woods et al 2016).
Slow warming gives enough time for the small intracellular ice crystals to recrystallize, join and grow into harmful ice crystals (Pegg 2007). Therefore, although there is no change in the amount of ice in the cells, what causes injury is the size and shape of the ice. In addition, during slow warming the cells would be exposed to concentrated solutes for a longer period of time, which increases the chances of toxicity caused by the solutes (Woods et al 2016).
In contrast, during rapid warming (which is generally applied in a 37 °C water bath) (Bellon et al 1999) there is not enough time for the ice crystals to recrystallize, merge and grow bigger, so the ice crystals just melt (Pegg 2007). In this way, rapid warming prevents the formation of large ice crystals that can be damaging to the cells and thus helps rescue them (Mazur 1984, Gao and Critser 2000).
1.2. Cryopreservation of vascular tissue
Previous research has shown that blood vessel cells and tissues can be seriously damaged when they are subjected to cooling and warming (freezing and thawing). This damage can range from loss of contractility in the smooth muscle layer to loss of function in endothelial cells (Müller-Schweinitzer 2009). According to some studies, storage of blood vessels at −80 °C would provide better preservation than lower temperatures such as −160 °C/−190 °C (Ku et al 1994, Müller-Schweinitzer 1994a, 1994b, Bujan et al 2000). Storage of vascular tissues in temperatures of around −80 °C for a short period of time (3–4 weeks) can preserve the structural integrity of the tissues. For example, studies have shown that the viscoelastic properties of human veins would not change during the cryopreservation period (Bia et al 2006, Müller-Schweinitzer et al 2007). However, storage under these temperatures for a long period would not guarantee the survival of mammalian cells (Müller-Schweinitzer et al 1986, Ku et al 1994). According to previous clinical research, slow warming of arteries and animal vessels (with a rate of 1 °C min−1) would cause less damage to the endothelial cell layer in comparison to rapid warming (which can cause fractures in the vascular wall) (Hunt et al 1994, Wassenaar et al 1995, Pegg et al 1997, Bujan et al 2000, Pascual et al 2004, Pasquinelli et al 2006, Müller-Schweinitzer et al 2007).
1.3. Monitoring the quality of the frozen tissues
It is important to evaluate the viability and structural integrity of the cells and tissues after cryopreservation (freezing-thawing cycle) and before their clinical application (such as transplantation). Some of the monitoring methods that are currently employed for this purpose include microcomputed tomography imaging (Bischof et al 2007); magnetic resonance swift imaging (Zhang et al 2014, Manuchehrabadi et al 2017); FT-IR spectroscopy (Giugliarelli et al 2016); label-free fluorescence imaging with optical fiber (Alfonso-Garcia et al 2018); light and cryomicroscopy, scanning and transmission electrode microscopy imaging (Bujan et al 2000); and general histological evaluation methods (Merdassi et al 2011). In addition there are mechanical tests such as tonometry for evaluating the biomechanical properties and functionality of tissues, such as their viscoelasticity (Bia et al 2006). However, these methods are mostly invasive and destructive to the tissues as they require segmenting the tissue into tiny rings or pieces, and also staining. Therefore, there is a need for a non-destructive method which would be able to monitor the tissue quality after its cryopreservation in a non-destructive manner.
Electrical bioimpedance measurements facilitate non-destructive miniaturized methods for real-time evaluation of the state or properties of biological materials. The bioimpedance measurement technique has been used in clinical applications to determine fluid content, electrolytic composition, cellular morphology, organ perfusion, inflammation, body composition, etc. The spatial resolution can range from molecular-size to whole-body, and can be focused by proper development of measurement setup and electrode design (Grimnes and Martinsen 2015). Structural tissue properties are usually highly detectable in a bioimpedance measurement, as are the concentration of cells and their geometry. Destruction of cell membrane and cell death are examples of detectable vital signs in a tissue (Amini et al 2018).
Therefore our hypothesis is that a potential change in the viability of the cells due to freezing/thawing will be detectable with a properly designed bioimpedance setup. Bioimpedance spectroscopy is one of the bioimpedance measurement techniques, which records the electrical impedance of a tissue over a frequency range to detect the frequency-dependent electrical properties of the tissues. As electrical properties of the tissue are related to their physiological and morphological properties, bioimpedance spectroscopy can be employed for evaluation of the tissue of interest (Dean et al 2008, Grimnes and Martinsen 2015).
The aim of the present study was therefore to employ bioimpedance spectroscopy as a non-invasive and non-destructive measurement technique, and to evaluate its ability in monitoring changes in venous segments which have undergone multiple freezing-thawing cycles.
2. Methods
Six ovine jugular veins were subjected to seven cycles of freezing-thawing. Studies have shown that preservation in −80 °C freezers provides long-term storage of tissues and cells from weeks to months, and is one of the most commonly used storage methods. The combination of slow freezing and fast thawing methods employed in this study is considered to be an optimal cryopreservation method, which induces less injury and damage to the cells and tissue (Shu et al 2015).
For the freezing part, slow cooling down to −80 °C with a freezing rate of 1 °C min−1 was performed in order to minimize the effects of ice injury to the venous tissue. For the thawing part, fast warming was performed by using a water bath at 37 °C in order to reduce recrystallization and ice injury. The venous segments were preserved in the University of Wisconsin (UW) solution during the whole storage and freezing-thawing periods.
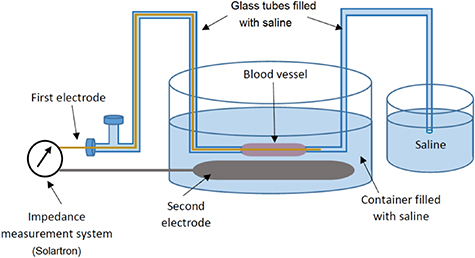
Bioimpedance spectroscopy measurement was performed on all veins after each freezing-thawing cycle, with at least a 48 h interval between each two consecutive measurements for each vein. A two-electrode bioimpedance spectroscopic measurement setup (figure 2) was applied and the same pair of electrodes was used as both current-carrying and voltage pick-up electrodes. The electrodes consisted of a gold wire going through the glass tubes and the venous segment, and a titanium plate placed at the bottom of the main container.
Figure 2. Bioimpedance spectroscopy measurement set-up with two-electrode configuration for monitoring veins after each freezing-thawing cycle.
Download figure:
Standard image High-resolution imageThe venous segments were fixed between two glass tubes in the main container. The container, glass tubes and venous segment were filled with saline, with a slightly positive pressure inside the vein to keep it expanded and prevent contact between the electrode and the wall of the venous segment. The measurement device employed was a Solartron complex impedance measurement system (SI 1260/1294, Solartron Group PLC., Hampshire, UK), using a controlled electrical excitation voltage of 500 mV rms to record the impedance modulus and phase of the veins in the frequency range of 1 Hz to 1 MHz (figure 3).
Figure 3. Block diagram of the bioimpedance spectroscopy measurement system.
Download figure:
Standard image High-resolution imageThe change in impedance over cycles was assessed statistically by selecting the impedance modulus at one frequency of interest and fitting these values from all veins in a linear mixed effects (LME) model. The change in impedance was modeled as a linearly dependent function ofthe number of cycles (as a fixed effect) and with a random intercept for the different veins. The LME was implemented using the fitlme() function in Matlab 2018b.
3. Results
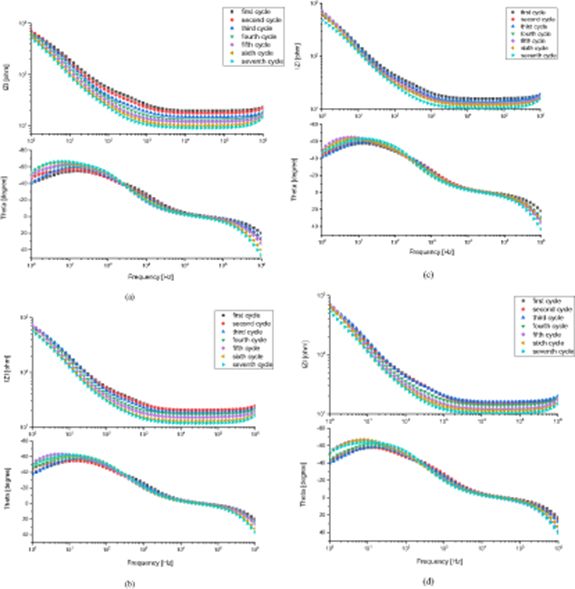
Comparison of the bioimpedance spectra recorded after each freezing-thawing cycle demonstrated obvious changes in both the modulus and phase angle of the measured bioimpedance. For four of the six monitored veins, this change followed a trend where the impedance modulus decreased systematically from the first to the seventh cycle. For the other two veins, following the measurement protocol and subsequently completing the measurements was impossible. This was due to the presence of tiny holes in the walls of the venous segments and consequent leakages from the walls; this made expanding the venous segment and preventing the venous wall from collapsing and coming into direct contact with the electrode inside the vein impossible. Therefore, measurements from these two veins were not reliable and were excluded from the results. In figure 4 the bioimpedance spectra from all four veins, including the impedance modulus (|Z|) and the phase angle (Theta), are shown.
Figure 4. Bioimpedance spectra from four veins recorded after each freezing-thawing cycle, including the impedance modulus |Z| and phase angle Theta of all four veins: (a) vein 1, (b) vein 2, (c) vein 3, and (d) vein 4.
Download figure:
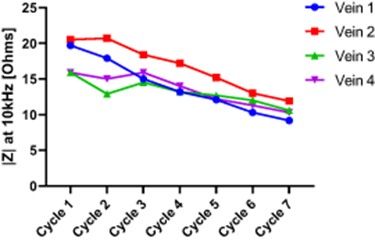
Standard image High-resolution imageFigure 5 shows the changes in impedance modulus (at the particular frequency of 10 kHz) over the seven cycles of freezing-thawing for all four veins. The reason for choosing 10 kHz was that this frequency is above the frequency range corresponding to contribution from electrode polarization impedance, and below the frequency range were inductive properties are demonstrated.
Figure 5. Changes in impedance modulus |Z| at the particular frequency of 10 kHz over seven freezing-thawing cycles for all four veins.
Download figure:
Standard image High-resolution imageBased on the spectra shown in figure 4, the impedance modulus at 10 kHz (figure 5) was selected for statistical analysis of the change in impedance over cycles. Fitting the 10 kHz impedance to the LME model gave an estimated change of −1.26 Ohms per cycle, with a 95% confidence interval from −1.49 to −1.04 Ohms per cycle (p < 0.001).
4. Discussion
Each step of the cryopreservation process can cause injury to the cells and influence the viability and functionality of the cells or tissues, therefore monitoring and evaluating the tissues after cryopreservation and before their clinical application is important. The majority of current monitoring methods are invasive and destructive. As an example, methods for histological evaluation of cells and tissues can be mentioned, which are highly accurate and considered as the gold standard. Thus, the use of an evaluation method that is not destructive to cryopreserved tissue is warranted. Bioimpedance measurement techniques such as bioimpedance spectroscopy have shown their suitability as real-time, non-invasive methods for characterizing tissues and evaluating their viability and integrity in a non-destructive manner (Grimnes and Martinsen 2015).
The main finding of the present study, which employed bioimpedance spectroscopy as the monitoring method, was observation of a decreasing trend in the impedance modulus and phase angle in four of the six measured veins (in the frequency range of 1 Hz to 1 MHz) after each freezing-thawing cycle. In the other two veins it was not possible to follow the measurement protocol and complete the measurements, due to leakage caused by the formation of tiny holes in the blood vessel walls.
The plots in figure 5 show that all four veins demonstrate linear changes in the same direction along with the cycles, and strongly suggest that these results are not random changes. In addition, the results of statistical analysis of impedance changes at 10 kHz, with the confidence interval of 95% and p-value < 0.001, indicate that the decrease in impedance modulus per cycle and for all veins is statistically significant.
The observed decrease in impedance measurements between each freezing-thawing cycle can be an indication of a decrease in the structural integrity of the endothelial cell layer. This can be due to solution-effect injury in addition to ice injury damage to the cells and their membranes during thawing, which would consequently affect the functionality of the venous segment.
Looking closer at the low frequency part of the plots in figure 4 (in the impedance modulus spectra from the first and second cycles in the frequency range around 1 kHz) a small arc can be detected, which flattens out for the subsequent cycles. The corresponding circle segments are also evident in the phase plot. Both of these findings strongly suggest the presence of an α-dispersion for the first and second cycles. The α-dispersion can usually be seen in the frequency range of 1 Hz to 100 kHz (Grimnes and Martinsen 2010, 2015) and is associated with tissue interfaces, such as cell membranes. Therefore, there is reason to believe that this α-dispersion can be used to detect a change in the properties of cell membranes or intracellular structures after consecutive freezing-thawing cycles (Ivorra et al 2005).
However, the arc representing α-dispersion (which is mostly evident from a few hundred Hz) should not be confused with the impedance modulus curve below 100 Hz, which has a large increase in impedance towards 1 Hz due to electrode polarization impedance (EPI). The presence of EPI or electrode impedance at the electrode interface with the tissue/solution in the frequency range below 1 kHz, and its contribution to changes in the impedance modulus and phase angle, is often inevitable — especially when a two-electrode configuration is employed (Grimnes and Martinsen 2015). Generally, electrode material and size, sample impedance, measurement frequency and some other factors affect the influence of EPI in the measurements. Therefore, by being aware of this dependency it is possible to design the measurement setup such that the contribution of EPI would be reduced or minimized (Kalvøy et al 2011, Amini et al 2018). In our measurement setup, which used gold as the electrode material (which demonstrated one of the lowest EPIs among the other electrode materials tested in our pilot measurements) and specific dimensions for the gold electrode (diameter of 1 mm and length covering the whole length of the venous segment), it is expected that the contribution of EPI would be low. In addition, changes in impedance modulus after each freezing-thawing cycle are most evident in higher frequency ranges (above 100 Hz), since the contribution from EPI is inversely proportional to frequency.
The positive phase angle seen in the MHz range can be interpreted as if the veins demonstrate inductive properties. However, studies have shown that the existence of inductive properties in tissue samples and cell membranes under stable conditions in passive bioimpedance measurements is not very likely (Cole 1972, Riu 2004). Nevertheless, the positive phase angle does not need to be a sign of measurement error and can be caused by the contribution of self-inductance when measuring low impedance values (<50Ω) at high frequencies, such as in our case. Especially when a two-electrode setup is used, the self-inductance of the measuring lead and wire would be in series with the tissue impedance and in this way contribute to the measurements. Hence, the lower the impedance value is, the larger the inductive influence would be (Grimnes and Martinsen 2007).
This study has shown that a bioimpedance spectroscopy technique is capable of detecting changes in venous grafts after freezing-thawing cycles. However, the mechanisms and types of morphological changes in the vascular grafts that have led to these distinct changes in the impedance spectra are unknown and require further investigations. In addition, optimization of the measurement setup and electrode configuration, in order to minimize the contribution of electrode impedance polarization, is recommended. In this way, the arcs representative of α-dispersion, which are the result of structural changes in the cells and the venous segment, would become even more visible.
5. Conclusion
From the results of this study, it can be concluded that bioimpedance spectroscopy has the potential to be developed into a novel method for non-invasive and non-destructive monitoring of the viability of complex tissue after cryopreservation.
Conflict of Interest
The authors do not have any potential conflicts of interest to declare.
Ethical approval
The research related to animal use has complied with all the relevant national regulations and institutional policies for the care and use of animals.