Abstract
Objective: As precision medicine is becoming a standard of care in selecting tailored rather than average treatments, physiological measurements might represent the first step in applying personalized therapy in the intensive care unit (ICU). A systematic assessment of respiratory mechanics in patients with the acute respiratory distress syndrome (ARDS) could represent a step in this direction, for two main reasons.
Approach and Main results: On the one hand, respiratory mechanics are a powerful physiological method to understand the severity of this syndrome in each single patient. Decreased respiratory system compliance, for example, is associated with low end expiratory lung volume and more severe lung injury.
On the other hand, respiratory mechanics might guide protective mechanical ventilation settings. Improved gravitationally dependent regional lung compliance could support the selection of positive end-expiratory pressure and maximize alveolar recruitment. Moreover, the association between driving airway pressure and mortality in ARDS patients potentially underlines the importance of sizing tidal volume on respiratory system compliance rather than on predicted body weight.
Significance: The present review article aims to describe the main alterations of respiratory mechanics in ARDS as a potent bedside tool to understand severity and guide mechanical ventilation settings, thus representing a readily available clinical resource for ICU physicians.
Export citation and abstract BibTeX RIS
1. Introduction
Since the first description in Ashbaugh et al (1967), respiratory mechanics have been widely studied as a useful monitoring tool to assess the severity of acute respiratory distress syndrome (ARDS). Indeed, stiff lungs requiring elevated ventilation pressure to obtain normal gas exchange are widely recognized as hallmarks of ARDS (Gattinoni and Pesenti 2005). Lately, technological advances and in-depth understanding of ventilator-induced lung injury (VILI) have further increased knowledge of the significance of global and regional respiratory mechanics in ARDS (Bellani et al 2011). Simple measures such as the airway driving pressure have been described as powerful independent predictors of ARDS mortality (Amato et al 2015), and complex assessment of alveolar interactions such as dynamic ventilation heterogeneity was suggested as a key mechanism underlying VILI (Mauri et al 2013).
In the present review article, we will present classical and more recent measures of respiratory mechanics and show how they can support the clinical, diagnostic and therapeutic work-up of ARDS. Respiratory mechanics might foster an understanding of the individual severity (i.e. the so-called ARDS 'phenotype') and become the target of personalized mechanical ventilation settings.
2. Respiratory system compliance
The lungs of patients with ARDS are mechanically heterogeneous and vary in their patterns of infiltration and mechanical properties. In its earliest phase, ARDS is characterized by high-permeability edema, and the alveolar airspace is occupied by proteinaceous fluid and cellular infiltrate. Destruction of surfactant-producing type II alveolar cell leads to decreased production with increased surface tension and tissue elastance, small airway collapse, alveolar flooding, and atelectasis (Ashbaugh et al 1967).
Thus, the filling of airspace by inflammatory cells and fluid and the contemporary loss in surfactant function result in severely reduced static respiratory system compliance (Crs), defined as tidal volume (Vt) divided by the difference between end-inspiratory plateau pressure (Pplat) and total positive end-expiratory pressure (PEEP), obtained during occlusions (figure 1):

Figure 1. Effects of increasing pressure on respiratory system compliance. Airway pressure, flow and tidal volume waveforms obtained during volume-controlled ventilation at two different PEEP levels in a passive lung simulator (Dual Adult Test Lung Simulator, Michigan Instruments, Grand Rapids, MI, USA). End-inspiratory and end-expiratory holds (black and white arrows, respectively) were performed to measure tidal volume (Vt) and driving pressure (ΔPrs) and to calculate respiratory system compliance (Crs). In panel A, Vt = 504 ml, ΔPrs = (Pplat – PEEPtot) is 20 cm H2O and Crs = Vt/ΔPrs = 25 ml cm−1 H2O at PEEP 5 cm H2O. In panel B, Vt decreased to 464 ml (6.2 ml kg−1 IBW), ΔPrs decreased at 19 cm H2O and Crs = Vt/ΔPrs decreased to 24 ml cm−1 H2O at PEEP 15 cm H2O. In this simulated case, increasing PEEP led to over-distension, reducing Crs and Vt.
Download figure:
Standard image High-resolution imageMost commercially available ventilators continuously display dynamic Crs during pressure-controlled ventilation, calculated as tidal volume divided by peak inspiratory pressure minus set PEEP. Among the advantages of dynamic Crs monitoring are that it is continuous and does not alter the breathing pattern of the patient. However, static and dynamic Crs can differ significantly in the presence of a short inspiratory time, elevated airway resistance, intrinsic PEEP, and active inspiration (Stahl et al 2006, Stenqvist et al 2008).
During assisted mechanical ventilation, Crs is measured in the same way, albeit with some patients it could be difficult to relax them and to obtain accurate Pplat and total PEEP values.
Compliance decreases during ARDS because of airspace loss due to inflammation; alveoli are collapsed and unable to expand during inspiration. This typical pattern of lung restriction during ARDS was originally called 'baby lung', defined as the fraction of apparently healthy lung parenchyma that still maintains inflation (Gattinoni et al 2016). Gattinoni and his group described how the size of the baby lung is linearly correlated with respiratory system compliance and thus with the stiffness of the ARDS lungs. In other words, they hypothesized that compliance might reflect the size of this baby lung, and they suggested that the lung during ARDS is small rather than stiff, and that the elastic properties of residual healthy lung are nearly normal, as indicated by the specific compliance (compliance/normally aerated tissue), which is similar between healthy subjects and ARDS.
They also described how the baby lung concept is not an anatomical one: considering the patient lying supine on the bed, lung units located in (gravitationally) dependent regions are compressed by lung weight above and consolidated by inflammation cells and fluid, while the residual non-dependent lung units are nearly normally inflated, and they represent the baby lung. The use of the prone position, in which the baby lung considered as an anatomical region was expected to become non-dependent, was expected to improve oxygenation by increasing its perfusion. However, at CT scan analysis it was described how the atelectatic and collapsed alveoli were redistributed to the dependent regions of the lung (Gattinoni and Pesenti 2005) and that the mechanisms improving oxygenation during proning are likely more complex.
The first attempt to correlate the severity of ARDS (lung size, edema, fibrosis, etc) and its mechanical properties assessed through the pressure–volume curve method (P/V curve) in acute respiratory failure (ARF) patients was reported by Matamis et al (1984). They used chest x-rays to monitor anatomical lung changes which were correlated with clinical derangements.
A few years later, Gattinoni and co-workers (Gattinoni et al 1987) studied the relationship between anatomical structure derived by CT scan (which offers more accurate regional information on lung densities than conventional radiography) and lung mechanics, derived by the P/V curve in ARDS patients. They correlated the mechanical proprieties of the lungs with CT scan analysis such as lung gas volume and tissue weight (over-aerated, or normally, poorly, or non-aerated lung tissue), and they found that the compliance appeared to reflect the amount of normally aerated lung tissue. Interestingly, respiratory system compliance was not related to the amount of poorly aerated or non-aerated tissue, commonly considered as the factor indicating ARDS severity.
However, seminal CT scan studies already indicated that lungs of ARDS patients are not homogenously affected. This topic will be more extensively covered later in this review. The authors constructed a three-zone model of an ARDS lung, with an H (health) zone, with normal morphologic features, an R (recruitable) zone and a D (diseased) zone, which is consolidated and unresponsive to pressure changes. Compliance appeared to be a useful index to indicate the extent of both the H and R zones: the smaller the H and R zones are, the more severe the lung injury.
One strategy proposed to re-inflate the collapsed region of the lung and improve compliance is to provide a brief increase in airway pressure to levels higher than for normal ventilation: recruitment manoeuvres (RMs) and Sigh have been used in this manner for the ventilation of ARDS patients. RMs are usually associated with positive short-term physiological effects such as increased pulmonary compliance and reduced intrapulmonary shunt (Hodgson et al 2016).
The positive effects of RMs on compliance were shown by Amato and co-workers (Amato et al 1995) in a study in which ARDS patients were randomized in two groups, the first ventilated in a conventional way (Vt = 12 ml kg−1, minimum PEEP guided by FiO2 and hemodynamic parameters and normal PaCO2 levels) and the second ventilated with another approach inducing recruitment (PEEP above the lower inflection point of the pressure–volume curve, Vt ⩽ 6 ml kg−1, peak pressure < 40 cm H2O and permissive hypercapnia). The results showed that the use of high tidal volume was associated with lung injury and a decrease in compliance due to the presence of alveolar overdistension and cycling reopening of collapsed alveoli, while patients in the recruitment approach showed a significant improvement in compliance, especially during the first week.
However, Ranieri et al (1991) showed that many ARDS patients may present an upward concavity of the pressure–volume curve without a lower inflection point. This situation probably reflects progressive alveolar recruitment with increasing PEEP levels, suggesting the presence of an inhomogeneous lung disease with different 'opening pressures' across the lung. They developed the stress index, which detects changes of compliance over time (i.e. at increasing airway pressure), which might be a better target for the optimization of PEEP and recruitment rather than compliance per se. One must note that the stress index can be evaluated only during volume-controlled ventilation in completely passive patients.
Considering the baby lung theory and the risk of cyclic alveolar opening and closing during mechanical ventilation, current treatment recommendations consist of limiting tidal volume, keeping plateau pressure below 28–30 cm H2O, and maintaining sufficiently high PEEP to improve oxygenation. This so-called 'protective ventilation strategy' is thought to reduce lung mechanical stress due to the repetitive opening and closing of alveoli maintaining alveolar aeration and reducing atelectrauma and VILI. To this end, PEEP selected on the best respiratory system compliance (i.e. the compliance associated with the maximum number of ventilated units) during a decremental PEEP trial has been described as effective and seems to be a physiologic way to personalize PEEP.
Considering that severe ARDS patients are unresponsive to conventional treatment with low compliance and/or extremely high plateau pressure (mortality rate > 50%), they may be candidates for extracorporeal membrane oxygenation (ECMO). Gattinoni et al applied a protocol in which patients meeting the ARDS criteria for ECMO on continuous positive pressure ventilation (CPPV) were switched to pressure-controlled inverted ratio ventilation (PC-IRV). If within 4 h there was no improvement in oxygenation, patients were initiated to ECMO. Instead, if during PC-IRV patients' gas exchange improved, they were switched to spontaneous breathing with continuous positive airway pressure (CPAP). The authors stated that the only predictive value for the success of assisted ventilation was respiratory system compliance. Patients with a compliance lower than 25 ml cm−1 H2O did not tolerate the switch to PC-IRV and CPAP, thus ending on ECMO. The authors concluded that this compliance is one of the most useful parameters in the management of severe ARDS patients' unresponsiveness to conventional treatment (Gattinoni et al 1984).
Franchineau and colleagues proposed to personalise PEEP titration in severe ARDS patients undergoing ECMO by monitoring them during a decremental PEEP trial through electrical impedance tomography (EIT). EIT can provide a dynamic index of lung over-distension and collapse based on regional compliances (see section 9 below). The authors, by minimizing this index, showed a broad variability in the optimum PEEP level (in the range 5–20 cm H2O), strengthening the need for personalized physiology-based PEEP titration in these patients with severe ARDS (Franchineau et al 2017).
3. Airway resistance
Data from several animal models of ARDS suggest that airflow resistance is increased in the case of diffuse lung injury (Esbensahde et al 1982, Brigham and Meyrick 1986, Christley et al 2013). Total respiratory resistance in ARDS patients includes airway resistance and 'additional' respiratory resistance that results from dynamic dissipation due to altered viscoelastic properties of the respiratory system (lung and chest wall) and time-constant inequalities within the lung parenchyma (Bernasconi et al 1988, Eissa et al 1991, Antonaglia et al 2005). Thus, the increase in airway resistance in ARDS can be ascribed to airway flooding, reduced lung volume, vagal reflex and airway hyperactivity.
During volume-controlled square flow ventilation of paralyzed patients, respiratory system resistance (Rrs) is easily measured during end-inspiratory occlusion (figure 2) as peak airway pressure (Ppeak) minus Pplat divided by the inspiratory flow (ϕ):

Figure 2. Measuring airway resistance at the bedside during volume-controlled ventilation. Airway pressure, flow and volume curves obtained during volume-controlled ventilation in a passive lung simulator (Dual Adult Test Lung Simulator, Michigan Instruments, Grand Rapids, MI, USA) at PEEP 5 cm H2O. End-inspiratory hold enables accurate measures of peak airway pressure (Ppeak) and plateau pressure (Pplat). Airway resistance (Rrs) can then be calculated as Ppeak − Pplat/ϕ = (27–24)/0.37 = 8 cm H2O/l * s−1.
Download figure:
Standard image High-resolution imageDuring pressure-controlled and assisted ventilation, inspiratory resistances are more difficult to measure and usually they are not routinely monitored.
Pesenti and colleagues (Pesenti et al 1991) investigated the acute effects of PEEP upon respiratory resistance in ARDS patients. They studied an ARDS group of patients and compared them to normal anesthetized patients. In both groups, they measured elastance (the reciprocal of compliance) and resistance at three levels of PEEP (0, 5, and 10 cm H2O) in normal subjects and five PEEP levels (0, 5, 10, 15, 20 cmH2 O) in the ARDS group. Considering inspiratory resistance, they found that Rrs was significantly higher in the ARDS group compared to normal intubated subjects, and it changed significantly with PEEP. Moreover, the authors measured the difference between resistance (ΔRrs) measured at the end of the inspiratory flow (Rrs-min) and after obtaining a stable Pplat (Rrs-max):

It was noted that ΔRrs was significantly different between the groups (normal versus ARDS patients) and it increased at higher PEEP levels. Moreover, in both groups (normal and ARDS patients), Rrs-max did not decrease until PEEP was lower than 10 cm H2O. Considering these results, the authors suggested that Rrs-max reflects the stress relaxation of the parenchyma and time-constant inhomogeneity between lung units, i.e. between over-distended units with short time constants and collapsible units with long time constants. Higher levels of PEEP could further over-distend some lung regions, increasing lung inhomogeneity. This could determine an increased 'pendelluft' phenomenon at high PEEP level with increased Rrs-max and ΔRrs.
Pelosi and co-workers (Pelosi et al 1995) confirmed similar results a few years later, when measuring Rrs-max and ΔRrs in mild and severe ARDS patients in comparison to normal subjects. They found that Rrs-max was markedly increased in ARDS, mainly by an increase in lung respiratory resistance, while the contribution of chest wall resistance was rather small. In respect to PEEP, they found that increasing levels of PEEP markedly augmented ΔRrs of the lung, resulting in increased Rrs-max of the lung and unchanged Rrs-max of the chest wall. Considering this result, as ΔRrs should reflect stress adaptation and time-constant inequalities within the lung parenchyma, they speculated that PEEP may change the viscoelastic properties of the lung by over-distending inflated lung regions, thus increasing maximum and additional lung resistance.
However, in mechanically ventilated patients, Smith and Marini found that PEEP of up to 10 cm H2O may determine a reduction in expiratory resistance as suggested by decreased driving pressure, increased compliance and unchanged expiratory flow. A possible explanation is that this happens in patients with expiratory flow limitation outset by higher PEEP levels (Smith and Marini 1988).
Eissa and co-workers studied the effects of inspiratory flow and inflation volume on the mechanical properties of the respiratory system of ARDS patients in comparison to normal subjects, by using the technique of rapid airway occlusion during constant-flow inflation (Eissa et al 1991). They found that airway resistance in the ARDS patients was higher than in healthy paralyzed volunteers. In normal subjects, it also decreased slightly but significantly with increasing volume. They supposed that the decrease in airflow resistance in healthy subjects with increasing lung volume is likely to be concomitant with an increase in airway diameters.
Considering that increased resistance may be due to airway hyper-reactivity secondary to inflammation, Wright and colleagues (Wright et al 1994) studied the reversibility of increased airflow resistance in ARDS patients. They designed a randomized, placebo-controlled cross-over study, in which ARDS patients received either an aerosol of β2-agonist or a placebo. They found that the aerosolized β2-agonist significantly reduced airflow resistance compared to baseline and placebo values. Furthermore, the β2-agonist increased both dynamic and static lung compliance. Thus, they supposed that it could result in a reduction of the risk of lung parenchymal trauma during mechanical ventilation.
In conclusion, all the studies mentioned report that total resistance is increased in ARDS patients in comparison to normal subjects. An adequate PEEP level may reduce resistance in the presence of flow limitation, but, to the contrary, a high PEEP level (more than 10 cm H2O) (Pesenti et al 1991, Pelosi et al 1995) and flow and volume variation (Eissa et al 1991), may worsen the viscoelastic properties of the lung, increasing resistance. However, even if increased respiratory resistance in ARDS patients may be considered as a hallmark (of decreased compliance), resistance is rarely measured and treated, unlike in cases of obstructive pulmonary disease. One simple approach could be to use bronchodilators when higher inspiratory resistances are detected, especially if using a PEEP level higher than 10 cm H2O.
4. End expiratory lung volume and PEEP
The functional residual capacity (FRC) is the volume of gas present in the lungs at the end of passive expiration. When PEEP is applied, the FRC is commonly named as the end expiratory lung volume (EELV). EELV is the minimum volume of gas present in the lungs throughout the entire respiratory cycle upon which the tidal volume is added during inspiration. The EELV is mainly determined by the balance between the elastic recoil forces of the lungs and the opposing forces of the chest wall and the applied PEEP. The EELV is the sum of the expiratory reserve volume, which is the maximal volume of gases that can be exhaled from the end-expiratory position, and the residual volume, which is the volume of gases remaining in the lungs after a maximal exhalation. The expiratory reserve volume can be measured through spirometry while measurement of the residual volume requires techniques such as nitrogen washout, helium dilution, body plethysmography or CT scan.
The EELVs of adult men with healthy lungs is commonly above 3000 ml when a PEEP of a few centimetres of water is applied (Patroniti et al 2008). In diseased lungs the EELV may decrease to a value as low as 500 ml (Patroniti et al 2010), which is highly impairing to the lung gas exchange function and the Crs. The reduction in EELV may be due primarily to alveolar collapse, pulmonary edema with alveolar flooding, pulmonary inflammation, or reduction of thoracic compliance.
Modern ventilation strategies aim to optimize the EELV to improve the gas exchange while preventing VILI. EELV is the primary volume of gases contributing to the gas exchange with the blood. If the lung parenchyma, no longer being aerated and causing a reduction in the EELV, preserves its perfusion, the blood that irrigates such parenchyma does not participate in the gas exchange. The intrapulmonary shunt thus increases, leading to hypoxemia. Luckily, there are mechanisms which reduce the pulmonary perfusion in unventilated regions, mainly hypoxic vasoconstriction, reducing therefore the amount of intrapulmonary shunt. But lung diseases may alter such protective mechanisms, thus maintaining elevated intrapulmonary shunt fractions.
The number of ventilated alveoli is a major factor in determining the compliance of the respiratory system. For example, during ventilation at constant volume, a 50 percent reduction in the number of ventilated alveoli (approximately, as it occurs during one lung ventilation) will lead to an approximately doubled driving pressure. Thus, a decreased EELV may contribute to the onset or maintenance of VILI, since the inspired tidal volume can be distributed in a smaller volume, leading to possible barotrauma or volutrauma.
In the era of personalized medicine, measuring the EELV seems to be a promising technique to properly set the mechanical ventilator and monitor the progress of lung disease. Unfortunately, until recently, no simple bedside techniques were available to measure EELV. Therefore, this promising technique has never entered clinical practice and consequently data supporting its use are lacking.
Indeed, in the last 40 years computerized tomography has been used to evaluate diseased lungs (Gattinoni et al 1987). A CT scan can be employed to measure EELV, but that exposes patients to radiological risks and also requires the patient to be transferred to the CT facility, with the attendant risks and expense.
EELV can be measured at the bedside by employing one of several techniques based on the dilution of tracer gases (Patroniti et al 2004, Chiumello et al 2008). Some of these techniques require ventilation of the patient with a closed circuit of known volume and baseline concentration of an inert gas, commonly helium (rebreathing techniques). Within the space of a few breaths, by measuring the final concertation of the tracer inert gas, it is possible to compute the EELV. This technique, to be applied in mechanically ventilated patients, requires either discontinuation of basal ventilation or complex modification of the breathing circuit to maintain the basal ventilation during measurement.
Other techniques, employing open circuits (non-rebreathing techniques), measure the EELV by changing the inspiratory concentration of a gas used as a tracer (either oxygen or nitrogen or an inert gas such as helium or sulphur hexafluoride SF6) and measuring the amount of such gas required to reach the new equilibrium (Richard and Guerin 2013). Some of these techniques, which require fast and sensitive gas analyzers and a precise synchronization between gas concentration and airway flow signals, are now (thanks to improved technology) implemented in commercially available mechanical ventilators and monitors. By changing the fraction of inspired oxygen, these devices can give a reliable measure of EELV, especially during controlled mechanical ventilation. It is to be hoped that these new devices could lead to a more widespread diffusion of EELV measurement and will help to clarify the clinical role of EELV measurement.
As previously mentioned, EELV is reduced in the presence of ARDS, as described also in the 'baby lung' concept introduced in the mid-1980s (Gattinoni and Pesenti 2005). The first CT examinations of adult patients suffering from ARDS showed non-homogeneous lung parenchyma with an estimated aerated lung tissue of the size of a healthy young baby. Since the mechanical properties of the aerated lungs are almost normal, the compliance of the respiratory system, in the presence of normal chest wall compliance, reflects well the volume of the aerated lung (EELV if measured at end expiration).
Subsequently, it was also discovered that the aerated parenchyma was primarily located in the non-dependent regions of the chest cavity. These data helped in the development of the concept of 'sponge' lung, which suggests that the diseased lung has an impaired permeability leading to interstitial edema. The increased lung weight causes the collapse of the dependent regions, unless an airway pressure (i.e. PEEP) higher than the superimposed pressure is applied. The sponge lung model seems to be likely applicable to patients suffering from ARDS of extra-pulmonary origin (as in sepsis), while the interstitial edema seems to be located more inhomogeneously in ARDS of pulmonary origin (as in pneumonia).
The current ventilation strategy aims to prevent the reduction of EELV by averting lung collapse through application of an adequate pressure (PEEP). However, once the alveoli are de-recruited, in order to open them it is necessary to apply a pressure higher than that required only to keep them open. Such recruitment pressure can be as high as 45 cm H2O (recruitment manoeuvre). By means of an effective recruitment and an adequate PEEP level it may be possible to minimize the intrapulmonary shunt, thus maximizing the oxygenation properties of a diseased lung. Moreover, a recruited lung has a higher EELV, and thus the tidal volume will then be distributed in a higher number of alveoli (i.e. lower Vt/EELV ratio), reducing the risk of VILI (Bellani et al 2011). Too high a PEEP, in contrast, may favour the onset of VILI by inducing over-distension (figure 1).
While selecting PEEP, it is also important to assess the volume status of the patient, as a higher PEEP might increase the intrathoracic pressure and have a negative impact on the patient's cardiac output. Consequently, in the acute phase of respiratory failure, when substantial levels of PEEP are needed, it is commonly required to achieve a positive fluid balance in order to maintain an adequate oxygen delivery. When compliance and oxygenation improve and PEEP can be lowered, a negative fluid balance may be desirable. An optimal level of PEEP will seek the right balance between the oxygenation target, VILI prevention, and hemodynamic stability.
5. Alveolar recruitment
At a constant PEEP and under steady state conditions, the application of a recruitment manoeuvre may cause an increase in the EELV (if there is recruitable lung volume), which would be mainly attributable to an increase in the number of aerated alveoli. This represents an accurate method of assessing alveolar recruitment. In this condition, if EELV measurement is not available, we may expect an increase in oxygenation and respiratory system compliance (i.e. an increased tidal volume at constant pressure ventilation or a reduced driving pressure at constant volume ventilation). Improved CO2 clearance may be noted, but it is not always present.
Assessing lung recruitment at different PEEP levels is more challenging. An increase in PEEP (and the associated increase in plateau pressure) commonly leads to an increased EELV, but such higher EELV may be solely due to an increase in the distension of previously opened alveoli (where no recruitment occurred) or a combination of distension of previously opened alveoli plus recruitment and expansion of previously collapsed alveoli (recruitment occurred). A distinction is to be made between these two situations for the optimizing of the ventilator setting. Many strategies can be used to evaluate lung recruitment, each of them measuring specific aspects of the alveolar recruitment. Computed tomography can show the amount of lung tissue that shifts from a non-aerated to an aerated state (figure 3).
Figure 3. Alveolar recruitment assessed at higher PEEP level by CT scan imaging. Static end-expiratory chest computed tomography images showing significant lung recruitment obtained by increasing PEEP from 12 cm H2O (image on the left) to 20 cm H2O (image on the right) in a sedated and paralyzed representative severe ARDS patient. Note reversal of alveolar collapse at higher PEEP in the mid-dorsal lung, while upper non-dependent units were more at risk of over-distension during tidal breathing.
Download figure:
Standard image High-resolution imageCT exposes the patient to the risks and inconveniences listed above. At least two scans are necessary, and it requires rather complex image analysis, which is not yet standardized.
Lung ultrasound (Lichtenstein 2014) is a non-invasive dynamic tool applicable at the bedside. Learning the technique takes time but it is proving to be a reliable instrument, useful to diagnose the presence of lung collapse, to identify the opening and closing pressures, and to assess the hemodynamic.
By measuring EELV at two PEEP levels and the respiratory system compliance at the lower PEEP, it is possible to estimate the recruited volume. Assuming there is no lung recruitment, the compliance at the lower and higher PEEP would be similar (if no lung over-distension occurs). Thus, the higher PEEP EELV could be easily computed (EELV at low PEEP plus the compliance multiplied for the pressure difference between low and high PEEP) and it should be similar to the EELV measured at the higher PEEP. If the measured high PEEP EELV is higher than the computed figure, one might speculate that lung recruitment occurred.
Similarly, recruitment may also be measured as the volume difference between two P/V curves measured at different starting PEEP levels (Maggiore et al 2003). The inspiration P/V curve shape can provide information on the recruitability of the lungs. A curve with an upward concavity, defining an inflection point (commonly named lower) and the presence of hysteresis in the inflation–deflation curve suggest a recruitable lung. The standard method of use of the P/V curves is still debated.
Also, the EIT may be employed to estimate lung recruitment by evaluating the variation in the end expiratory impedance values at different PEEP levels or changes in homogeneity (see below) (Frerichs et al 2017).
Alveolar recruitment minimizes lung strain. Thus, measuring it at the bedside to guide ventilation settings might be an effective clinical strategy to improve ARDS outcomes.
6. Plateau pressure
The equation of motion of the respiratory system states that the pressure applied to the system (Prs) during ventilation is the sum of the pressure generated by the ventilator (measured at the airway opening, Paw) and the pressure developed by the respiratory muscles (Pmus); this pressure is needed to overcome airway resistance and generate airflow (Pres) and to overcome elastic forces (Pel) (Lucangelo et al 2005). During controlled mechanical ventilation, Pmus is zero and the equation of motion becomes
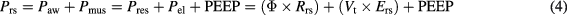
where Rrs is airway resistance, Vt is tidal volume and Ers is respiratory system elastance. Pplat is the pressure measured at the airway opening at end inspiration during static conditions (i.e. absence of airflow), after complete equilibration among alveolar and airway pressure. In the absence of flow, Pres is zero and alveolar and airway pressures coincide. Under these conditions, Pplat reflects the elastic recoil pressure of the entire respiratory system (Lucangelo et al 2005). Pplat is measured during an end-inspiratory occlusion, simply by pushing the 'inspiratory hold' button available on all commercially-available ICU ventilators. During the pause, the flow drops to zero, the volume is trapped inside the lung and static airway pressure is measured. It is important to perform an occlusion of at least 2 s, to allow equalization of the pressure in alveoli with different time constants.
This is valid both for volume-controlled ventilation (VCV) and for pressure-controlled ventilation (PCV). It is noteworthy that during PCV, Pplat measured by means of an end-inspiratory occlusion can significantly differ from the set value of inspiratory pressure.
Finally, Pplat can also be measured during pressure-support ventilation (PSV), an assisted mode of ventilation. In this case, Pmus is not zero since the patient's inspiratory muscles generate a certain amount of pressure, and the patient and the ventilator share the work of breathing. During the end-inspiratory occlusion manoeuvre, if the patient relaxes the inspiratory muscles, a stable plateau can be identified on the airway pressure tracing. Just like during controlled mechanical ventilation, this represents the elastic recoil pressure of the system. This value of Pplat can be higher than, equal to or lower than the pressure delivered by the ventilator (which is the sum of PS + PEEP); the difference is called Pmus index (PMI) and corresponds to the pressure developed by the respiratory muscles at end inspiration (Foti et al 1997). However, performing an end-inspiratory occlusion manoeuvre in a spontaneously breathing patient requires the complete cessation of any inspiratory effort during the manoeuvre. If a stable plateau is not identifiable, the value is not reliable and the manoeuvre must be repeated.
Monitoring Pplat during mechanical ventilation is extremely important for at least two reasons.
First, it is necessary to assess the elastic properties of the respiratory system and to calculate the compliance of the system (Crs). It is well known that Crs in ARDS patients is directly related to the size of the 'baby lung' (the portion of lung parenchyma available for ventilation), which is in turn related to the severity of the disease.
Second, since during static conditions the airway and alveolar pressures are in equilibrium, Pplat is considered to be a reliable estimate of the lung-distending pressure and consequently of the amount of lung stress, a major determinant of VILI.
This has been clearly demonstrated by the seminal ARMA trial, in which 861 ARDS patients were randomized to low Vt ventilation (6 ml kg−1 of predicted body weight or adjusted to keep Pplat lower than 30 cm H2O) or to 'traditional' Vt (12 ml kg−1 or Pplat lower than 50 cm H2O). The patients in the low Vt − low Pplat group had a significantly lower mortality (31 percent versus 40 percent, p = 0.007) (ARDSnet 2000). Based on these results, limiting Vt and keeping Pplat to < 30 cmH2 O are still considered the cornerstone of ventilatory strategy for ARDS patients.
However, the threshold of 30 cmH2 O is debated. Terragni et al using CT scan images (Terragni et al 2007) and Bellani et al using positron emission tomography (Bellani et al 2011) demonstrated that tidal hyperinflation and lung inflammation can be detected in a significant proportion of patients, despite the use of 'protective' Vt and Pplat. Based on these observations, several experts recommend a lower target of Pplat (usually lower than 27 cm H2O). The main limitation of the use of Pplat as a crude surrogate for lung stress depends on the fact that the true lung-distending pressure is not static airway (alveolar) pressure but rather transpulmonary pressure (discussed in the following paragraph), that considers the pleural pressure variations due to chest wall elastance. In practice, a value of Pplat of 30 cm H2O can both over- and under-estimate lung stress in patients with different mechanical properties of the lungs and the chest wall.
7. Driving pressure
During mechanical ventilation, airway driving pressure across the respiratory system (ΔPrs) is defined as the pressure delivered by the ventilator to inflate the system. ΔPrs equals the tidal pressure change between total PEEP (PEEPtot, the sum of external PEEP + intrinsic PEEP) and end-inspiratory Pplat:

Here, ΔPrs must be calculated under static (no-flow) conditions, by means of an end-inspiratory pause and an end-expiratory pause. It can be easily measured at the bedside in patients who are not making respiratory efforts.
Recently, many authors have affirmed that, in patients with ARDS, ΔPrs can be considered the best surrogate for assessing lung stress and strain at the bedside (Amato et al 2015). For this reason, the lowering of ΔPrs has been proposed as a means of reducing the risk of VILI. The concept of lung-protective ventilation is based on the application of lower Vt, lower Pplat and higher PEEP levels, to reduce the amount of external energy applied to the lung, mainly due to the dynamic stress and strain caused by tidal insufflation (Slutsky and Ranieri 2013). To this end, Vt should be scaled to the proportion of the lung available for ventilation, which corresponds to end-expiratory lung volume (EELV) and is known to be significantly reduced in ARDS patients ('baby lung'). Since measures of EELV are not routinely available, it is recommended not to exceed the limit of Vt of 6 ml kg−1 of ideal body weight (IBW). However, scaling Vt to IBW does not accurately reflect the amount of aerated lung tissue and is not related to lung stress.
On the contrary, compliance of the respiratory system is linearly related to the size of the 'baby lung' (Gattinoni and Pesenti 2005). Since the tidal ΔPrs is equal to the ratio between Vt and Crs according to the equation

it can be viewed as a way to normalize Vt for the lung's capacity to accept it, thereby integrating two key factors of mechanical stress. For this reason, limiting ΔPrs may be a better way to titrate the Vt to the actual size of the aerated lung, thus reducing lung stress and strain and possibly improving patient outcome.
In a recent important retrospective trial, Amato et al analysed the effect of ΔPrs on the survival of ARDS patients. They reviewed individual data from 3562 patients with ARDS enrolled in nine randomized trials and performed a mediation analysis to estimate the effect of ΔPrs changes resulting from different ventilator settings. ΔPrs was the ventilation variable that had the strongest association with survival; the relative risk of mortality significantly increased above a threshold of ΔPrs of 14 cm H2O, even in patients ventilated with 'protective' Vt and Pplat. Interestingly, in patients with higher Pplat values, mortality increased only when high Pplat was associated with higher ΔPrs; similarly, higher PEEP levels had a protective effect only when associated with low ΔPrs. Finally, at constant Pplat, a lower Vt was a strong survival predictor when normalized to Crs (i.e. ΔPrs) but not to IBW (Amato et al 2015).
Also, the recently published Lung Safe study confirmed the strong prognostic value of ΔPrs, with mortality rate increasing with ΔPrs (Bellani et al 2016a, Laffey et al 2016) (figure 4).
Figure 4. Correlation between driving airway pressure and mortality in ARDS. In a large international cohort of ARDS patients enrolled in the Lung Safe study, driving pressure higher than 14 cm H2O was associated with worse outcome of moderate and severe ARDS. Adapted from Laffey et al (2016). Copyright © 2016, Springer-Verlag Berlin Heidelberg and ESICM. With permission of Springer.
Download figure:
Standard image High-resolution imageA similar effect on survival was reported by Guerin et al, who retrospectively reviewed the data from two large randomized studies on 787 ARDS patients, in whom Vt and Pplat were strictly maintained within the 'safe' limits of 6 ml kg−1 and 30 cm H2O, respectively. They found a significant association between ΔPrs and mortality, observing a 5 percent increase in the risk of death for each 1 cm H2O increase in ΔPrs and a significant mortality reduction when ΔPrs was lower than 13 cmH2 O (Guerin et al 2016). However, at variance with Amato's study, ΔPrs was not superior to Pplat, Vt and Crs as a predictor of death, and the authors concluded that when protective ventilator settings are strictly applied, ΔPrs shares the same prognostic information as Pplat and Crs.
An inherent limitation of the concept of airway driving pressure resides in the fact that it represents the pressure change across the entire respiratory system, which is composed of two structures in series, the lung and the chest wall. As we have seen in the previous paragraph, the portion of ΔPrs used to expand the lung and the chest wall depends on their relative compliances (or elastances).
Thus, transpulmonary driving pressure (ΔPL) should better reflect lung stress than airway driving pressure. For example, in a patient with normal lung compliance (CL) but very low chest wall compliance (Ccw), a certain Vt can generate a high ΔPrs but a low ΔPL and consequently a low lung stress.
ΔPL is the difference between the static value of driving airway and pleural pressures, with pleural pressure estimated by esophageal pressure (Pes):
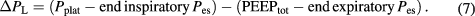
To evaluate the difference between ΔPrs and ΔPL, Cortes-Puentes et al (2015) performed an experimental study causing reversible modifications of Ccw in animals with different lung pathologies. As expected, they found that ΔPrs is significantly affected by changes in Ccw and by underlying lung properties.
However, Chiumello et al recently measured ΔPrs, ΔPL, lung stress and partitioned compliance in 150 ARDS patients at two PEEP levels (Chiumello et al 2016). At both PEEP levels, ΔPrs showed significant correlation with ΔPL. Moreover, patients with higher ΔPrs had a significantly higher lung stress, and a ΔPrs above 15 cm H2O accurately detected a lung stress exceeding 24 cm H2O.
Airway and transpulmonary driving pressures are promising measures for the identification of patients at risk of VILI, given their correlation to patient outcome. However, their role as therapeutic target for titration of mechanical ventilation settings is still unclear and should be evaluated in prospective randomized studies.
8. Transpulmonary pressure
The term "transmural pressure" refers to the pressure inside a compartment, relative to the outside. In hollow structures, transmural pressure is the pressure across the structure walls and, under static conditions, is equal to the elastic recoil pressure of the compartment. The transmural pressure of the lungs is transpulmonary pressure (PL). Since the pressure inside the lung is airway pressure (pressure at airway opening, Paw) and the pressure surrounding the lungs is pleural pressure (Ppl) (Sahetya and Brower 2016):

Here, PL can be divided into two parts: the pressure needed to overcome airway resistance and generate the airflow between the airway opening and the alveoli (resistive pressure), and the pressure needed to expand the alveolar compartment (which is due to the elastic recoil of the lung and is called transalveolar pressure;  ) (Bellani et al 2016b). Transalveolar pressure is the pressure across the alveolar wall and represents the actual stress imposed on the lungs during ventilation. It is equal to the difference between alveolar and pleural pressure
) (Bellani et al 2016b). Transalveolar pressure is the pressure across the alveolar wall and represents the actual stress imposed on the lungs during ventilation. It is equal to the difference between alveolar and pleural pressure

However, under static conditions (i.e. zero airflow), airway and alveolar pressure are in equilibrium and transpulmonary and transalveolar pressure almost coincide. At variance, under dynamic conditions, pressure at the airway opening and alveolar pressure are not the same, due to the resistive pressure drop.
It should now be clear that transpulmonary (and not airway) pressure is the 'real' pressure that distends the lungs, causing lung stress and VILI, and that mechanical ventilation settings should be adjusted to target 'safe' values of PL rather than of Paw. Since the respiratory system is composed of two elastic structures connected in series, the lung and the chest wall, the pressure applied at the airway opening is used in part to inflate the lungs and in part to expand the chest wall according to their relative compliances (or elastances) (see below). Thus, transpulmonary and airway pressures can be significantly different in the presence of chest wall abnormalities causing elevated pleural pressure values. In other words, the same Pplat of 30 cm H2O can be non-injurious in a patient with relatively normal lung compliance but a very stiff chest wall, or highly biotraumatic in a patient with stiff lungs and normal chest wall compliance (Mauri et al 2016).
Unfortunately, there are no simple and safe techniques allowing direct measurement of Ppl and the most common method used to estimate it at the bedside is esophageal manometry. The pressure measured by means of an air-filled catheter equipped with a long thin-walled balloon positioned in the lower third of the esophagus (Pes) is commonly considered to be a reasonable surrogate of the average Ppl surrounding the lungs in upright, healthy subjects (figure 5).
Figure 5. Driving transpulmonary pressure as a more sensitive measure of lung stress. Airway pressure (Paw), flow, esophageal pressure (Pes) and transpulmonary pressure (PL) waveforms recorded from a patient on volume-controlled ventilation (Vt 500–7.1 ml kg−1 IBW, respiratory rate 20, PEEP 8 cm H2O) with moderate ARDS. The difference between Paw and Pes is PL. As Pplat is 17 cm H2O and end-inspiratory Pes ( ) is 7 cm H2O, the resulting
) is 7 cm H2O, the resulting  is 10 cm H2O, while PEEP 8 cm H2O minus end expiratory Pes (
is 10 cm H2O, while PEEP 8 cm H2O minus end expiratory Pes ( ) of 3 cm H2O yield
) of 3 cm H2O yield  of 5 cm H2O. Thus, driving transpulmonary pressure (ΔPL = 5 cm H2O) in this patient is well within safe limits (i.e. 8–10 cm H2O) Mauri et al (2016).
of 5 cm H2O. Thus, driving transpulmonary pressure (ΔPL = 5 cm H2O) in this patient is well within safe limits (i.e. 8–10 cm H2O) Mauri et al (2016).
Download figure:
Standard image High-resolution imageHowever, the reliability of Pes as an estimate of Ppl in supine, mechanically ventilated patients is questionable, due to several possible sources of errors (Mauri et al 2016). Several studies have evaluated PL as a target for mechanical ventilation. The most relevant was conducted by Talmor et al in 61 patients with ARDS who were randomly assigned to mechanical ventilation with PEEP adjusted based on Pes measurement or on the PEEP-FiO2 table from the ARDS Network recommendations (Talmor et al 2008). PL was assessed with the 'direct calculation' method, i.e. by simply subtracting the absolute value of Pes to the pressure measured at the airway opening (PL = Paw − Pes). In the Pes-guided group PEEP was titrated to obtain positive values of end-expiratory PL, while maintaining end-inspiratory PL lower than 25 cm H2O; at variance, control patients frequently had negative end-expiratory PL values. Patients in the intervention group had significantly higher PEEP levels associated with a significant improvement in oxygenation and lung compliance.
Given the uncertain reliability of the absolute Pes values as an estimate of the actual Ppl, some authors have proposed to use the 'elastance-derived' method (Chiumello et al 2016) to calculate PL instead of the direct calculation method used by Talmor. This method is not based on the absolute Pes values, but uses tidal Pes variations to calculate chest wall elastance:

As explained above, the pressure at the airway opening is the sum of the pressure needed to inflate the lung (PL) and of that needed to expand the chest wall (Pcw):

As the change in volume of the lung and the chest wall is identical, the equation can be written as

being the elastance of the respiratory system (Ers) = Paw/Vt and lung elastance (EL) = PL/Vt, the equation can be written as

Grasso et al applied this method in a small cohort of 14 patients with influenza A referred for ECMO, in whom PEEP was titrated to target an end-inspiratory PL of 25 cm H2O, which is considered the upper physiological limit (Grasso et al 2012).
Gulati et al retrospectively compared the direct Pes measurement and the elastance-derived methods to estimate Ppl in 64 ARDS patients. The two methods did not correlate, giving Ppl estimates different by as much as 10 cm H2O for a given patient. They also compared the two strategies of PEEP optimization (i.e. targeting a positive end-expiratory PL using absolute Pes values versus targeting an end-inspiratory PL of 25 cm H2O using the elastance-derived method) and again found significantly discordant and unrelated results (Gulati et al 2013). In general, we might hypothesize that inequalities in Ppl values across the thorax might have led to these discrepancies.
9. Inhomogeneity
Healthy lungs are homogenous lungs; at end expiration, lung collapse is not present and the lungs appear uniformly expanded. During inspiration, then, the forces that distend the parenchyma to generate the negative alveolar pressure and the inspiratory airflow are homogenously distributed in structures with similar mechanical characteristics per unit of tissue, yielding uniform expansion. During ARDS, increased lung weight by lesion edema causes collapse of the alveolar structure. Collapse, most of the time, is in the dependent lung regions (i.e. dorsal collapse).
However, collapse of non-dependent regions positioned above well-aerated units is common, too, maybe because of heterogenous distribution of inflammation cytokine and cells. Lately, this inhomogeneity has increasingly been recognized as a relevant indicator of ARDS severity as well as a major determinant of VILI, with the two notions likely being closely connected (Cressoni 2014).
Some confusion arises as inhomogeneity in published studies has been intermutually referred to as both static end-expiratory aeration heterogeneity and as dynamic inspiratory ventilation inhomogeneity. The first refers to the inhomogeneous distribution of aerated and collapsed lung regions in the ARDS lung at end-expiration; it is normally assessed by CT scan and it represents a static picture of the specific ARDS phenotype (Cressoni et al 2014). The demonstrated correlation of this measure with clinical severity of ARDS likely derives from the fact that aeration heterogeneity causes ventilation heterogeneity. Indeed, a study on an animal model of ARDS showed that homogenization at end inspiration with small variations from the average expansion volume of the alveolar units is reached only in lungs that are already homogenous at end expiration (Mascheroni et al 1988, Yoshida et al 2013).
Thus, end-expiratory aeration inhomogeneity might be a marker of the more specific ventilation hetereogenety (i.e. inequal expansion of alveolar units during inspiration). Indeed, the early work of Mead and colleagues (Mead et al 1970) showed how in the presence of a distending force (change of transpulmonary pressure), local stress to alveolar walls is multiplied by the following equation:

Here, ΔPL represents change in transpulmonary pressure, V is the volume expansion of surrounding regions and V0 is the volume expansion of the target region.
Another study performed with PET (Wellman et al 2014) showed that local inflammation in the injured lung is proportional to the regional expansion divided by the starting volume, showing that alveoli which expand more from the starting volume might undergo more relevant stress. This indicates how ventilation inhomogeneity might be a causing factor of VILI and explain the correlations between aeration and ventilation heterogeneity with the outcome of ARDS.
A CT scan allows precise measurement of aeration heterogeneity at end expiration. A study by Cressoni et al quantified the ratio between air and surrounding tissue at the alveolar level and showed that more scattered distribution is correlated with ARDS severity (Cressoni et al 2014). A CT scan might be used to assess ventilation heterogeneity, too, but this would require two CT scans performed at end expiration and end inspiration, which would necessitate considerable exposure to radiation. Dynamic ventilation heterogeneity could be assessed at the bedside in patients undergoing controlled volume ventilation by assessing changes in ΔRrs, as mentioned before. The higher this value is, the more pendelluft and redistribution occur at end inspiration, testifying to an uneven distribution of tidal volume.
Another radiation-free bedside method with which to assess ventilation homogeneity is EIT (Luepschen et al 2007, Constantin et al 2014, Frerichs et al 2017), which allows dynamic continuous monitoring of tidal ventilation distribution (figure 6).
Figure 6. Effects of lower support on ventilation homogeneity of spontaneously breathing ARDS patient. These images represent the topographic distribution obtained by EIT of tidal ventilation in an ARDS patient undergoing pressure support ventilation (PSV). On the left, PSV is higher (i.e. 12 cm H2O) and the dependent regions of the lung are poorly ventilated (region 3 receives 9 percent of tidal volume, and region 4 receives 17 percent) in comparison to the non-dependent (region 1 receives 31 percent and region 2 receives 42 percent). On the right, PSV is lower (i.e. 3 cm H2O) and the dependent regions now receive 36 percent of the tidal volume, while the non-dependent was reduced to 63 percent. The red arrows indicate redistribution of tidal volume towards dependent lung. Setting PSV on a lower level might increase the diaphragm activity and improve ventilation homogeneity.
Download figure:
Standard image High-resolution imageInhomogeneity indexes derived from EIT ranges from the simple ratio of tidal volume reaching non-dependent/dependent regions to more complex dynamic intra-tidal inhomogeneity measures to the pixel-level global inhomogeneity index or the ventilation center. Whichever index is considered, they all refer to the presence of a dynamic imbalance of regional lung expansion, resulting in increased local transpulmonary pressure and risk of VILI.
Once heterogeneity is measured, ventilation settings aimed at minimizing it might be regarded as a valuable addition to protective ventilation strategies. To date, many studies have shown that PEEP modifies homogeneity (Zhao et al 2010). Interestingly, it is not simply a higher PEEP but rather personalized settings that obtain the best homogeneity in each patient. In fact, both low PEEP levels with excessive lung collapse and high levels with over-distension yield heterogeneity. Evidence in animals and indirectly in adults suggests that the prone position might also be associated with higher homogeneity (Valenza et al 2005). During assisted breathing, instead, lower support (Froese 2006, Mauri et al 2013), the presence of some degree of ventilation variability (Bellani et al 2012, Blankman et al 2013, Mauri et al 2017) and cyclic expansion by Sigh (Mauri et al 2015) seem to be associated with increased homogeneity.
Homogeneity is a key determinant of ARDS severity and VILI, probably through increased local lung stress. Multiple personalized settings of assisted and controlled ventilation modes might help to decrease it.
10. Over-distension
Over-distension is commonly referred to as regional lung stretch up to specific vital capacity. For healthy subjects, the pressure at which this phenomenon occurs is extremely high and is almost never reached (around a transpulmonary pressure of 20 cm H2O). However, during ARDS, in the presence of alveolar collapse that induces intrapulmonary shunt requiring elevated PEEP and of substantially decreased compliance increasing driving pressure, lung regions can reach vital capacity even during low volume tidal ventilation (Terragni et al 2007). Over-distension causes direct barotrauma with rupture of lung parenchyma, inducing alveolar haemorrhage, formation of bullae, and pneumothorax. Moreover, indirect uncontrolled increase of alveolar wall length stimulates specific receptors and inflammation cells, leading to the release of cytokines and recruitment of cells and additional lung injury.
Thus, over-distension should be carefully avoided in order to limit the development of VILI (Hong et al 2008, Kuipers et al 2012). Static end-expiratory over-distension is normally quantified by CT scan as the amount of hyper-aerated lung units (i.e. units with Hounsfield units value below that of normal lung parenchyma). These units are clearly subject to tidal over-expansion and to the above-mentioned risks, but this method might underestimate the amount of tidal over-distension. Indeed, regions that are normally aerated at end-expiration might be subject to over-distension at end-inspiration.
Dynamic assessment of tidal volume-related expansion should be desirable in the assessment of over-distension. Terragni and colleagues performed end-expiratory and end-inspiratory CT scans and showed that a substantial fraction of over-distension is present in the lungs of ARDS patients even within protective ventilation settings, thus suggesting the need for ultra-protective ventilation with alternative methods to remove CO2 (Terragni et al 2009). However, performing two CT scans might not be feasible in everyday clinical practice and could not provide a method of monitoring patients over time.
The upper inflection point of a low-flow P/V curve indicates the sudden change in compliance of the lungs when over-distension is reached, and it is a classic way to assess it at the bedside. Keeping Pplat below the upper inflection point might be a simple and safe method to avoid massive over-distension. Another method is the pressure–time stress index, which identifies major over-distension by the appearance of an upward concavity in the airway waveforms on the ventilator during square flow ventilation (see above). However, both the global P/V curve and the stress index methods identify the average pressure above which lungs are over-distended, while some regions might already be stretched at far lower pressures.
EIT assists the identification of regional over-distension by at least two methods. A pixel-level P/V curve might be recorded and a map of its concavities displayed, so as to choose the pressure associated with most pixels having a linear trend (Becher et al 2017). Moreover, a decrease of a regional value of static compliance between two conditions such as two PEEP levels is considered an EIT-based sign of over-distension, particularly if this happens in the non-dependent lung.
Liu et al introduced a method to classify EIT images into normally ventilated, overinflated, recruited, and tidally recruited/derecruited regions that gives important bedside information (Liu et al 2016).
In current clinical practice, the CT scan is considered the gold standard to establish lung over-distension (i.e. CT pixel densities of less than −850 HU), but this represents a static measure that does not correspond to the dynamic mechanism leading to stress and alveolar rupture, which, instead, is more appropriately assessed by dynamic EIT measures.
Finally, EIT facilitates the identification of over-distension during spontaneous breathing caused by strenuous inspiration causing air shift and occult pendelluft from the non-dependent to the dependent lung (Yoshida et al 2017).
Dynamic assessment of regional over-distension might be a fundamental task to set protective ventilation and decrease local transpulmonary pressure. Reduction of over-distension might be obtained directly through a decrease of tidal volume and PEEP, or indirectly through improvement of local mechanics and homogeneity.
11. Conclusions
Respiratory mechanics represent an extremely valuable bedside tool to assess the severity of ARDS, stratify patients and guide treatment. Several parameters can be obtained at the bedside to measure lung stress, strain, heterogeneity and over-distension, for the whole respiratory system or separated for the lung and chest wall. Time spent in measuring respiratory mechanics should not be viewed as a physiologic exercise for experts but rather as an up-to-date clinical work-up of ARDS patients.
Acknowledgments
The authors have no conflicts of interest to disclose. The authors would like to acknowledge departmental funding.







