Abstract
Diffusion magnetic resonance imaging (dMRI) tractography is currently the only imaging technique that allows for non-invasive delineation and visualisation of white matter (WM) tracts in vivo, prompting rapid advances in related fields of brain MRI research in recent years. One of its major clinical applications is for pre-surgical planning and intraoperative image guidance in neurosurgery, where knowledge about the location of WM tracts nearby the surgical target can be helpful to guide surgical resection and optimise post-surgical outcomes. Surgical injuries to these WM tracts can lead to permanent neurological and functional deficits, making the accuracy of tractography reconstructions paramount. The quality of dMRI tractography is influenced by many modifiable factors, ranging from MRI data acquisition through to the post-processing of tractography output, with the potential of error propagation based on decisions made at each and subsequent processing steps. Research over the last 25 years has significantly improved the anatomical accuracy of tractography. An updated review about tractography methodology in the context of neurosurgery is now timely given the thriving research activities in dMRI, to ensure more appropriate applications in the clinical neurosurgical realm. This article aims to review the dMRI physics, and tractography methodologies, highlighting recent advances to provide the key concepts of tractography-informed neurosurgery, with a focus on the general considerations, the current state of practice, technical challenges, potential advances, and future demands to this field.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Since its inception in the mid-1980s, diffusion magnetic resonance imaging (dMRI) has been eminently successful to aid investigation and clinical management of neurological disorders in the central nervous system. One of its first applications is for detection of reduced local tissue diffusivity early on following cerebral ischaemia, which had led to revolutionary changes in acute stroke practice and improvements in post-stroke outcomes (Barber et al 1998, Baird et al 2001, Hjort et al 2005, Chalela et al 2007, Merino and Warach 2010, Macintosh and Graham 2013, Nagaraja et al 2020). In the 90s, the invention of Diffusion Tensor Imaging (DTI) (Basser et al 1994a, 1994b), enabled estimation of per-voxel average white matter (WM) fibre orientation based on the ensemble diffusion directionality, from which virtual streamlines can be reconstructed and visualised as 3D objects, mimicking true WM fibre connections between specific brain regions (i.e. the WM tracts or fibre pathways). This imaging post-processing technique is known as dMRI tractography or fibre tracking.
Over the past two decades, dMRI tractography has evolved tremendously in its methodologies. There are two principal classes of methods. The first one is called whole-brain tractography, which aims to generate comprehensive WM tracts for the human brain. This has led to the emergence of brain structural connectomic research; advances made in this field represent an important breakthrough for neuroscience research (Sporns et al 2005, Bullmore and Sporns 2009). Another class of method that is more directly relevant to clinical neurosurgery practice is called targeted (streamline) tractography, also referred to as WM tract segmentation or 'virtual dissection' (Catani et al 2002, Catani and Thiebaut de Schotten 2008). Targeted tractography is used to aid pre-surgical planning and intraoperative image guidance (neuronavigation) by mapping out the important WM tracts adjacent to the surgical corridor and the intended resection lesion. Surgical injury to the WM tracts can lead to permanent neurological and functional deficits, making the accuracy of tractography reconstructions paramount.
Research over the last 25 years has significantly improved anatomical accuracy of tractography, with advances in the dMRI acquisition, development of more reliable techniques for voxel-wise WM modelling and tractography reconstructions. An updated technical review about tractography methodology in the context of neurosurgery is now timely given the thriving research activities in dMRI, to ensure more appropriate applications in the clinical neurosurgical realm. This review focuses on addressing the technical basis of performing targeted tractography, and the related technical constraints imposed on neurosurgical applications. The article is organised with a question-and-answer (Q&A) format. These Q&As are intended for non-imaging scientists and clinician-researchers with interests in acquiring knowledge about the brain WM anatomy relevant to neurosurgical practice (Q&A 1); the principles of dMRI physics (Q&A 2), and technical nuances of different dMRI modelling and tractography methods (Q&A 2–4), including the challenges faced in tractography reliability and validation (Q&A 6 and 7); the current state (Q&A 5), technical constraints and practical challenges faced in tractography-informed neurosurgery, and the recent research advances attempting to overcome these challenges (Q&A 8). They are also written for imaging scientists, and MRI vendors and related industry partners, to provide them with greater insights into the clinical neurosurgery tractography needs, outlining the authors' viewpoints on potential future demands, with an intention to foster future technical developments tailored to these needs. A 'highlights' section is included at the end of each Q&A, summarising the pertinent information, and is intended as 'take-home messages' for all readers.
Q&A 1. Tell me about WM anatomy
1.1. WM anatomy of the human brain
The cerebral WM forms the deeper component of the human brain. It contains bundles of nerve fibres (i.e. axons), the extended cellular processes from the nerve cell bodies (i.e. neuronal soma) located in the cerebral grey matter (GM). Cerebral WM provides the physical pathways through which the neurons interconnect and communicate. Similar to insulated electric cables, many axonal fibres are surrounded by laminated sheaths of lipid-dense tissue called myelin (figure 1), which gives WM its colour. In the central nervous system, myelin is produced by a type of glial cells, called oligodendrocytes. Each myelin sheath wraps around a segment of axon multiple turns (known as a myelin sheath or internode). One oligodendrocyte can form myelin internodes to as many as 50 axons (Lee et al 2021b). Between the neighbouring myelin internodes are approximately 1 μm wide gaps of bare axonal membrane, known as the Nodes of Ranvier (Susuki and Rasband 2008, Kiernan 2009, Lee et al 2021b). Ion channels are confined to these nodes. Action potential jumps from one node to the next, resulting in saltatory (rapid) conduction along neighbouring myelin internodes, providing up to 100 times faster conduction than through unmyelinated nerve fibres (Susuki and Rasband 2008, Kiernan 2009, Lee et al 2021b). Besides the axons, glial cells (neuroglia) make up the remaining cellular constituents in the cerebral WM; these include astrocytes, the aforementioned oligodendrocyte, its precursor cells (oligodendrocyte progenitor cells), and microglia (Walhovd et al 2014). The precise numbers and proportion of different cellular constituents of cerebral WM in humans are unknown 11 .
Figure 1. (a) Schematic representation of neurons with myelinated axons. (b) Cross-section schematic of the myelin sheath, showing the laminated structure. Adapted with permission from Springer Nature Customer Service Centre GmbH: Springer, Magnetic Resonance Materials in Physics, Biology and Medicine, Nilsson et al 2013. (The role of tissue microstructure and water exchange in biophysical modelling of diffusion in white matter, Nilsson M, van Westen D, Stahlberg F, Sundgren P C and Latt J), Copyright © 2013, The Author(s). CC BY 4.0.
Download figure:
Standard image High-resolution imageAt a macrostructural level, the axonal fibres in the cerebral WM are often conceptualised as tightly packed, parallel bundles of myelinated axonal fibres, known as WM tracts or fibre bundles. The WM tracts are conventionally categorised into three main classes based on their connectivity patterns (see also figure 2):
- (1)Commissural WM tracts—they provide inter-hemispheric cortical–cortical connections. The corpus callosum is the largest commissural tract in the human brain. Other examples include the anterior commissure, posterior commissure, and habenular commissure.
- (2)Association WM tracts—they provide either long-ranged or short-ranged intra-hemispheric cortical–cortical connections. Arcuate fasciculus is an example of long association WM tract, providing perisylvian frontal, parietal and temporal cortical connections, critical for subserving language function. Other examples of long association tracts include the superior longitudinal fasciculus, cingulum, uncinate fasciculus, inferior fronto-occipital fasciculus, and inferior longitudinal fasciculus. Short association fibres, also known as the subcortical U-fibres, forming U-shaped connections between adjacent cortical gyri running just below the deepest portion of sulci.
- (3)Projection WM tracts—they connect the cortex with subcortical structures, including the brainstem and spinal cord. They are afferent (outgoing) or efferent (incoming) fibres with respect to the cortex, forming the corona radiata and the internal capsule. Examples include the corticospinal tract subserving voluntary motor function, optic radiation, subserving primary visual function, and fornix, which forms part of the limbic system.
Figure 2. Examples of major white matter tracts in humans, highlighted in yellow, based on classic post-mortem fibre dissection. Adapted with permission from Ludwig E and Klingler J 1956, S. Karger AG, Basel. (a) anterior fibres (forceps minor) of corpus callosum; (b) arcuate fasciculus; (c) projection tracts (including the corticospinal tract), from cranial to caudal: corona radiata, internal capsule, and projection fibres enter/exit the brainstem; (d) inferior fronto-occipital fasciculus; and (e) optic radiation.
Download figure:
Standard image High-resolution imageWhile conceptually useful to aid neuroanatomical learning, the aforementioned terms, the 'association, commissural, and projection WM tracts', represent a rather over-simplistic view of the WM tract anatomy and patterns of its fibre arrangement. A WM tract can contain axons of different lengths, different sizes (diameters) and with different myelination extents. For example, the cingulum is often simply referred to as a long-range association WM tract, providing direct fronto-temporal connections. Evidence from both human cadaveric fibre dissection and non-human primate axonal neurotracer studies (Tournier et al 2011, Thiebaut de Schotten et al 2012) suggested it comprises both long- and short-range association fibres. The longest fibres connect the frontal subgenual region to mesial temporal lobe structures; while the shorter subcortical U-fibres pass in and out the cingulum, joining the adjacent cingulate, frontal and precuneus cortices. Similarly, histology studies of the human corpus callosum in the mid-sagittal plane revealed regional differences in axonal diameters and myelination extent, reflective of the need for different functional purposes and the associated nerve conduction speed (Aboitiz et al 1992).
Next, these WM tracts are arranged in a necessarily complex way, forming a 3D structural chassis through which the neurons communicate between and within the cerebral hemisphere, the cerebellum, and the brainstem. Locally, axonal fibres from different WM tracts, such as those contained within an MRI voxel, can be arranged either simply as tightly packed parallel axonal bundles; or organised in more complex ways, such as containing axonal fibres that are bending, kissing and crossing with each other, collectively termed the 'crossing fibre' phenomenon in the field of dMRI (Tuch et al 2002, Behrens et al 2007, Tournier et al 2011). Distinguishing different WM fibre sub-populations in these crossing fibre regions may not be possible, even with the meticulous cadaveric fibre dissection technique. It has been shown that such crossing fibre arrangement is highly prevalent, present in up to 90% of the brain WM regions in the human brain (Jeurissen et al 2013). This is an important point to bear in mind when considering the validity of dMRI models since the dMRI signal is sensitised by structural factors governing tissue microstructural environment, including axonal fibre arrangements (more to this in Q&A 2 and 3).
1.2. Applied WM tract anatomy in neurosurgery
Achieving gross total lesion resection is the most important factor affecting survival following glioma neurosurgery (Kuhnt et al 2011, Capelle et al 2013, Li et al 2016), and the strongest predictor of achieving seizure freedom following lesion-based focal epilepsy surgery (Hamiwka et al 2005, Krsek et al 2009, Fallah et al 2015). Planning for and performing neurosurgery requires an in-depth understanding of individualised neuroanatomy, including the WM tract anatomy, as affected by pathology, to help survey surgical risks and to determine a safe surgical strategy. Not all WM tracts are considered equally important by neurosurgeons. This is particularly relevant when the lesion resides within or adjacent to eloquent brain cortices, such as areas controlling movement, language and vision, and the related WM tracts. Inadvertent injury to the WM tracts during surgery can lead to permanent functional damage and adverse neurocognitive sequelae (Kinoshita et al 2005, Duffau 2014, Nimsky 2014). The need for subcortical WM preservation makes having accurate and precise WM tract reconstructions foremost paramount for pre-surgical planning.
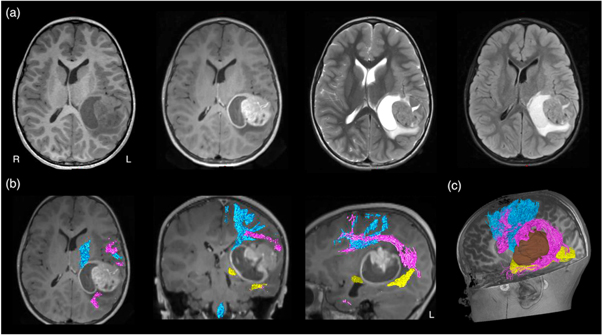
MRI-based neuroimaging is indispensable for modern neurosurgical practice, with critical roles in aiding pre-surgical planning and providing intraoperative imaging guidance (Upadhyay and Golby 2008, Enchev 2009). During surgery, the patient's head is co-registered with his or her preoperative MRI data using the image-guided neuronavigation software. Akin to a Global Positioning System, this enables subsequent intraoperative neuronavigation by accurately tracking the positions of the surgical instruments, and displaying these in relation to the patient's MRI dataset. Additionally, predefined image segmentations, such as the tumour volume, and the tractography images can be displayed into the operating microscope eyepiece, via augmented reality technology, and thus, be overlayed on the live microscope view as either semi-transparent or outlined objects (Kuhnt et al 2012, Sommer et al 2013). The ability to confirm the live resection view with MRI makes surgery more precise, while minimising injury to surrounding healthy brain structures. Conventional structural MRI sequences, such as T1-weighted, gadolinium contrast enhanced T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) imaging, provide rich information concerning the relevant surgical anatomy. This includes demonstrating the regional surface sulcal-gyral and vascular anatomy, lesion extent, and the clarity of the tumour-brain interface. They also provide clues about pathological tissue properties and makeups, such as the presence of solid versus cystic component; the presence of acute intra-lesion haemorrhage; and suggestion of histopathologically high-grade tumour component with gadolinium-contrast enhancement. Despite being informative, none of these routine structural MRI sequences can depict the WM tract anatomy, specifically to demonstrate the tract disruption and/or displacement from its conventional trajectory, adjacent to the target surgical lesion (see figure 3(a)).
Figure 3. (a) Conventional structural MRI sequences (from left to right: T1-weighted, T1-weighted post-gadolinium contrast, T2-weighted, and FLAIR imaging) enable the characterisation of brain tumour and its effect on peri-lesion white matter. However, they were unable to demonstrate both the location and disruption of adjacent white matter tracts to the tumour; (b) diffusion MRI tractography aids pre-surgical planning and intraoperative imaging guidance by enabling in vivo mapping and visualisation of the corticospinal tract (in blue), arcuate fasciculus (in pink), and optic radiation (in yellow), demonstrating their spatial relationship with the tumour. All MRI are displayed in radiological orientation. (c): 3D rendered tractography images and tumour (segmented in brown). Material acronyms: L (left), R (right).
Download figure:
Standard image High-resolution imageWithout being able to delineate affected WM tract location under direct vision, direct brain electrical stimulation (DES) performed during surgery is widely accepted as the surgical 'gold standard' confirming the WM tract position and its functional relevance (more details about the DES procedure is described in Q&A 7) (Penfield and Boldrev 1937, Ojemann et al 1989, Berger and Hadjipanayis 2007, Duffau 2015). Pre-surgical localisation of intrinsic tumours and functional cortical regions may be achieved by multiple non-invasive imaging modalities, such as positron emission tomography (PET); single-photon emission computed tomography; blood oxygen level-dependent functional MRI (BOLD-fMRI); magnetoencephalogram (MEG); and navigated transcranial magnetic stimulation (nTMS). Diffusion MRI tractography remains the only non-invasive technique enabling visualisation of in vivo WM tract anatomy (Jones 2008a), making it a powerful technique to complement neurosurgical planning and intraoperative execution (figures 3(b) and (c)). Coupled dMRI tractography with functional brain mapping strategies, such as BOLD-fMRI, or with intraoperative DES findings can improve the functional relevance of tractography reconstructions and offer insights into brain structural-functional relationship between the cortical–subcortical structural networks and patient's functional states both before and after the surgery (Berman et al 2004, Kinoshita et al 2005, Bello et al 2008, Sanvito et al 2020) (more to this in Q&A 5).
1.3. Highlights for Q&A 1
- The brain WM consists of myelinated and unmyelinated axonal bundles forming a complex 3D structural chassis, important for information relay between connected cortical and subcortical GM regions and brainstem.
- The majority of brain WM regions contain crossing fibres or more complex fibre arrangements, rather than simply tightly packed parallel fibre bundles.
- WM tract preservation is imperative for optimising post-surgical functional outcomes.
- Diffusion MRI is currently the only non-invasive imaging technique enabling in vivo mapping of WM tracts throughout the human brain.
Q&A 2. How to measure water diffusion using MRI?
Water is naturally abundant in living humans, thus, they are readily available as a signal source for hydrogen-based MRI technique, like dMRI. The dMRI signal is generated based on measuring the ensemble distribution of water diffusion-driven spin displacements in the studied biological tissue (Merboldt et al 1985, Taylor and Bushell 1985). The term 'diffusion' here refers specifically to the 'self-diffusion' that occurred stochastically owing to the thermal energy carried by the water molecules. It can be considered as a multi-scale integrated process, as random microscopic fluctuations of a vast number of water molecules can be inferred from observations at a larger scale using statistical physical models (Wesbey et al 1984a, Wesbey et al 1984b, Le Bihan et al 1986). In biological tissues, such as the brain WM, the ensemble diffusion distribution of water molecules is shaped by the local tissue environment. The microscopic length scale of water diffusion makes dMRI a sensitive and powerful tool to probe tissue cytoarchitecture and microstructural properties (Le Bihan 2003), that are otherwise unrecognisable through other MRI methods. This section provides an overview of dMRI physics.
2.1. Pulsed gradient spin-echo (PGSE) sequence
Diffusion-weighted imaging (DWI) is a class of MRI method, whereby the signal obtained is sensitised by the diffusion of water molecules. In 1965, Stejskal and Tanner pioneered the famous PGSE sequence (Stejskal and Tanner 1965), which is still widely used by the typical dMRI scans nowadays. The PGSE sequence forms the basis of many modern dMRI methods. Following their PGSE experiments, they also proposed the Fourier relationship between the diffusion-weighted (DW) signal and the spin displacement distribution, which has laid the foundation of the q-space theory in diffusion imaging (Callaghan et al 1988) (see next paragraph). Stejskal–Tanner's PGSE pulse sequence employed a pair of magnetic field gradients with short duration (so-called 'diffusion gradients'), with equal magnitude and opposite gradient polarity, to record the diffusion-driven net dephasing of the spins (see figure 4) (Stejskal and Tanner 1965). The basic principle is that one diffusion gradient 'labels' the spins according to their spatial position (within the gradient), and the other diffusion 'unlabels' the spins—if no diffusion is present, the two effects cancel out; however, diffusion introduces a mismatch in the contribution from both gradients, leading to a net phase accumulation for each spin related to their diffusion. The PGSE sequence has a clear distinction between the diffusion encoding time and the diffusion time, defined as the duration and separation of the diffusion gradient pair, respectively. To correlate DW signal with molecular diffusion, Torrey considered the magnetisation transfer induced by diffusion and re-formulated the Bloch–Torrey equation by including an additional term (Bloch 1946, Torrey 1956). For an isotropic medium, the solution for such an equation is given as
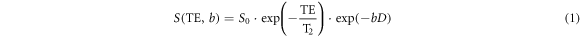
in which D is the diffusion coefficient of the medium, S0 is the initial MR signal without diffusion weighting at time t = 0, S is the MR signal obtained at the echo time (t = TE), T2 is the transverse or spin–spin relaxation time at which the transverse magnetisation decays to approximately 37% of its initial value (i.e. S0), and b is the diffusion sensitising/weighting factor, or the so-called b-value, defined as
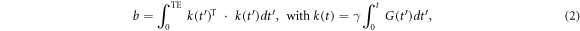
where δ is the nuclear gyromagnetic ratio, G(t) denotes the time-dependent gradient strength, and T means transpose operation. The diffusion gradient with a symmetric trapezoidal shape produces b-value as
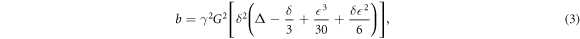
where δ is the duration, ∆ the separation, and ε the gradient rise time; the effective diffusion time (∆eff) is usually defined as ∆eff = ∆ − δ/3. Notably, without the diffusion weighting factor, i.e. at b = 0, the MR signal S in equation (1) is modulated by the T2 relaxation time of local tissue, thus producing a T2-weighted image contrast using the PGSE sequence.
Figure 4. The diagram of Stejskal–Tanner's PGSE pulse sequence (Stejskal and Tanner 1965). Following an excitation RF pulse (rf90), a pair of diffusion gradients (highlighted in red) are placed before and after the refocusing RF pulse (rf180). δ and Δ are the duration and separation of the two diffusion gradients. GS, GP, and GR are the slice selection, phase encoding, and readout gradients respectively; TE is the echo time of DW signal.
Download figure:
Standard image High-resolution image2.2. Q-space imaging
When using Stejskal–Tanner's PGSE pulse sequence (Stejskal and Tanner 1965), the accumulated phase shift for a single spin in the magnetic field gradients is given as
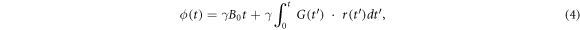
where the first term represents the phase due to the static magnetic field B0, and the second term is due to the effects of a magnetic field gradient. For a diffusing spin, the degree of phase accumulation owing to the applied gradient of a PGSE sequence is proportional to the spin displacement along the direction of the applied gradient. At TE, when the spin echo is formed, the net phase shift (φ) of one individual spin is therefore
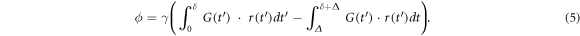
If the diffusion gradient pulse duration in a PGSE sequence is infinitely short (i.e. δ ≪ ∆), the diffusion distance under the diffusion gradient pulse duration is substantially smaller than the pore size of the medium. Under this narrow pulse approximation, the spin phase is then
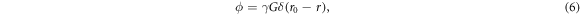
where r0 and r are the spin's position at the first and second instantaneous gradient pulse, respectively. For a given proton density ρ, the diffusion signal taking into account the spin displacement probability or diffusion propagator  which is the conditional probability of finding a single spin at position r from its initial position r0 after any diffusion time interval τ = t − t0, is given as
which is the conditional probability of finding a single spin at position r from its initial position r0 after any diffusion time interval τ = t − t0, is given as
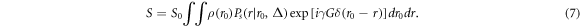
Assuming that R = r0 − r, this can be reformulated as
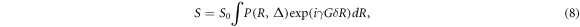
where P(R,∆) expresses the average probability for a spin diffusing a distance R within a time interval ∆. It is sensible to introduce the effects of the diffusion gradient pulses into the analysis by defining the reciprocal spatial vector q given as
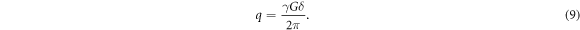
Hence, this q-space formalism can be rewritten as
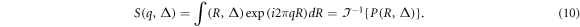
Based on this Fourier relationship, a mathematical q-space analysis method was developed by Cory and Garroway (1990) and by Callaghan (1993). They proposed that at a sufficient diffusion time, the displacement probability function may relate to the size and shape (e.g. spherical, cylindrical) of the compartment where diffusion occurs. These microstructural parameters will be reflected by the diffusion-diffraction peaks in the echo signal attenuation. Therefore, the q-space imaging technique can reveal direct microstructures of the biological tissues.
2.3. Apparent diffusion coefficient (ADC)
The driving force of diffusion MRI is to monitor the diffusion-driven displacements of water molecules at a microscopic level, which is well beyond the image resolution of both typical clinical and modern research-orientated MRI scanners that are at millimetre scale. The overall signal observed in DWI represents the statistical integration of all microscopic water molecular displacements presented in an MRI voxel. Accordingly, the complex diffusion processes that occur in a biological tissue on a voxel scale are often described with a global statistical parameter, named ADC (Le Bihan et al 1986):
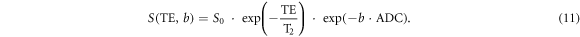
Such parameterisation of diffusion by a global ADC is intended to represent those physical processes that occur at scales smaller than the scales resolved by the method. The scale of an MRI voxel is imposed by technical limitations, e.g. by the strength of the imaging gradient field, whereas the actual scale of the diffusion processes and interactions with the local tissue environment is determined by physical phenomena at microscopic scale. The so-called partial volume effect averages and smooths some spatial inhomogeneity, making ADC parameter a summary metric of microscopic diffusion process within an MRI voxel in millimetric scale.
2.4. Anisotropic diffusion
For isotropic free water diffusion, i.e. when the spin displacement probability is a three-dimensional Gaussian distribution, DW signal attenuation can be modelled using a monoexponential function characterised by ADC, as shown by equation (11). In biological tissue, water molecular diffusion or spin displacement is hindered by local tissue architecture. The movement of water molecules under typical diffusion time can be interfered by many biological elements, such as cell membranes, myelin sheaths, water contents and other macromolecules, leading to diffusion anisotropy (Moseley et al 1991, Beaulieu 2002).
In biological tissues containing some forms of coherent structural organisation, such as the brain WM tracts, water diffusion is hindered to a greater extent in a direction perpendicular to the principal axonal axis than parallel to it. Importantly, this means that DW signal and the derived ADC value within the same voxel would vary depending on the applied DW gradient direction. For example, the measured DW signal attenuates to a much greater extent in the direction aligned with the principal axonal axis than perpendicular to it, leading to a higher ADC value along this direction. This suggests that a set of gradient directions are necessary to obtain DW signal as a function of orientation, from which certain models can be imposed to estimate the underlying fibre organisation within a local MRI voxel. In 1994, Basser et al proposed the first model for this purpose, which is now well recognised in the field as the famous diffusion tensor model for analysing dMRI data, also known as 'DTI' (Basser et al 1994a, 1994b); see the next Q&A.
2.5. Highlights for Q&A 2
- The dMRI signal is sensitised by the underlying tissue microstructural environment, making it a unique and powerful tool to probe brain WM macro- and microstructures.
- The classic Stejskal–Tanner's PGSE pulse sequence is the basis of q-space theory that describes the diffusion-driven MRI signal attenuation.
- Isotropic free water diffusion can be modelled as a Gaussian distribution parameterised by the diffusion coefficient.
- Water diffusion in the brain WM tracts is anisotropic and non-Gaussian distributed. The ADC value parallel to the principal axonal axis is greater than those derived perpendicular to this axis.
- The DW signal is a function of gradient orientation in the brain WM.
Q&A 3. How to obtain WM trajectories using DTI?
This section provides an overview of the principle and limitations of the diffusion tensor model (shortened to tensor model from hereon) and tensor-based tractography.
3.1. Diffusion tensor imaging (DTI)
The tensor model and tensor-based tractography remain the most widely adopted technique for the studying of in vivo brain WM tracts and their microstructural properties. The tensor provides a means to characterise the degree of diffusion anisotropy within DWI voxels, through an imposed assumption that the distribution of spin displacement is Gaussian. The term 'DTI' is often misinterpreted as synonymous with 'DWI', whereas it is not: DTI is only one of the many dMRI local modelling methods to analyse DWI data. The tensor model can be represented as a 3 × 3 matrix:

The diagonal elements of the diffusion tensor correspond to the ADC value in the corresponding direction (e.g. Dxx = ADCx ); the off-diagonal elements reflect the correlation between diffusion in the corresponding two directions (e.g. Dxy is a measure of the correlation of the diffusion along the x and y directions). As the diffusion tensor, D, is a symmetric and positive definite matrix, it has six unknown coefficients to be estimated (as Dxy = Dyx , Dyz = Dzy , and Dxz = Dzx ). Hence, the tensor model requires at least six DWIs (with diffusion sensitisation in six non-collinear directions) and one reference image without diffusion weighting to perform tensor decomposition:
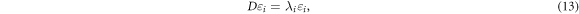
where εi is the eigenvector of its corresponding eigenvalue λ i (i = 1, 2, 3). The largest eigenvalue λ1 gives the principal direction of the diffusion tensor, ε i , and the other two eigenvectors span the orthogonal plane to it. Schematically, the DTI model can be represented as an ellipsoid with the principal axis parallel to the principal eigenvector (ε1). The other two minor axes of the ellipsoid represent eigenvectors, ε2 and ε3, in orthogonal planes to the principal eigenvector. The relative difference between the three eigenvalues determines the size and the shape of the tensor ellipsoid. Some rotationally-invariant scalar measures have been defined based on this eigensystem decomposition in the literature, the most widely used are the mean diffusivity (MD, which reflects the overall size of the tensor ellipsoid) and the fractional anisotropy (FA, which is a quantitative measure of how the tensor ellipsoid deviates from a spherical shape) (Basser and Pierpaoli 1996). Figure 5 illustrates the maps of scalar metrics, ellopsoids, and principal eigenvectors that can be obtained using the tensor-based modelling.
Figure 5. Results of voxel-wise modelling dMRI data using the diffusion tensor model—DTI. Top left: an anatomical T1-weighted image showing a coronal slice of a human brain. Top middle: the fractional anisotropy (FA) map computed from the diffusion tensor. Top right: the directionally encoded colour (DEC) map where voxels are coloured according to the orientation of the principal eigenvector (ε1) of the tensor, by weighting the x/y/z component of ε1 with the red/green/blue colour components, respectively. Middle left: visualisation of the tensor using the ellipsoid model. Note that voxels with high FA correspond to 'long' ellipsoids (e.g. at corpus callosum), otherwise to spherical shapes (e.g. at ventricles filled with cerebrospinal fluid (CSF)). Middle right: zoomed-in view showing the 'crossing fibre' brain region (the white block). Here, the tensor-based modelling cannot provide correct fibre orientations. Bottom left and right: the ε1 map from the diffusion tensor, which can only provide one direction per voxel. Similar to the DEC map, all ellipsoids and ε1 are coloured-coded based on the orientation of ε1. For comparisons, see figure 7, in which the fibre orientation estimation of the same brain is based on higher-order modelling techniques.
Download figure:
Standard image High-resolution imageTo complement these scalar maps with orientation information of the diffusion tensor, a directionally encoded colour (DEC) map is also typically generated based on the principal eigenvector. By convention, this DEC map uses a red–blue–green colour scheme (Pajevic and Pierpaoli 1999). The red colour represents the component of the principal eigenvector along the left–right orientation, the blue colour represents the component in the superior–inferior orientation, and the green colour represents the component in the anterior–posterior orientation.
Based on the analysis of the local tensor for every voxel within the brain, it is possible to infer the structural morphology of WM tracts using diffusion MR tractography (see below), and its cellular microstructure properties in both the healthy and disease states by quantifying diffusion tensor metrics.
3.2. Tensor-based tractography
As shown by equation (13), the decomposition of a local diffusion tensor yields three eigenvalues and the corresponding eigenvectors. A notable feature is that when an MRI voxel is occupied by coherent axonal fibres, there is a strong correlation between the fibre orientation and the direction of the principal eigenvector (ε1) (Basser et al 2000, Lin et al 2001). The voxel-wise tensor decomposition provides a vector field of principal eigenvectors that can be connected between neighbouring voxels to form a curve or a streamline. This is the original concept of dMRI streamline tractography (Conturo et al 1999, Jones et al 1999, Mori et al 1999, Basser et al 2000), where WM tracts are delineated by streamlines generated through a stepwise integration process using a mathematical algorithm, based on the local fibre orientation distributions obtained from an applied signal or biophysical model. The diffusion tensor was the earliest model to provide such a vector field, and hence this is the information that most initial fibre-tracking algorithms were based upon. The simplest tractography algorithm is based on a deterministic model, which follows step-wise the estimated local WM fibre directions through the data until some termination criterion is reached. These methods are labelled as 'deterministic' since the trajectory is uniquely determined for a given starting point or so-called the seed point.
The first algorithm operating as such was the Fibre Assignment by Continuous Tracking algorithm (FACT) using the principal eigenvector of the tensor as the direction of propagation (Mori and van Zijl 2002), which became the most popular algorithm for over a decade. Starting from a predefined seed point, this method works by evaluating the direction of the fibre at the current location, stepping along this direction by a small fixed step size, and repeating until the track or streamline is terminated. Termination criteria typically include that the streamline has entered an area of low FA (since these methods typically rely on DTI to estimate fibre orientations), or that the streamline has made a sudden sharp turn (high curvature), deemed biologically implausible.
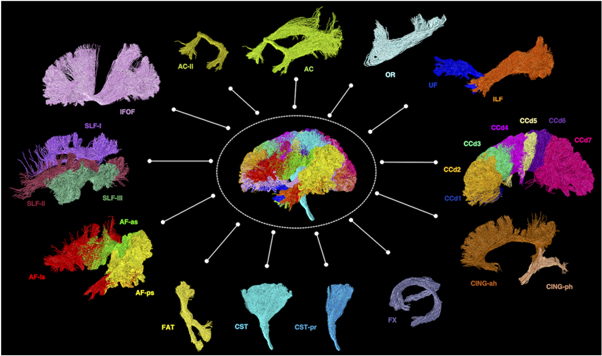
Using dMRI tractography, streamline trajectories can be generated that seem to follow the path of known WM tracts (figure 6). Importantly though, tractography does not reconstruct actual WM fibres or axons but only streamlines (or curves in 3D space) that are mathematical representations that delineate possible WM tracts—a crucial concept that has been misused quite frequently for applications in the field (Jones et al 2013, Jeurissen et al 2019).
Figure 6. Examples of diffusion MRI tractography of known major white matter tracts in the human brain 12 . Material acronyms: AC (anterior commissure), AC-ll (anterior commissure, the lateral (temporal) limb component), AF-as (arcuate fasciculus, the anterior indirect segment), AF-ls (arcuate fasciculus, the long direct segment), AF-ps (arcuate fasciculus, the posterior indirect segment), CCd1-7 (corpus callosum, segments 1-7), CING-ah (cingulum, the anterior horizontal segment), CING-ph (cingulum, the parahippocampal segment), CST (corticospinal tract), CST-pr (corticospinal tract, the peri-Rolandic component), FAT (frontal aslant tract), FX (fornix), IFOF (inferior fronto-occipital fasciculus), ILF (inferior longitudinal fasciculus), OR (optic radiation), SLF-I-III (superior longitudinal fasciculus, components I–III), UF (uncinate fasciculus).
Download figure:
Standard image High-resolution image3.3. Tracking algorithms: deterministic versus probabilistic
Deterministic tractography algorithms, like the FACT method, exploit the local orientation distribution information of the diffusion process (or fibres) to reconstruct from a starting seed, a virtual connection that corresponds to the most probable propagation path within this local orientation field. This retrograde and anterograde propagation process from the initial seed corresponds to a numerical integration scheme whose order can be adjusted to vary the integration step and accelerate the integration process. Deterministic tractography algorithms are inherently very sensitive to the presence of noise in the DWI data, which can lead to both false positive and false negative errors in estimating the local direction of the fibres in tractography output reconstructed over the whole brain. The quality of the estimated local orientation also depends on the ability of the chosen model to correctly represent the underlying reality.
To reduce this dependency on the noise present in the data and to compensate for the limitations of the tensor model to efficiently map the fibre configurations, probabilistic tractography methods have been introduced that fall into two broad categories: statistical sampling and Bayesian inference methods:
- (1)In statistical sampling approach, probabilistic streamlines are generated in most aspects similar to deterministic streamline methods, except that streamline propagation is sampled from the distribution of fibre orientation during the integration process (Parker et al 2003, Jones 2008b), rather than a 'fixed' direction in the deterministic tracking algorithm. It is a convention to derive these candidate directions from a Gibbs sampler to favour the most plausible directions. Due to its probabilistic nature, these algorithms require generating many streamlines, to properly sample the available directional configurations. The tractography outcomes obtained from such methods are generally populated with good anatomical connections, but also with more false positive connections that should be removed by a posteriori filtering.
- (2)The Bayesian approach does not reconstruct individual streamlines. Instead, it aims to construct for each point of interest within the brain, the connection probability maps to all points on the target region of interest (ROI) (Behrens et al 2007). The Bayesian approaches are also contaminated with false positives as the streamline approach above, whereas a posteriori cannot be applied to remove these errors.
It is important to understand that the choice of the local model is independent of the choice of the tractography algorithm (e.g. the tensor model can be combined with either the deterministic or probabilistic tracking strategies). Both components can be responsible for errors and lead to the creation of false positives in the connectivity maps.
3.4. Limitations of tensor modelling and tensor-based tractography
The tensor model is simple and fast in terms of data acquisition and analysis, making it a popular technique to be applied in acute neurosurgical settings. In addition, tensor-based analysis including tractography has been implemented as a package by the major MRI scanner and surgical neuronavigation vendors, and thus is the default post-processing approach for the majority of clinical tractography analysis.
The fundamental problem of DTI framework is the assumption that spin displacement follows a Gaussian or normal distribution in the brain WM. As outlined previously (section 2.4), water diffusion can be hindered or restricted by several brain WM microstructural properties within the typical diffusion time. On the other hand, although the tensor model can deliver an accurate depiction of the fibre orientation in regions where there is only one coherent fibre population, a tensor can only model a single fibre population within a voxel. When a voxel is filled with 'crossing fibres' with more than one orientation, the principal eigenvector of the tensor will become ambiguous (shown in figure 5). Given the high occurrence of crossing fibres in the human brain (Jeurissen et al 2013), this will cause subsequent qualitative and quantitative tractography errors, if the principal eigenvector field serves as the input data upon which the fibre tracking algorithm operates. Furthermore, the tensor-based anisotropic metrics, such as FA, would also be affected by the incoherent fibre distributions (Tournier et al 2011). Limitations of the tensor model to deal with intravoxel incoherent fibre population motivated the invention and development of several 'higher-order' local methods. They are discussed in the following Q&A.
3.5. Highlights for Q&A 3
- The diffusion tensor model can be schematically represented using an ellipsoid, with its principal axis corresponding to the direction of the principal eigenvector of the diffusion tensor.
- The deterministic tensor-based fibre tracking is the simplest tractography algorithm that takes the principal eigenvector of the tensor model as the fibre direction.
- Probabilistic tractography samples the distribution of fibre orientations at each tracking step to provide a likely distribution of the WM tracts.
- The diffusion tensor and tensor-based tractography are problematic since the tensor model cannot differentiate 'crossing fibres' or other complex fibre arrangements that appear frequently in WM voxels of the human brain.
- DTI is not synonymous with DWI. It is only one of the many dMRI local modelling methods.
Q&A 4. How to implement modern tractography techniques?
This section will begin with an overview of some of the high-order dMRI models, followed by a discussion of the differences between a tensor- and a non-tensor-based tractography. It will end by providing a brief overview of state-of-the-art quantitative tractography methods, beyond the local fibre modelling techniques.
4.1. Local modelling beyond diffusion tensor
Obtaining voxel-wise orientation distribution functions (ODFs) is the main target of local modelling. The so-called 'high angular resolution diffusion imaging' (HARDI) data are acquired using a higher b-value (≥3000 s mm−2) and more non-collinear diffusion directions (≥45 directions), than those acquired for the tensor model (typical b-value is 1000 s mm−2 with <30 diffusion directions) (Tournier et al 2011). As such HARDI estimates the additional higher angular frequency features of the DW signal that are not adequately modelled by the tensor model. The HARDI local modelling techniques can be broadly categorised into two main classes from their principles—one estimates diffusion ODF (dODF) and the other estimates fibre ODF (fODF). The following subsections provide a general description of these key ideas. To gain further methodological insights into various modelling techniques, the readers are referred to Daducci et al (2014) for more details.
4.1.1. Methods for deriving dODF
Relying on the Fourier relationship (Cory and Garroway 1990, Callaghan 1993), diffusion spectrum imaging (DSI) (Wedeen et al 2005) was the first proposed technique to model the complex WM fibre architecture using the diffusion propagator. Computing the dODF from a DSI propagator requires dense Cartesian q-space sampling, and reduces the 3D diffusion propagator to its 2D radial projections. Performing dense Cartesian q-space sampling is time demanding, limiting DSI being used for routine clinical applications. The use of sparse sampling and dictionary learning techniques can reduce the scan time. Sparse sampling can be achieved with DW signal decomposition based on spherical harmonics (Ozarslan et al 2013), where the q-space is sampled using three concentric spheres (or 'shells'), each with an increasing number of DW samples. This is similar to the 'multi-shell' sampling of multi-tissue constrained spherical deconvolution (CSD) methods (see next section).
Numerous alternative ODF reconstruction methods have been proposed which are based on a more restricted q-space sampling, providing a more decent acquisition time for routine clinical applications. Among these methods are those which seek to formulate the logarithm of the DW signal in the form of a Taylor expansion. These methods include high-order tensors (Ozarslan and Mareci 2003) and kurtosis analysis (Jensen et al 2005, Lazar et al 2008, Fieremans et al 2011, Neto Henriques et al 2015). Other methods assume a 'single-shell' spherical sampling of the q-space, which relies on the use of orthonormal basis functions on the sphere. Examples of these methods include the diffusion orientation transform, which relies on a pointwise convergent expansion of the plane wave (Ozarslan et al 2006, Canales-Rodríguez et al 2010); the numerical Q-ball model, which relies on the Funk Radon transform (Tuch 2004); the analytical Q-ball model, which relies on the use of spherical harmonics with the Funk–Hecke transform (Descoteaux et al 2007). Later, the sharpening deconvolution transform that performs the deconvolution in the ODF space was introduced to reconstruct fODF from dODF, and a further Laplace–Beltrami regularisation to increase its robustness to noise (Descoteaux et al 2009).
4.1.2. Methods for deriving fODF
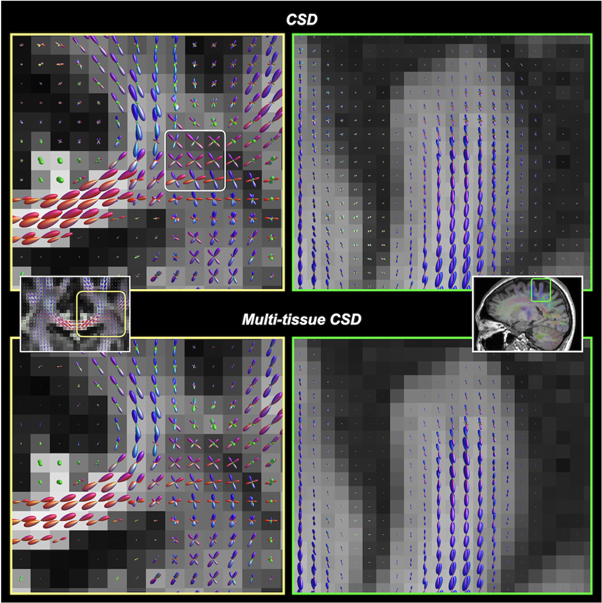
This class of technique directly reconstructs the fODF which is represented as a mixture model of distinct fibre populations. The model-dependent reconstruction techniques can be divided into two categories: those based on an impulse response function of the fibre bundle to the diffusion process expressed as a parametric function, and those where the impulse response is estimated directly from the acquired data. Various parametric functions on the sphere have been used, such as Gaussian mixtures (Alexander et al 2001), Ball and Sticks (Behrens et al 2003), and among others (see (Jelescu and Budde 2017) for review). Back in 2004, the idea of spherical deconvolution of the DW signal was emerged, assuming a Gaussian kernel self-estimated from the most anisotropic voxels of a single-shell DWI dataset (Tournier et al 2004, Anderson 2005). The technique based on spherical harmonics was further improved, known as the non-negativity CSD technique, by ensuring the positiveness of the ODF values (Tournier et al 2007, 2008). While there is still no consensus about which local modelling technique outperforms the other, the CSD-based fODF estimation is increasingly widely used. A further improvement of CSD was achieved using a multi-shell q-space sampling and extending the deconvolution approach to take into account the existence of several diffusion kernels corresponding to the various brain tissue types that can be encountered within the voxels (i.e. the GM, WM, and CSF) (Jeurissen et al 2014; see figure 7). This multi-shell multi-tissue CSD technique is used growingly, providing more reliable fODF information for tractography. It is now also possible to perform reliable local multi-tissue CSD estimations using either a single-shell HARDI data (Dhollander and Connelly 2016), or a single-shell, non-HARDI dataset consisting of a lower b-value and/or lesser diffusion directions (Calamuneri et al 2018, Arrigoni et al 2020, Fekonja et al 2021). Of note, the tractography examples shown in figure 6 were reconstructed using the multi-tissue CSD model.
Figure 7. Reconstruction of voxel-wise fODFs using CSD (upper row) and multi-tissue CSD (bottom row). Left column: the same slice location shown in figure 5 (where the tensor model results in incorrect fibre orientation estimates). Right column: fODFs near a cortical gyrus. Multi-tissue CSD (Jeurissen et al 2014) decomposes the signal contribution from different tissue types (GM, WM, and CSF), and which significantly reduces the partial volume contamination between tissues. The resultant WM fODFs from multi-tissue CSD are more accurate at tissue interfaces. Notably, ventricles should normally contain CSF voxels (i.e. no WM compartment), the multi-tissue CSD technique completely suppresses the spurious fODFs that occur with the (single-tissue) CSD. Such an improvement is advantageous to any ODF-based tractography algorithms.
Download figure:
Standard image High-resolution image4.1.3. Methods for quantifying tissue microstructure
A dMRI gradient scheme that combines both spherical and radial q-space sampling (i.e. a 'hybrid' acquisition) enables estimation of tissue parameters beyond the dODF or fODF information. For instance, this makes the quantification of microstructural features characterising the axonal populations possible (Assaf et al 2008, Alexander et al 2010, Zhang et al 2012). In addition, multicompartmental models have been developed during the last decade relying on the definition of several compartments within each voxel, each contributing to the DW signal with respect to their volume fractions according to specific models: the intra-axonal compartment is often represented by a distribution of sticks or cylinders whose dispersion can be controlled by a Watson or Bingham distribution, the extra-axonal compartment including the permeable glial compartment is represented by a tensor model whose main axis is parallel to the main direction of the axon population, and the CSF compartment is represented by an isotropic tensor (Jespersen et al 2007, Assaf et al 2008). On the other hand, the development of non-Gaussian dMRI techniques such as anomalous diffusion could provide new insights into various tissue properties (Liang et al 2016, Gatto et al 2019, Capuani and Palombo 2020), which has been applied to axon diameter mapping with in vivo human dMRI data (Yu et al 2017, 2018). Besides, machine learning and large-scale numerical simulations are opening up an avenue to the development of computational models benefiting from a higher degree of realism. These techniques have potential to go beyond the analytical approaches for which the absence of analytical solutions to the diffusion equation for geometries other than the sphere, ellipsoid and cylinder and the low robustness of regression methods are a strong obstacle to improving their realism (Yeh et al 2013, Ginsburger et al 2019, Palombo et al 2019, Lee et al 2021a). More recently, a novel technique called multidimensional diffusion imaging has been proposed based on the use of time-varying diffusion gradient profiles, rather than the trapezoidal gradients used classically in the PGSE sequences. It relies on the possibility to shape the q-vector according to the encoding time, thus providing further degrees of freedom to explore linear, planar or spherical encodings being more informative to prevent fit degeneracy of multicompartmental models (Westin et al 2016).
All these aforementioned microstructural models can provide advanced proxy microstructural features that can be advantageously exploited as prior knowledge to further constrain the tractography techniques (Daducci et al 2016). Readers are invited to read recent reviews (Afzali et al 2021, Novikov 2021) for a detailed summary of the present and the future of microstructural dMRI.
4.2. ODF-based tractography
Improving fibre orientation estimates is an essential step for voxel-wise ODFs reconstruction in tractography. While providing more accurate fibre orientation estimates over the crossing fibre regions than those estimated by the tensor model, ODF-based tractography methods are not problem-free. Concerns remained regarding the anatomical accuracy (Thomas et al 2014) and the propensities of generating many false positive streamlines for ODF-based tractography methods (Maier-Hein et al 2017). The discrepancy between the estimated WM fibres (in micrometre scale) and the dMRI voxel dimension (in millimetre scale) causes the partial volume effect. This leads to a 'bottleneck effect' on the accuracy of the fibre orientation estimate that can be achieved (Maier-Hein et al 2017). In WM regions with complex fibre configuration, the ODF-based algorithm may indiscriminately reconstruct all possible configurations that are compatible with the ODF field, leading to the downstream bias of retaining many false positive streamlines (Tournier 2019). This is particularly critical for whole-brain tractography and structural connectomics 13 analysis, where there is a need to address the tractography reconstruction bias by deriving quantitative metrics from the streamlines to approximate true WM fibre properties (see next section). In targeted tractography adopted in neurosurgical applications, the goal is to reconstruct a known WM tract. The use of a priori anatomical knowledge about the WM tract is fundamental to make the tractography technique 'less blinded' to the underlying biological reality. It is quite well established now that targeted tractography can be highly accurate if it can be a priori constrained by knowledge of where the WM tract starts, ends, and where it does not go (more to this in Q&A 5) (Schilling et al 2020a).
4.3. Quantitative tractography
A step further to tractography reconstruction is to derive quantitative parameters for individual WM tracts. For instance, a common 'along WM tract profiling' (tractometry) approach combines WM tract segmentation with voxel-wise MRI metrics, allowing tract-wise microstructural quantification (Colby et al 2012, Yeatman et al 2012). While measures such as tensor-based FA and MD have been used frequently as imaging surrogates of tissue microstructures, the field has now gravitated toward implementing more direct imaging metrics representing proxies of the underlying cellular geometries as described in section 4.1.3. Such quantitative analysis is an important basis for brain structural connectomics research (Sotiropoulos and Zalesky 2019, Yeh et al 2021). Direct tissue validations of these tractography-based quantitative DWI metrics, in the healthy and the pathology states, remains an open issue in both the fields of neuroscience and clinical neuroimaging research. In recent years, DWI-based quantitative metric analysis, has moved on from tractography visualisation in pre-surgical planning to an entire field of clinical neuroimaging study, attempting to investigate the impacts of various neurosurgery-related pathologies on the tractography diffusion metrics, and to address postoperative outcome predictions based on DWI-based metrics derived from the preoperative tractography data. For example, in a clinical series of 30 mixed adult brain tumour patients, nTMS-based corticospinal tract tractography reconstructed using individualised FA thresholds, had been demonstrated to improve both the precision and functional relevance of the reconstructions, which in turn affected pre-surgical planning by directly modifying the surgical strategies and facilitating intraoperative neuronavigation and electrostimulation (Frey et al 2012). Both the tract-averaged and peri-tumoural FA reduction and ADC increase derived from the preoperative nTMS-based corticospinal tract tractography had been shown to predict postoperative motor deterioration in motor-eloquent adult high-grade gliomas (Rosenstock et al 2017). A recent publication by the same group extended the investigation to 65 motor eloquent high-grade glioma adults, using a more fibre-specific metric based on the CSD model derived along the corticospinal tract profile, showing they were more specific to tumour-induced changes compared to the ADC or FA values (Fekonja et al 2021). Nonetheless, detailed discussion about advanced quantitative tractography inferring brain microstructures, is beyond the scope of the present review and will only be briefly covered here with a short introduction.
Tractography is a mathematical computing process that has no direct quantitative physical and biological attributes (Jones et al 2013). One intuitive (but wrong) manner to make conventional tractography quantitative is by measuring the streamline density or streamline counts, within a WM tract. These streamline metrics are commonly adopted as an edge metric in connectome analysis to indicate the connection 'strength' of a WM tract. It has been increasingly recognised in the field that the reconstructed streamline density from conventional tractography cannot represent a valid imaging biomarker of the biological WM fibre density (Jones et al 2013). Studies have adopted other edge metrics, such as the mean FA value along a WM tract, as surrogates to biological WM connectivity (see Yeh et al (2021) for more details). There is still no clear evidence to verify which of these imaging metrics more closely approximates the true biological connectivity. Nevertheless, some clinical investigators have adopted the large-scale network connectome approach to assess or predict the post-surgical properties of WM tracts in clinical neurosurgical tractography reconstructions (Bonilha et al 2013, 2015, Hutchings et al 2015, Aerts et al 2018, Gleichgerrcht et al 2018, 2020, Henderson et al 2020). Together with the functional connectome derived from the resting-state fMRI data, such a network-based approach has the added benefit of addressing the issue of localism versus distributed function that arises in targeted tractography.
Outstanding progress made about advanced quantitative tractography methods in recent years has led to improved biological relevance of the reconstructed quantitative WM connectomes. Many of them exploit the quantitative microstructural information derived from advanced local modelling to estimate the contribution of reconstructed streamlines. For a more comprehensive introduction of this area, readers are advised to check out the recent articles (Daducci et al 2016, Jeurissen et al 2019, Rheault et al 2020, Zhang et al 2021) and book chapters (Smith et al 2020) dedicated to reviewing the key aspects of quantitative tractography reconstruction.
4.4. Highlights for Q&A 4
- Local models beyond the diffusion tensor aim at resolving the 'crossing fibres' problem by improving the accuracy of fibre orientation mapping; they can be broadly categorised into the method measuring dODF and fODF.
- Modern ODF-based tractography is not problem-free. The anatomical accuracy and false positive streamline generation are two major issues.
- Incorporating a priori anatomical knowledge about the WM tract into tractography is the key to improve the biological accuracy of the streamlines generation.
- Many advanced quantitative tractography methods exploit the brain WM tissue microstructural information derived from the advanced local modelling techniques. They are more relevant for whole-brain tractography reconstructions and quantitative structural connectomics research, and less relevant for tractography used in neurosurgery.
Q&A 5. Targeted tractography: What is the current state of practice in neurosurgical applications?
Contrary to the need to address quantitative tractography reconstruction bias for brain structural connectomic research, targeted tractography applied in neurosurgery aims to visualise selected WM tract(s) anatomy adjacent to the surgical target. Thus, the focus is 'qualitative', as long as the reconstructed tractography mimics the true WM tract anatomy (the so-called virtual dissection of WM tracts (Catani et al 2002, Catani and Thiebaut de Schotten 2008)). This Q&A will start by defining targeted tractography, then provide a brief overview of the current state of targeted tractography applications in neurosurgery.
5.1. Targeted tractography: WM tract segmentation
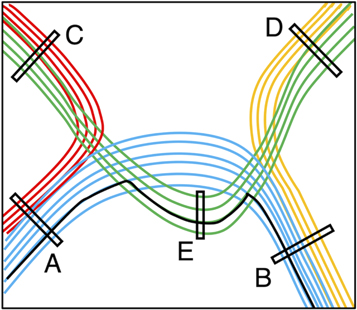
Targeted tractography utilises strategically placed ROIs typically based on a priori knowledge about the WM tract anatomy, to impose anatomical constraints for streamline propagation/tracking (see figure 8). They include: the seed ROI, which defines the starting point of streamline tracking; the inclusion ROI (also known as the 'AND' ROI or 'waypoints'), which defines the obligatory tract passage where the tract is known to pass through; and the exclusion ROI (also known as the 'NOT' ROI), which defines the region the tract is known not to pass through. Streamlines intersecting the exclusion ROI are rejected.
Figure 8. A schematic illustration demonstrating the regions-of-interest (ROIs) strategy used for targeted tractography. Here, we have four different coloured white matter tracts organised in complex fibre arrangements, including crossing, kissing, and overlapping patterns. To reconstruct the blue-coloured white matter tract, the tracking ROIs are placed based on a priori anatomical knowledge about all four white matter tracts. ROI-A and ROI-B can be used as either the seed ROI (representing the tracking starting point) or the inclusion ROI (representing the obligatory passage of this tract). ROI-C, ROI-D, and ROI-E are used as exclusion ROIs (representing regions where the blue-coloured tract is known not to pass). Note that without using the ROI-E as an exclusion ROI, false positive reconstruction can be produced (i.e. the solid black-coloured streamline) due to streamlines propagating over the crossing-fibre region between the blue- and green-coloured white matter tracts.
Download figure:
Standard image High-resolution imageThe streamline tracking in targeted tractography is also dependent on the spatial and angular resolution of DWI data (Vos et al 2016, Schilling et al 2017), and many algorithmic variables that can be subjectively selected by the tracking operator. These include the dMRI model (e.g. DTI versus high-order models, such as CSD), the tracking algorithm of choice (e.g. deterministic versus probabilistic); and a set of pre-defined tracking parameters, e.g. the retained streamline numbers, the maximum and minimum retained tracking lengths, the size and angle between successive per-voxel streamline steps, and the criteria defined for track termination (Jeurissen et al 2019). Thus, performing targeted tractography in neurosurgical settings requires delineation of tracking ROIs by operators with expert anatomical knowledge, experience in adapting the ROI strategies in the presence of pathology, and expertise with chosen tractography techniques and their related technical limitations.
The following section will describe the two main classes of ROI strategies for targeted tractography in more detail: the anatomy-based ROI and functional-based ROI.
5.1.1. Using anatomy-based ROIs
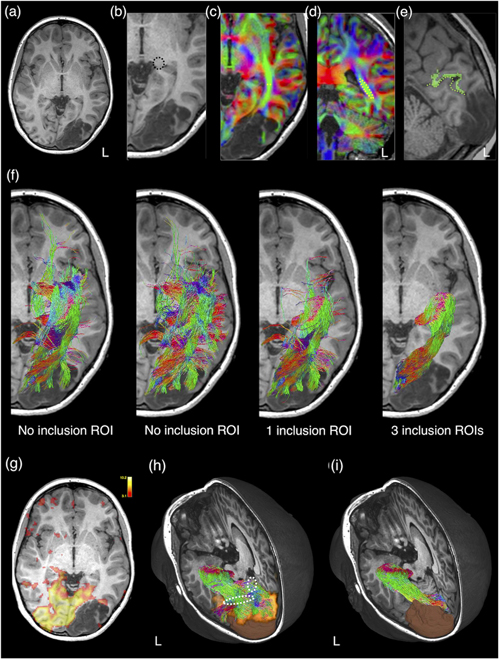
These are typically delineated manually based on the recognisable anatomical structures or regions (such as the cerebral peduncle for corticospinal tract reconstruction) or based on the WM regions delineated by the contrast related to the principal diffusion direction, as evident on the DEC map (Pajevic and Pierpaoli 1999, Calamante et al 2010). For example, the sagittal stratum, a deep WM region adjacent to the occipital ventricular trigone, is an obligatory passage for optic radiation, thus commonly adopted as the inclusion ROI for its tractography reconstruction (Yang et al 2019) (see also figure 9). The sagittal stratum can be identified and delineated on the DEC map as a green-colour region adjacent to the ventricular trigone, containing predominantly anterior-to-posterior oriented WM fibres (Yang et al 2019). In addition to manually defined ROI, further anatomical constraints to specific cortical and subcortical GM regions can be defined by using automated individual brain GM parcellation schemes derived from structural MRI or using warped atlas-based brain regions into the subject native imaging space. Targeted tractography based on manually placed or template-driven anatomical constraints can result in reconstructions that very accurately reflect the ground truth WM connections mapped by axonal neurotracer in sectioned macaque brain (Schilling et al 2020a). Increasing adding well-chosen ROIs, based on a priori anatomical knowledge about the WM tract, can further improve the anatomical precision of the tractography result (see figure 9).
Figure 9. The impact of different regions-of-interest (ROIs) strategies used in targeted tractography of the left optic radiation. This is a 13 year old girl with refractory visual focal epilepsy referable to a left occipital developmental brain tumour (dysembryoplastic neuroepithelial tumour; as shown on anatomical T1-weighted image in (a)). The optic radiation tractography can be reconstructed entirely using anatomy-based ROIs. The seed ROI is placed at the lateral geniculate body ((b); dashed black circle). An inclusion ROI is placed at the sagittal stratum, an occipital periventricular deep white matter region, containing anterior-posteriorly oriented optic radiation fibres, identified as a green coloured region on directional encoded colour maps (dashed yellow line and circle in respective axial (c) and coronal (d) planes). Note that blue colour encodes the superior–inferior orientation, and red colour encodes the left–right orientation. (e) Another inclusion ROI is placed at the pericalcarine cortex on either side of the left calcarine sulcus (dashed green line). Automated anatomical segmentation (in green) produces erroneous parcellation of the left pericalcarine cortex. The pericalcarine cortex is delineated manually in this instance. (f) The anatomical accuracy of the optic radiation tractography reconstruction improves with increased numbers of well-defined ROIs. All tractography results are shown in the same colours as those used for the directionally encoded colour maps. Here, we show the differences in the tracking results by using different numbers of inclusion ROIs. The same exclusion ROIs (not shown here) are used in all reconstructions. Note the far left panel in (f), a seed ROI is placed at the lateral geniculate body. In the next panel, a seed ROI is placed at the sagittal stratum. An alternative way for targeted tractography reconstruction is to use ROIs based on functional data. In this case, visual cortical activation derived from visual-task BOLD-fMRI (g) is used as an inclusion ROI. (h) A 3D rendered image of the resultant optic radiation tractography, the brain tumour segmentation (in brown) and the activated visual cortex (in orange). The tracking result should be interpreted with caution. Since direct geniculate-extrastriatal connections are rare in humans (Locke 1967, Ellis 2005, Clatworthy et al 2010), the portion of the tracking result may represent false positive reconstructions (dashed white outlines). (i) A 3D rendered image of the same optic radiation tractography using entirely anatomy-based ROIs, as described previously, is included here for comparison purpose. Material acronyms: L (left), ROI(s) (region-of-interest(s)).
Download figure:
Standard image High-resolution imageThe aforementioned ROI strategy will need to be modified if the brain anatomy is obscured by the presence of pathology or by the previous neurosurgical intervention (tracking in the post lesion resection regions, for example). While the decision is likely to be individualised, the general principle is to place ROIs only in areas with recognisable anatomy, and avoid placing precise ROIs in areas affected by pathology or surgery, which would introduce tracking bias due to ambiguous a priori anatomical knowledge. The presence of pathology may also render automated brain parcellation regions useless (see figure 9), or manual editing by expert raters is mandatory to ensure the delineated ROI satisfactorily encompasses the targeted anatomy (although the editing process may itself introduce further bias). This process, however, can be more time consuming than manually defining the ROI in the first instance.
5.1.2. Using functional-based ROIs
Another method is to use functional brain data to guide ROI definition, resulting in functionally more relevant targeted tractography reconstruction (Staempfli et al 2008, Tournier et al 2011) (see figure 5). This type of ROI strategy may be indicated when the delineated brain anatomy is obscured by the presence of pathology, and/or questions arise to visualise WM tract components subserving specific functions that may have reorganised topological functional representation (e.g. using finger-tapping BOLD-fMRI to help map out the finger-motor fibres of the corticospinal tract, for a brain tumour residing in the hand knob portion of the primary motor cortex). Examples of functional imaging modalities used to localise eloquent cortical regions include (but not limited to) the peak activation regions defined by task-based BOLD-fMRI (Smits et al 2007, Kleiser et al 2010, Sanvito et al 2020), nTMS (Frey et al 2012, Conti et al 2014, Krieg et al 2015, Picht et al 2016, Rosenstock et al 2017, Weiss Lucas et al 2017, Rosenstock et al 2020, Fekonja et al 2021), and MEG (Gaetz et al 2010). Similarly, functional brain mapping obtained through either cortical DES (Berman et al 2004) performed with electrophysiology monitoring (Maesawa et al 2010); or intracranial electrodes (i.e. the grid electrode used for intracranial electrocorticography in epilepsy surgery or electrodes used in deep brain stimulation surgery) can be used to define the tracking ROI.
An alternative approach is to first reconstruct the tractography using the anatomy-based ROI. The result is then visually assessed with respects to concordant streamline projections into eloquent cortices, mapped by the functional brain data or by intraoperative subcortical DES (Kamada et al 2005, Berman et al 2007, Bello et al 2008, Diehl et al 2010, Jeong et al 2013).
5.2. Survey of neurosurgical clinical practices
Targeted tractography has been widely applied in neurosurgery, with indications ranging from, resective surgery for wide spectrums of intrinsic brain lesions, examples including both high-grade and low-grade gliomas, cerebral metastases, vascular lesions (such as arteriovenous malformation and cavernoma); epilepsy surgery (such as resection of focal cortical dysplasia; anterior temporal lobectomy for hippocampal sclerosis and mesial temporal lobe epilepsy); cranial nerve mapping for skull-base neurosurgery (identify facial nerve location in vestibular schwannoma surgery, for example); functional neurosurgery (identifying thalamocortical connections adjacent to the targeted deep GM nuclei in deep brain stimulation surgery, for example), to intramedullary spinal cord tumour neurosurgery. The readers are referred to several comprehensive updated reviews and related studies for further information (Potgieser et al 2014, Egger et al 2016, Essayed et al 2017, Antherieu et al 2019, Henderson et al 2020, Vanderweyen et al 2020).
In high-grade glioma surgeries, DTI-based functional neuronavigation led to improved gross total resection with corticospinal tract involvement, prolonged survival, and a significant decrease in a postoperative motor deterioration compared to the control surgery group carried out without DTI-based tractography (Wu et al 2007). Similarly, improved lesion resection extent, survival rates, and greater motor functional preservation had been reported in retrospective DES-assisted glioma surgical series, complemented with DTI- and fMRI-informed neuronavigation (Bello et al 2008, Ius et al 2012). In a recently reported language dominant, insular-opercular paediatric epilepsy surgical case series, when performing DES or awake surgery was contraindicated (Yang et al 2020), combining expert generated probabilistic CSD-based targeted tractography and BOLD-fMRI were used to inform pre-surgical planning and intraoperative functional neuronavigation. The authors reported good surgical outcomes with minimal post-surgical morbidities. These children also avoided the added anaesthetic and surgical risks needed for further invasive intracranial electrode monitoring to localise epileptogenic focus and eloquent brain. The reported seizure freedom rates and post-operative morbidity profiles were comparable to other surgical series utilising high-density stereo-electroencephalography and open resection (von Lehe et al 2009, Dylgjeri et al 2014, Weil et al 2016, Freri et al 2017) or with invasive laser interstitial thermal ablation therapy (Freri et al 2017, Hale et al 2019). Finally, the use of targeted tractography in functional neuronavigation has been shown to improve efficiency in cortical and subcortical DES brain mapping (Bello et al 2008, Gonzalez-Darder et al 2010).
Presently, targeted tractography applications in neurosurgery are dominated by the use of the tensor model and FACT tracking algorithm as originally proposed in dMRI research 25 years ago. Contextually, this is likely due to the fibre-tracking tools from available commercial navigation software packages only supporting the use of this outdated dMRI tractography technique.
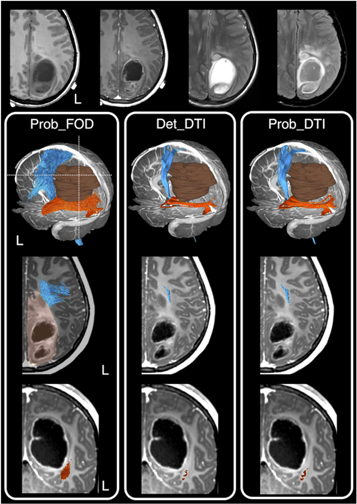
In a recent survey conducted from 36 out of all 40 neurosurgical units in the UK and Ireland, 90% of the neurosurgical units use tractography regularly, and they are predominantly DTI-based reconstructions. Concerningly, many neurosurgeons remain unfamiliar with the underlying methods used to produce tractography visualisations (Toescu et al 2020). An alternative way to provide a snapshot of clinical practice is to look at relevant tractography surgical cohort reporting. An author-initiated PudMed-based search for relevant literature over the last 25 years, demonstrating the striking contrast between the advanced dMRI-informed tractography research and what is being utilised and published from the clinical neurosurgical practice. Overwhelmingly, 94.7% of neurosurgical publications utilised DTI-based tractography, with the remaining 5.3% of studies utilised higher-order dMRI modelling techniques. Importantly, although many of these studies recognised the limitation of DTI-based tractography, and emphasised the need to introduce more advanced methods, none had actually proceeded with furthering research and industry partnership, working towards translating advanced dMRI methodologies into neurosurgical practice (Kuhnt et al 2012, Bucci et al 2013, Farquharson et al 2013, Kuhnt et al 2013, Zhang et al 2013, Lim et al 2015, Mormina et al 2015, Ashmore et al 2020, Fekonja et al 2021). Figure 10 shows a clinical example of differences in tractography appearances based on the selected dMRI modelling and tracking techniques.
Figure 10. A clinical case example showing tractography reconstruction using a combination of different modelling techniques and tracking algorithms and the impact on pre-surgical planning and intraoperative image-guidance. This is an 11- year-old girl presented with early clinical features suggestive of raised intracranial pressure, referable to a large left parieto-occipital high-grade glioma (glioblastoma multiforme). The corticospinal tracts (in blue), and optic radiations (in orange), and the brain tumour segmentation (in brown) are shown. The dashed white lines are the approximate image planes for the axial and coronal MR images, which are displayed in radiological convention. Note both the deterministic and probabilistic DTI tractography (Det_DTI and Prob_DTI) lead to the impression of gaps observed between the tumour margins and both white matter tracts; and failure to reconstruct the lateral projections of the corticospinal tract (i.e. the 'too-few', false-negative tracking problem). The use of the probabilistic tracking algorithm only partially recovers some of the 'missing' lateral corticospinal tract fibres. Using these tractography images in surgery can lead to inadvertent surgical injuries to both fibre tracts and associated functional consequences. On the other hand, reconstructions using a probabilistic fibre orientation distribution based (FOD) technique (Prob_FOD), combined with carefully placed regions-of-interest based on anatomical priors, limit the 'too-many', false-positive tracking problem. The reconstructed fibre tracts are anatomically more plausible in their appearances and are abutting the tumour margins—a critical piece of information for surgical approach and resection of this tumour. Material acronyms: Det_DTI (deterministic tracking algorithm, diffusion tensor imaging model), Prob_FOD (probabilistic tracking algorithm, fibre orientation distribution based), Prob_DTI (probabilistic tracking algorithm, diffusion tensor imaging), L (left).
Download figure:
Standard image High-resolution imageNonetheless, there remains a pressing need to bridge the evidence-practice gap between dMRI-informed tractography research on the one hand and clinical neurosurgery practice on the other. Establishing close clinical, research, and industry partnerships are key to further translate these novel techniques into the clinical neurosurgery realm (more to this in Q&A 8).
5.3. Highlights for Q&A 5
- Pre-surgical planning and intraoperative neuronavigation in neurosurgery utilise targeted tractography to visualise WM tracts adjacent to the resecting lesion.
- Expert manual delineations of tracking ROI based on a priori anatomical knowledge about the WM tract remains the reference standard for target tractography reconstruction used in the clinical setting.
- Alternatively, the tracking ROI can be based on eloquent cortical regions derived from functional brain data to improve the functional relevance of the tractography reconstructions. This is particularly the case in scenarios with distorted anatomy and concerns of functional reorganisation.
- Presently, targeted tractography applications in neurosurgery are dominated by the use of the tensor model and FACT tracking algorithm, as originally proposed in dMRI research 25 years ago. There remains a pressing need to 'move beyond DTI', and bridge the evidence-practice gap.
Q&A 6. Is tractography sufficiently reliable for neurosurgical applications?
The anatomical accuracy of fibre tracking is a primary concern in neurosurgical applications. This Q&A will begin by outlining typical sources that lead to anatomically inaccurate and variable tractography results, and will then comment on the factors to consider, when determining the reliability of tractography use in neurosurgery.
6.1. Source of tractography uncertainties
6.1.1. DWI acquisition and artefacts
The quality of DWIs is determined by the capability of the MRI system and the acquisition parameters. MRI hardware, such as the magnetic gradient field strength, is crucial to the signal-to-noise ratio (SNR) of MR images. Clinical MRI scanners are usually equipped with a limited gradient strength (commonly less than 80 mT m−1). Under such a condition, the diffusion pulse duration and separation (i.e. δ and ∆, see 2.1) must be prolonged to achieve the required high b-value for HARDI acquisition (Cho et al 2008, Tournier et al 2008, Yeh et al 2010, Tournier et al 2013) leading to longer echo time and stronger T2 signal decay. In DSI, for example, the SNR of individual DWI volumes can drop to noise level, as a result of strong DW signal attenuation and T2 decay. The DWI acquisition protocol and sampling strategy will need to be optimised to ensure the robustness of modelling and tractography results. While these optimisation processes are model specific, they generally consider the trade-off between the achievable b-value and the SNR of DWIs (Jones 2004, Cho et al 2008, Tournier et al 2013).
The number of unique diffusion gradient directions and the spherical uniformity of the gradient sampling scheme are other key factors to achieve the required angular resolution (Jones 2004, Tournier et al 2013). Increased sampling of DW directions can compensate for the low SNR and improves the precision of fibre orientation mapping. However, this requires an increased total scan time, renders it impractical for neurosurgical applications.
The DWI acquisition is sensitive to physiological motion, including voluntary head motion and involuntary motion relating to cardiac or CSF pulsation. The fast single-shot echo-planar imaging (EPI) is the main dMRI pulse sequence, which allows each slice to be acquired in a sub-second snapshot and a whole-brain DWI volume to be scanned usually around 10 s (depending on other imaging parameters). The single-shot EPI-based sequence in parts reduces motion artefact as compared to non-EPI methods, by confining head motion only within a short scan time. Restriction of DWI acquisition to quiet periods of the cardiac cycle via navigator echo (or cardiac-gating) compensates for the cardiac pulsation artefact. However, the associated longer acquisition time usually renders it impractical in most clinical settings.
The DWIs are subject to all the artefacts existing in EPI, making visual inspections of the image data necessary. Among them, susceptibility-induced inhomogeneity of static magnetic field (i.e. B0) causes localised signal loss (due to dephasing) and images to appear non-linearly warped along the phase-encoding direction. This occurs particularly in the anterior temporal and the frontal brain regions near the brain and bone/air interface (e.g. the skull base and sinuses) and the periventricular surface. Estimation of the magnetic field map or using parallel imaging acquisition can partly correct the distortion (Wu et al 2008).
The application of strong diffusion gradient pulses can induce eddy-currents and non-linearity effects that alter the applied gradient field profile, resulting in distortions of DWIs. Furthermore, the image distortion induced by eddy-currents is a function of the applied diffusion gradient direction, thus causing misalignment between DWI volumes. Modern MRI scanners have the in-built dMRI pulse sequence that can minimise this problem (Reese et al 2003). It can also be partially corrected via imaging registration approaches (Andersson and Sotiropoulos 2016).
Other artefacts including Gibbs ringing, DWI signal drifting, and the RF-driven bias field can all influence the quality of DWIs. Gibbs ringing is associated with the truncated sampling in using Fourier transforms to reconstruct signals into images, which typically occurs at high-contrast interfaces with an appearance of a 'ripple' pattern. DWI signal drifting is a consequence of MRI system instability, often leading to a drop of signal during the scan. The imperfect RF waves result in intensity non-uniformity in MR images, known as a bias field.
All of the aforementioned factors can exert downstream effects on the accuracy of the estimated diffusion model and the tractography. Corrections of these DWI artefacts are increasingly regarded as essential (Glasser et al 2013). Several dMRI freeware have robust DWI preprocessing pipelines for preparing DWI data for subsequent fibre-tracking analysis.
6.1.2. Diffusion models and tractography parameters
The quality of tractography results is also related to the choice of diffusion model that influences the accuracy and angular resolution of per-voxel fibre orientation estimation (described in Q&A 2–3). Studies have shown how changing tractography algorithms and their parameters affects the reconstruction of WM tracts. For a streamline generation, the fundamental tracking parameters include the step size, integration method, interpolation method, seeding strategy, and termination criteria (see Jeurissen et al (2019) for a review of these factors). As there are many elements involved, there is no one-for-all solution that can work for all WM tracts in the brain. Tractography reconstruction is inherently limited by a trade-off between sensitivity (i.e. to maximise the true positive rate) and specificity (i.e. to minimise the false positive rate).
For targeted tractography, the optimal setting may vary between WM tract under investigation. Nevertheless, it does suggest that deriving accurate per-voxel measurements is critical (i.e. optimal data acquisition, preprocessing, and voxel-wise diffusion modelling), since even a few degrees of error in the fibre orientation mapping can propagate and be magnified due to the step-by-step streamline integration of the tracking process.
6.1.3. ROI placements
The delineation of anatomically-based ROI is inherently biased by the accuracy of the a priori of WM tract anatomy knowledge. Any uncertainties from these empirical anatomical data, for example, the potential erroneous conclusions drawn from early histological and cadaveric dissection works, will directly impact the precision of ROI definition and the tractography output (Dick and Tremblay 2012). This is also relevant when the tractography results are post-edited manually to remove spurious and/or false positive streamlines, which also requires a priori anatomical knowledge. For these reasons, it is recommended to investigate the test-retest reliability of the tractography outputs, in both the intra- and the inter-rater scenario, and such assessments are likely to be specific to the tractography rater(s) and the practising institution. Using functional ROIs, such as cortical activation regions from task-based BOLD-fMRI, is subject to the patient's performance of the functional task, and the technical limitations imposed on these functional data themselves (Haller and Bartsch 2009).
Currently, the adopted WM tract nomenclature, and definition, with respect to its cortical projections, and intracranial course, in both the post-mortem dissection and dMRI tractography literature can vary considerably. There are now suggestions emerging from the clinical settings to address the issue about standardisation or consensus methodology on dissecting specific WM tracts using targeted tractography (Fekonja et al 2019), with some beginning to explore the clinical utility of implementing automated or atlas-derived tractography reconstruction techniques (Yendiki et al 2011, Tunc et al 2014, Labra et al 2017, Garyfallidis et al 2018, Wasserthal et al 2018, Zhang et al 2018, Warrington et al 2020, Zhang et al 2021) in adult brain tumour and epilepsy tractography cases (Tunc et al 2016, O'Donnell et al 2017, Mancini et al 2019). The results from international collaborative efforts using either the same clinical brain tumour or research dMRI datasets show a large variety in the tractography virtual dissection methods employed by different groups, and significant inter-rater variations of the tractography outputs (Pujol et al 2015, Schilling et al 2020b). Thus, there remains a need for clinical-research partnership to provide a consensus definition of WM tract anatomy for both dMRI research and clinical tractography use.
6.2. Tractography reliability
To answer whether tractography is reliable for neurosurgical applications, we must consider and ideally satisfy three levels of evaluation: 'algorithmic', 'biological' and 'clinical' reliability. In this section, we will delve into these concepts. Issues concerning tractography validation will be discussed in Q&A 7.
The first level is algorithmic reliability, which is the primary goal for all tractography technical development. This includes features such as the sensitivity to the imaging noise and curvature overshoot. The second level is the biological reliability that defines how accurate tractography outputs can delineate the true WM tracts, in both the healthy and the pathological brains. These two levels of reliability are fundamentally different. An algorithmically reliable tractography method operates properly on a given FOD field (i.e. the tractography output is highly precise and reproducible). However, the tractography output is only biologically reliable if the underlying FOD field is biologically plausible or meaningful (i.e. the tractography output is anatomically and biologically accurate). For example, in WM voxels containing crossing fibres, an algorithmically reliable fibre tracking algorithm would not reconstruct an anatomically accurate WM tract if the underlying vector field is derived from an over-simplified diffusion model, such as the tensor model (as described in Q&A 2). The tensor-based deterministic tracking approach is thus highly reproducible but biologically inaccurate due to the intrinsic systematic modelling bias (Farquharson et al 2013). Likewise, it is generally not sufficient to claim biological reliability purely based on an assessment of algorithmic reliability.
The third level is clinical reliability, which describes whether the use of tractography can improve surgical outcomes or not. This level of reliability can only be indirectly assessed using clinical or functional outcome measures following surgery. Prospective comparison of different tractography techniques is not possible within the same surgery, since there is no way to 'redo the same surgery' in the same patient.
Paradoxically, existing objective evidence concerning the clinical reliability of tractography applications in neurosurgery has so far been disappointing due largely to inadequate clinical outcome reporting from uncontrolled retrospective clinical surgical cohorts. Notably, a Cochrane review concluded low to very low-quality evidence that intraoperative DTI-based tractography image guidance benefited the extent of resection and found no survival benefit in adult high-grade glioma surgeries (Barone et al 2014), contrary to the reporting by Wu et al which remains the only randomised control trial to date which demonstrated functional neuronavigation incorporating tractography led to prolonged survival in adult motor-eloquent high-grade glioma patients (Wu et al 2007). While this review reaffirmed the prevalence of utilising DTI-based tractography in neurosurgery, it has not evaluated (thus does not necessarily discredit) emerging clinical targeted tractography studies utilising advanced dMRI data acquisition, higher-order dMRI models, and a probabilistic tracking algorithm (Fernandez-Miranda et al 2012, Chamberland et al 2014, Yang et al 2019). While there is currently not enough evidence to support that the application of advanced tractography techniques gives better surgical outcomes than conventional approaches, the clinical reliability should be established on the basis of algorithmic and biological reliability. In our opinion, tractography methods that are superior in all three levels will ultimately provide more meaningful and trustworthy results for clinical neurosurgical use.
Reportedly, the same Cochrane review also identified a significant concern regarding the risk of bias in the studies being investigated, with incomplete reporting of post-surgical adverse events, and high attrition bias in follow-up data, highlighting the need for prospective study design, high-quality randomised clinical trials and improve quality of clinical outcome reporting for future neurosurgical tractography studies (Barone et al 2014, Vanderweyen et al 2020).
6.3. Highlights for Q&A 6
- The accuracy and reliability of targeted tractography output is strongly dependent on how the dMRI data are acquired and processed.
- Sources of tractography uncertainties include the different DWI artefacts, the chosen diffusion model and the tracking parameters, and the chosen method of ROI placement strategies.
- A reliable tractography technique for neurosurgical application should ideally fulfil algorithmic, biological and clinical reliability.
- An algorithmically reliable tractography method does not necessarily equate to a biologically reliable tractography method. The tensor-based deterministic tractography used routinely in the neurosurgical setting is a good example of this.
- Assessing for clinical reliability of the tractography method is difficult, and can only be achieved indirectly with post-surgical functional outcome measures or indirectly via functional brain imaging.
Q&A 7. How to validate tractography outcomes?
Performing tractography validation in the living human brain, as in the case for neurosurgical applications, is fundamentally challenging due to lack of ground-truth. Preclinical validation work has been performed via various approaches: in silico or with physical phantoms, post-mortem fibre dissection and myelin-stained sections of ex vivo human brains and animal models, and axonal autographic neurotracer studies in ex vivo animal models. Clinical tractography validation is conducted indirectly using intraoperative DES and concordant information derived from functional brain imaging data. This section provides an overview and discusses both the strength and limitations of each validation method.
7.1. Synthetic or phantom data
Various software tools have been developed to validate the algorithmic reliability of fibre tracking (e.g. Close et al (2009), Neher et al (2014)). One can synthesise DWIs through a numerical model or simulate the FOD field directly. Synthetic DWIs can also be generated via using more sophisticated productions of simulated fibre configuration, combining with Monte Carlo simulations of water diffusion within the virtual tissue substrate, and then computing MRI signal based on the Bloch equation. This allows a tractography algorithm to be assessed in a controlled way against various settings, such as varying b-value, the number of gradient directions, SNR, and artefacts (e.g. Balls and Frank (2009), Yeh et al (2013)).
More recently, a synthetic DWI dataset was 'reversely' generated based on the predefined outcomes of targeted tractography (Maier-Hein et al 2017). Those predefined WM tracts, identified by one expert radiologist who performed and checked the quality of reconstruction, were utilised as the ground-truth WM pattern in the human brain. While more complex and realistic than previous numerical phantoms, this synthetic phantom does not fully capture the brain complexities, as only a subset of WM tracts are included in the model. Currently, such targeted tractography based on the manual delineation of ROIs would likely remain the ground-truth, which is dependent on raters' anatomical expertise. This highlights the need for a well-regulated and validated tractography methodology, to allow expert raters to assess and maximise the anatomical accuracy of tractography outputs, as alluded in section 6.1.3.
The other option is to construct a physical phantom model (Poupon et al 2008, Tournier et al 2008). Compared to computational synthetic data, building a physical phantom is challenging, sometimes expensive and time-consuming. It is also not as flexible as the computer simulation in terms of designing different fibre configurations for testing purposes. Nevertheless, this allows the testing to be performed using a realistic data acquisition and can be used to evaluate the stability of DWI scans, including cross-scanner calibration.
7.2. Post-mortem fibre dissection
Post-mortem fibre dissection studies are fundamental for understanding the organisation of WM tract architecture (Dejerine 1895, Dejerine 1901, Klingler and Ludwig 1956, Türe et al 2000, Schmahmann and Pandya 2007, Fernández-Miranda et al 2008, Lawes et al 2008, Martino et al 2013). When comparing the tractography outputs with the existing or classic descriptions of anatomical references about WM tract anatomy based on human post-mortem dissection studies, there are some important caveats to consider: despite over centuries of works by many, there is still no agreement to the exact anatomy of many WM tracts. In particular, disagreements exist with regards to the precise cortical origin or terminations of these WM tracts (Martino and Brogna 2011). Classic post-mortem fibre dissection technique requires WM tracts to be identified by an 'outside-in' approach through peeling away the cortex (Klingler and Ludwig 1956). Even with a meticulous dissection technique using an operative microscope, delineation of adjacent WM tract populations cannot be reliably guaranteed (Yasargil et al 2004). In addition, post-mortem fibre dissection is relatively coarse, and cannot reliably trace WM fibres through crossing fibre regions (Fernández-Miranda et al 2008). Tissue fixation-related artefacts during cadaveric specimen preparation can also affect the accuracy and validity of the dissection findings. For this very reason, the existence of some WM tracts, as distinct fibre tract entities, has been questioned (Tusa and Ungerleider 1985).
7.3. Histological sections and axonal tracing
Ex vivo dMRI of tissues, in combination with histological stains or axon tracing techniques, is an important approach to investigate information of in vivo dMRI data (Schilling et al 2018, Yendiki et al 2021). This allows validation of WM orientation and tractography estimates to be performed on the same tissue sample, either from post-mortem human brains or animal models (Seehaus et al 2015). The advances in imaging techniques on ultra-high field MRI systems (with B0 at 7 Tesla or above) can achieve high-quality high-resolution image acquisition at tens to few hundreds of micrometres, making the comparisons of fibre orientation mapping with histological sections even more feasible (Sato et al 2017, Roebroeck et al 2019, Yendiki et al 2021). Further, in vivo studies in mammalian tissue using endogenous fluorescent axonal labels have been an additional strategy to unveil the relationships between the dMRI signals and WM microstructures. Such experimental strategy has been adopted to provide quantitative tissue validation of advanced dMRI biophysical model metrics (Sato et al 2017), or to study the sensitivity of dMRI metrics for early detection of presymptomatic degenerative neurological diseases (Gatto et al 2018a, Gatto et al 2018b).
Histochemical staining of sectioned brain tissues is a classic way to study WM tract anatomy. Axonal anatomy is identified through tracing the course of Wallerian degeneration, or via staining the myelin content of either developing or degenerating axons (Englander et al 1975, Kretschmann 1988, Yagishita et al 1994). Histochemical staining studies can also be performed in animal brains through axoplasmic transport of either retrograde or anterograde tracers (Schmahmann and Pandya 2006, Schmahmann et al 2007, Morecraft et al 2009). These techniques offer the advantage of reducing the number of misidentified crossing fibres, and can reliably identify both the origin and the terminations of different WM tracts (Morecraft et al 2009). In particular, myelin-stained histology is popular for generating the ground-truth fODFs from the histological orientation profile using a structural tensor analysis approach.
Many studies have used manganese-enhanced MRI (MEMRI) for tractography validation (Lin et al 2001, Knösche et al 2015). The paramagnetic manganese ion (Mn2+) is a potent T1-shortening agent and can be uptaken and transported actively along the axon. Hence, manganese tracers can be identified on T1-weighted images and thereby used to characterise the connectivity of an injection site within the same brain, which is comparable to the connectivity profile of a seed region obtained by tractography (Knösche et al 2015). To date, the mechanism of Mn2+ within the brain is not very well understood, making the interpretations of the results obtained by MEMRI sometimes uncertain. For example, the apparent transport rates of Mn2+ could potentially vary among axons according to experimental modelling from dynamic MEMRI (Deng et al 2019).
More recently, tractography validation has moved towards using a multi-modality approach. Methods such as three-dimensional polarised light imaging can provide a multiscale reconstruction of FODs to evaluate diffusion modelling and tractography (Salo et al 2018, Alimi et al 2019). Tissue clearing methods combined with immunohistochemical staining, provide neuron-to-neuron connectivity at microscale cellular level, potentially to be adopted as ground-truth connectome data (Goubran et al 2019, Leuze et al 2021). These advanced neuroimaging techniques are promising but currently only applicable to imaging small blocks of biological tissue.
7.4. Indirect validations using empirical clinical evidence
Performing DES is considered as the 'gold standard' in neurosurgery to validate the eloquent cortex location, WM tract position and its functional relevance (Penfield and Boldrev 1937, Ojemann et al 1989, Berger and Hadjipanayis 2007, Duffau 2015). A meta-analysis demonstrated brain tumour surgeries assisted by DES resulted in more extensive resection of the eloquent lesion, and fewer late severe neurological deficits compared to resections performed without DES (De Witt Hamer et al 2012). During DES-assisted surgery, a small amount of electrical current is passed directly to the targeted cortex, which elicits transient functional perturbation that could be identified by testing the patient's response during awake surgery (as in identifying transient language deficits in counting or object naming objects, for example), or while the patient is asleep under general anaesthesia (i.e. to elicit limb motor response following motor cortex stimulation, as evident by electrophysiological monitoring, i.e motor evoked potentials). Similarly, in subcortical stimulation, the anatomical location of the WM tract is confirmed if the functional perturbation was elicited when stimulating subcortically at the tractography-informed WM location. One should be aware that DES procedure is subjected to its own methodological consideration, including the stimulation parameter used, the stimulation types (i.e. monopolar versus bipolar stimulation), the size of the electrode tips and its effective stimulation radius (about 5–10 mm), false positive stimulation due to current afterdischarges, and false negative stimulation due to dysmyelination or neuronal migration disorders causing epilepsy (such as focal cortical dysplasia, and tuberous sclerosis) in paediatric cases (Chitoku et al 2001, Ng et al 2009, Tuxhorn 2010) or due to patient fatigue, as in the case of language mapping in awake surgery (Tuxhorn 2010, Mandonnet 2011).
Evidence concerning DES-based tractography validation studies was primarily derived from clinical glioma series performing motor stimulation and DTI-based corticospinal tractography reconstruction (Kamada et al 2005, Berman et al 2007, Mikuni et al 2007, Bello et al 2008, Kamada et al 2009, Gonzalez-Darder et al 2010, Zhu et al 2012, Bonney et al 2017). The correspondence between the tractography and subcortical DES findings were generally high, with reported sensitivity ranged between 90% and 98% and specificity ranged between 85% and 100% (Mikuni et al 2007, Bello et al 2008, Zhu et al 2012, Bonney et al 2017). Despite this, these studies consistently observed an up to ∼1 cm discrepancy between the tractography position and the positive direct stimulation points. This spatial discrepancy may represent cumulative errors relating to the stimulation procedure, image registration, technical nuances concerning the dMRI model and tractography method, and intraoperative brain shift (more to this point about brain shift in Q&A 8; (Berman 2009)). This has led to a recommended practice of a '1 cm' safety margin for tractography-informed neuronavigation, based on preoperative deterministic DTI-based tractography.
Considering DES is both invasive and time-consuming to perform, and contraindicated for some (i.e. young children, for example), it may not be a viable surgical adjunct in certain clinical scenarios. In these instances, functional imaging modalities, such as task-based BOLD-fMRI, and MEG, can be used to indirectly validate tractography by co-localising tractography streamline terminations with the eloquent cortices. This observation can be used as indirect means to improve the clinician's confidence about the tractography findings, although a perfect spatial concordance is not to be expected due to differences in the underlying imaging principles. Interestingly, quantifying the spatial extent of this structural-functional co-localisation has been adopted as a strategy to indirectly validate exploratory automated tractography techniques applied in temporal lobe epilepsy and brain tumour patients (O'Donnell et al 2017, Mancini et al 2019).
Lastly, functional outcomes following surgery can serve as indirect reminders about the validity of preoperative tractography, with knowledge of tract preservation or damage during the surgery or demonstrated by postoperative tractography. This form of indirect validation using functional interpretation is only possible if a WM tract or tract component has clearly defined topological functional representation. A good example is surgical injuries to the anterior temporal fibres of optic radiation (i.e. Meyer's loop) results in upper quadrant visual field defects (Hughes et al 1999). Preoperatively reconstructed Meyer's loop tractography and its distance from the temporal pole could predict the degree of postoperative visual field deficits following anterior temporal lobectomy epilepsy surgery performed in adults (Piper et al 2014, Lilja et al 2015). Incorporating intraoperative optic radiation tractography in anterior temporal lobectomy could significantly reduce the frequency of postoperative visual field deficits (Piper et al 2014, Cui et al 2015). In other scenarios, the possibility of functional reorganisation with either an adaptive or maladaptive neuroplasticity mechanism (Thomas et al 2007), makes speculation about the direct functional relevance of the tractography appearance uncertain. To this end, it is important to emphasise that tractography only infers WM structures. Combining it with postoperative functional imaging studies are mandatory to provide further insights into brain structural-functional relationships.
7.5. Highlights for Q&A 7
- In vivo dMRI tractography validation is fundamentally limited and remains one of the greatest challenges in the field due to the lack of ground-truth information.
- Tractography validation can be performed using numerical or physical phantoms, post-mortem fibre dissection, myelin-stained histological sections, or axonal neurotracers in non-human primates. Direct translation of these findings to in vivo tractography is confined by the methodological limitations of these techniques.
- Concordance of multimodal structural-functional imaging findings improves clinical confidence of the tractography findings.
- DES remains the 'gold standard' for functional brain mapping in neurosurgery, including validation of both the position and functional relevance of WM tracts during the surgery.
- Functional neuronavigation informed by tractography and functional imaging modalities, such as BOLD-fMRI, are used to complement the DES findings, and to improve the efficiency of the stimulation procedure performed during surgery.
Q&A 8. What are the practical challenges and future demands of tractography-informed neurosurgery?
This section highlights some key practical challenges of current tractography-informed neurosurgical practice, emerging techniques aiming to overcome these challenges, and demands for future improvements, with an aim to introduce advanced tractography methodologies into the routine clinical realm.
8.1. Practical challenges
8.1.1. Intraoperative brain shift
Conventional intraoperative image-guidance is based on preoperatively acquired MRI. The main drawback is the inability to account for the effects of brain shift that occurred throughout the course of surgery (Dorward et al 1998, Nabavi et al 2001) (see also figure 11). The brain shift is a progressive, dynamic process affecting the whole brain and is influenced by multiple intraoperative factors, such as the craniotomy and dural opening sizes, resection size, and the presence of brain oedema (Romano et al 2011, Gerard et al 2017, Yang et al 2017). The direction of WM tract shift can be unpredictable, impacted by the type of WM tracts studied, and the proximity of WM tracts to the lesion (Nimsky et al 2006, 2007, Maesawa et al 2010, Yang et al 2017). It remains unclear how different surgical factors contribute to the magnitude and direction of the intraoperative WM tract shift. As alluded to previously, the presence of brain shift contributes to the proposed '1 cm' safety resection margin relating to neuronavigation errors guided by preoperatively reconstructed deterministic DTI-based tractography (Kamada et al 2005, Berman et al 2007, Mikuni et al 2007). A recent probabilistic CSD-based tractography study in paediatric epilepsy and brain tumour surgeries had demonstrated intraoperative WM tract shift accounts for one-third to half of this distance (Yang et al 2017). A revised '5 mm' resection margin was proposed, representing combined errors from electrical stimulation, tractography methodology, and image registration.
Figure 11. A clinical case showing evidence of progressive intraoperative brain shift affecting neuronavigation accuracy using preoperatively reconstructed tractography. This is the same patient used for case illustration in figure 9. She had full visual fields on formal perimetry testing before the surgery. (a): Preoperative axial T2-weighted MRI showing the tumour location and with left optic radiation tractography reconstruction (in orange), showing the tract is antero-medially displaced by the tumour. (b)–(d): Serial intraoperative photos showing dynamic brain shift occurred throughout the surgical course. Different degrees of brain herniations are noted at craniotomy, prior to (b) and following dura opening. (c) The view at the completion of tumour resection, showing the surgical cavity collapses away from the surgeon's view (d). Note the cavity is lined by both the occipital white matter (asterisk) and the resection enters into the occipital horn of the left lateral ventricle (crosshair). Two intraoperative MRI scans were performed, first during (e), and second at completion, confirming gross total tumour resection was achieved (f). The intraoperative MRI is shown with and without the preoperative tractography overlays. In both instances, using this preoperatively prepared tractography would result in inaccurate image-guidance during the surgery. This is evident by the overlaps observed between the tractography, the residual tumour (dashed white-coloured outline), and the lateral ventricle (dashed white-coloured outline) in (e); and between the tractography and the surgical cavity (solid white-colour line) in (f). Material acronyms: iMRI (intraoperative MRI), L (left).
Download figure:
Standard image High-resolution imageAcquiring updated intraoperative images using intraoperative MRI scans remains one of the most practical solutions addressing the registration inaccuracy caused by brain shift (Dorward et al 1998, Nabavi et al 2001). While other imaging techniques, such as intraoperative ultrasound, can be performed directly by the operating surgeon allowing for rapid assessment of updated lesion status, it does not enable tractography visualisation (Sosna et al 2005, Liang et al 2019, Yeole et al 2020). Intraoperative MRI remains the most direct method that can obtain updated tractography during the surgery. Recent developments in MRI have produced intraoperative high-field 3 Tesla MRI scanners acquiring high-resolution DWI data possible during surgery. The accuracy of surgical neuronavigation can be improved by the real-time update of WM tract positions, compensating for the effects of intraoperative WM tract shifts. Open-source software tools enabling live, interactive visualisation of streamline reconstructions are now emerging (e.g. Fibernavigator 14 (Chamberland et al 2014)). In line with the aforementioned challenge of bringing advanced dMRI data and higher-order dMRI WM modelling technique to neurosurgical settings, there remains a pressing need to examine the computation efficiency of dMRI data acquisition and tractography processing pipeline, in order to bring intraoperative real-time tractography into surgical reality.
8.1.2. Optimising peri-lesion WM fibre tracking
The presence of brain pathology poses unique challenges for tractography reconstruction relating to the distortion of normal brain anatomy and the associated cerebral vasculature (Zhang et al 2013, Abhinav et al 2014). While generally speaking (thus not applicable to all cases), a disease process that behaves aggressively, such as high-grade glioma, can lead to axonal loss and disruptions of adjacent WM tract fibres (Goebell et al 2006). Alternatively, a slow-growing lesion, such as low-grade glioma and epileptogenic developmental brain tumour, for example, can cause mechanical displacement of the tract fibres, or can infiltrate the WM tract (Leclercq et al 2011). Peri-lesion WM oedema may be present, which can be vasogenic in origin for high-grade glioma (Goebell et al 2006), or cytotoxic in origin for an acute haemorrhagic lesion, for example. Similarly, WM injury/gliosis may be present following hypoxic-ischaemic cerebral insults (Wang et al 2016), and treatment-related WM injury, in the form of demyelination or axonal degeneration due to tissue necrosis, that may be presented in brain tumour cases following cranial irradiation therapy (Greene-Schloesser et al 2012). These lesion-tissue effects introduce uncertainties in WM modelling based on the diffusion signal (Provenzale et al 2004). They typically introduce noisier, and less anisotropic diffusion signal, leading to inaccurate local modelling of orientation estimates, and premature tracking terminations and thus false negative tractography results ensued (Roberts et al 2005, Leclercq et al 2011).
Presently, there are emerging methods that better deal with peri-lesion fibre trackings compared to the DTI model. They included methods based on higher-order dMRI models (McDonald et al 2013, White et al 2013a, 2013b, Zhang et al 2013, Abhinav et al 2015, Paquette et al 2016), multi-tissue CSD accounting for signal contribution from the WM, GM, and CSF compartments (Jeurissen et al 2014); free water estimated based on the two-tensor model and unscented Kalman filter tractography (Pasternak et al 2009, Malcolm et al 2010, Lecoeur et al 2014, Chen et al 2015, O'Donnell et al 2017, Gong et al 2018, Parker et al 2020); oedema-assisted correction of fODF estimation using multi-shell dMRI data (Lecoeur et al 2014) and edema-invariant particle filter tractography informed by pathological tissue image segmentation (Deslauriers-Gauthier et al 2018). The more recently proposed microstructural-informed tractography, which takes into account the subtle diffusion features relating to cellular microstructures and microenvironment (such as cell shape and tissue permeability), also holds great potential (Nilsson et al 2013, Daducci et al 2016).
Several brain tumour cohort studies demonstrated improved anatomical plausibility of the reconstructed WM tracts using higher-order dMRI models combined with a probabilistic algorithm, compared with DTI-based tracking using a deterministic algorithm (Kuhnt et al 2012, Bucci et al 2013, Farquharson et al 2013, Kuhnt et al 2013, Zhang et al 2013, Lim et al 2015, Mormina et al 2015). The benefits of the advanced tractography technique were most pronounced when reconstructing WM tracts near to the pathology and in presence of peri-lesion oedema (Kuhnt et al 2013). In one study, this was validated using DES, confirming correspondence between the reconstructed tractography image and true WM tract positions (Bucci et al 2013).
Importantly, while these advanced methodologies hold great promise, the reporting remained limited to using preoperative MRI data from small retrospective surgical cohorts. They were conducted primarily with a research focus (i.e. methodological comparisons) rather than applied for tractography-informed neurosurgery in prospective clinical surgical cohorts. Evidence of clinical tractography application was limited to selected case(s) illustration in few of these papers and related publications, with some lacking clinical descriptions concerning the neurosurgical procedure and the post-surgical outcomes (Fernandez-Miranda et al 2012, Bucci et al 2013, Abhinav et al 2015). There remain pressing needs for in vivo validation works, examining the accuracy of these modelling findings. This can be done either with using tissue histopathology obtained during the neurosurgical procedure (i.e. look for the presence of axons within the lesion to address the question of whether the fibre tracts traverse through the lesion); or with direct cortical and subcortical DES, confirming the WM tract position, as was done in one of these studies (Bucci et al 2013).
8.2. Future demands: moving beyond DTI
Many neurosurgeons are now increasingly recognising the need to 'move beyond DTI' in neurosurgical practice (Kinoshita et al 2005, Tournier et al 2011, 2013, Duffau 2014, Nimsky 2014, Kinoshita et al 2016, Toescu et al 2020, Fekonja et al 2021). Unfortunately, to date, advanced MRI tractography techniques have yet to be incorporated into commercial neurosurgical navigation software, which are the ones most widely used in clinical practice. The impediment to this clinical translation is multifactorial, in part, stemmed from the clinician's lack of awareness or misconception surrounding the dMRI theory, and technical nuances that underpin the tractography outputs (Toescu et al 2020). Many perceive that the advanced techniques are 'too complex' to implement and involve 'long acquisition and processing times incompatible with clinical practice' (Kinoshita et al 2016). There remains a need to improve the technical efficiency of advanced methods to be comparable with acute neurosurgical time. For example, it is currently not feasible to apply the advanced methods to a high-grade glioma patient who presented with acute neurological decompensation, and brain herniation related to the tumour mass effect, which warrants immediate surgery for tumour debulk. On the contrary, the application of advanced tractography methodologies is highly feasible for many lesion-based neurosurgery performed on either a semi-urgent or elective basis. This includes most of the benign intrinsic brain lesions, such as low-grade glioma, developmental brain tumours, cavernoma, and majorities of the lobectomy and lesionectomy-based epilepsy surgeries (Mormina et al 2015, Caverzasi et al 2016, Mormina et al 2016, Yang et al 2017, Hales et al 2018, Yang et al 2019, Becker et al 2020, Yang et al 2020). Additionally, continual advances in MRI hardware and dMRI acquisition scheme, such as the simultaneous multi-slice imaging (Feinberg and Setsompop 2013) have reduced the data acquisition time down to clinically acceptable times.
On the other hand, many advanced MRI techniques were developed with little consideration to clinical use, driven mainly by imaging scientists, MR physicists and signal processing experts, with little input from clinicians and neuroanatomists. These were based on studies usually in healthy and typically developed individuals without demonstrating methodological feasibility and reliability using clinical neurosurgical patient data. These methods are only possible through open-source MRI software packages, and the tractography outputs are incompatible with current surgical navigation software, thus preventing immediate clinical uptake. Open-source tools are being developed and now made available in the public domain to transfer such tractography outputs and import them into selected surgical navigation platforms, enabling intraoperative image-based navigation by integrating tract visualisations with other image data (e.g. Karawun 15 ). Alternatively, there are novel tools (e.g. OpenIGTLink 16 ) to integrate open-source viewing platforms and real-time surgical neuronavigation data. This is an area of ongoing research development with the potential of significant clinical impact.
Thus, it is clear that clinicians, researchers, MRI and surgical navigation vendors need to work collaboratively to help bridge the evidence and practice gap, to introduce advanced tractography methodologies into the routine clinical neurosurgical realm. In line with the recommendations provided by a recent tractography review (Vanderweyen et al 2020), we believe continued and improved communication and education between all parties involved will likely lead to clinicians being better informed of the technical nuances; researchers and industry vendors to better appreciate the focus of clinical needs; and greater potential for translating advanced sequences and modelling techniques into MRI and surgical navigation devices. Pleasantly, such collaborative efforts are already underway, as highlighted by a recent multi-institution, multi-disciplinary dMRI tractography project with the aims to both survey the different in vivo fibre dissection approaches, and attempting to reach a consensus definition of WM tract anatomy and nomenclature between the clinical and research communities (Schilling et al 2020b).
Many international tractography challenges held to date, for example, ranging from using MRI phantom (Fillard et al 2011, Cote et al 2013), synthetic dMRI data (Maier-Hein et al 2017), high-resolution human dMRI data (Neher et al 2015, Schilling et al 2020b) and selected clinical brain tumour MRI data (Pujol et al 2015), all, more-or-less, revealed a common feature: a high degree of inter-rater tracking variability of tracking reliability and reproducibility; and different groups adopted their preferred in-house advanced tractography algorithms. (For a comprehensive review about these tractography challenges, see Schilling et al (2019).) Thus, before a methodological consensus can be considered for clinical neurosurgical tractography applications, more comprehensive surveys of clinical tractography usages in neurosurgery, like the recently conducted UK-based clinical survey mentioned previously (Toescu et al 2020), and more high-quality studies are required to quantify the performance of different advanced methodologies using in vivo clinical MRI data (Vanderweyen et al 2020). Similarly, more in vivo tractography validation studies are needed in DES-assisted neurosurgical cases to map out WM tract position. We advocate for all future clinical tractography studies to include comprehensive clinical outcome reporting, preferably with formal neurological and objective functional assessments conducted both before and after surgery. Lastly, high-quality prospective randomised clinical trials involving a tractography intervention and a patient control group, similar to the study performed by Wu et al (2007), are desired in order to formally evaluate the clinical value of advanced dMRI tractography methods in a less biased manner.
8.3. Highlights for Q&A 8
- Intraoperative brain shift compromises the accuracy of tractography-informed neuronavigation based on preoperatively acquired MRI, highlighting the need for intraoperative real-time imaging updates.
- The accuracies of dMRI modelling and fibre tracking are uncertain within the pathology and in the peri-lesion WM. Promising methods are being proposed but remaining largely confined to the research domain.
- Clinical translation of advanced dMRI modelling and tractography methods requires a collaborative approach involving close clinical, research and industry partnership.
- Further high-quality methodological and prospective studies are required to: (1) survey more comprehensively the current landscape of clinical tractography use worldwide; (2) quantify tracking variability based on different advanced methods, using clinical MRI datasets; (3) further in vivo tractography validation works in DES-assisted neurosurgery and myelin-stained histopathology validation based on the resected surgical specimens; (4) improve clinical outcome reporting in tractography-informed neurosurgical case series.
9. Conclusion
Diffusion MRI tractography is a unique neuroimaging technique for in vivo visualisation of WM tracts in the brain. Despite 25 years of advances in dMRI research and tractography techniques, deterministic DTI-based targeted tractography reconstruction remains the most widely adopted method in clinical neurosurgical applications and the only method to be adopted by commercial neurosurgical navigation software. Barriers to this clinical translation are complex, and in large part, driven by the lack of high-quality evidence to support clinical benefits from more advanced tractography methods. Contextually, this was also related to the lack of clear knowledge transfer from the dMRI communities to the industry vendors, and clinician end-users, concerning the dMRI physics, and the technical benefits of the more advanced tractography methods. Starting with reviewing the WM anatomy, and dMRI physics, we demonstrated why it is imperative that techniques beyond DTI-based tractography should be implemented and adopted for neurosurgical applications. Importantly, there are fundamental differences between the methodological (algorithmic) and biological reliability of tractography. Highly reproducible tractography outcomes (e.g. such as those that can be achieved with deterministic DTI-based tractography) do not necessarily suggest biological accuracy.
This article reviewed the sources of tractography uncertainties, including factors relating to data acquisition, modelling, tracking algorithm and the adopted ROI strategy. For neurosurgical applications, considerable variations can occur when targeted tractography is performed by different raters. Recognising these technical limitations are crucial for using and interpreting tractography results, as well as to motivate new developments or improvements. There is an urgent need to standardise and optimise the dMRI scanning and processing paradigms, to minimise the variations induced by manual intervention.
Verifying the clinical reliability of dMRI tractography remains an open challenge to both the clinical and dMRI research communities. Uncertainties remain in the dMRI modelling accuracy within the pathology and the peri-lesion WM regions. Promising methods are being proposed but are largely confined to the research domain. Accuracy of tractography-informed neuronavigation is compromised by intraoperative brain shift, highlighting the need for intraoperative real-time imaging updates. As of now, clinically applied targeted tractography cannot be relied upon solely, particularly when used for intraoperative neuronavigation. Tractography should be used in conjunction with functional imaging modalities, such as BOLD-fMRI, to indirectly confer tracking accuracies by its functional relevance. The spatial concordance between brain structure and function should be interpreted carefully within the context of technical limitations of the employed tractography and functional imaging methods themselves. DES remains the gold-standard for functional brain mapping and in vivo tractography validation in neurosurgery. Functional neuronavigation utilising multimodal imaging approach, complement DES by improving procedural efficiency, surgical precision, and reducing postoperative surgical morbidities. With more neurosurgeons increasingly recognise the need to 'move beyond DTI', emerging evidence is now available, concerning the feasibility and clinical benefit of advanced tractography techniques employed by selected academic neurosurgical institutions. In these expert hands, questions may begin to be asked about the role of multimodal tractography and functional imaging, in cases where performing DES is clinically contraindicated or is not possible. Looking ahead, continued improvements in dMRI tractography methodology coupled with more accurate physics modelling, more efficient data acquisition and processing pipelines, real-time and interactive technology are all expected to bring more benefits to the neurosurgical environment. Ongoing communications between clinicians, basic scientists, and MRI and surgical navigation industry vendors, are necessary to understand mutual needs for future developments, thus maximising the full potential of dMRI tractography.
Acknowledgments
JYMY receives position funding from the Royal Children's Hospital Foundation (RCH1000), and acknowledges the support from the Murdoch Children's Research Institute, Royal Children's Hospital, The University of Melbourne Department of Paediatrics and the Victorian Government's Operational Infrastructure Support Program. CHY is grateful to the Ministry of Science and Technology of Taiwan (MOST 109-2222-E-182-001-MY3) for the support. FC acknowledges the support of the National Health and Medical Research Council of Australia (APP1091593 and APP1117724) and the Australian Research Council (DP170101815).
Footnotes
- 11
A volume of rodent WM equivalent to a 2 mm isotropic MRI voxel size of the human brain WM could contain around 0.5–5 million axons, 0.68 million oligodendrocytes, 0.18 million astrocytes, 52 thousand oligodendrocyte precursor cells and 76 thousand microglia. Despite having fewer numbers than axons, astrocytes including their extensive long processes contribute to about half of the volume (Walhovd et al 2014). Note that these numbers do not necessarily reflect the exact constitution in the human WM.
- 12
Probabilistic tractography were performed using MRtrix3 (Tournier et al 2019), based on a multi-tissue constrained spherical deconvolution model (Dhollander and Connelly 2016). The tracking regions-of-interest (ROIs) were derived by combining the manually delineated and the Desikan–Killany–Tourville atlas-based automated parcellations (Desikan et al 2006, Klein and Tourville 2012) available from FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). Decisions about the ROIs were based on a priori knowledge about the WM tract anatomy, similar to those described by Wassermann et al (2016), although with further modifications.
- 13
Connectomics is an active research area that aims at understanding the organisation and topology of functional and structural connectivity—an application of network theory onto brain sciences (Bullmore and Sporns 2009). A crucial procedure is the construction of connectome, which is analogous to a network graph used in the mathematics domain. The idea is to generate a complete brain connectivity diagram of all associations among a collection of vertices (also called nodes) and their pairwise links (also called edges). Nodes are brain ROIs, typically derived from a brain parcellation or atlas, but depending on the measurement technique, they can also come from more localised areas such as using electrodes. Edges are the actual measure of relations (or structural connectivity,) between every node pair.
- 14
- 15
- 16