Abstract
Positron emission tomography-magnetic resonance (PET-MR) scanners could improve radiotherapy planning through combining PET and MR functional imaging. This depends on acquiring high quality and quantitatively accurate images in the radiotherapy position. This study evaluated PET-MR image quality using a flat couch and coil bridge for pelvic radiotherapy. MR and PET image quality phantoms were imaged in three setups: phantom on the PET-MR couch with anterior coil on top (diagnostic), phantom on a flat couch with coil on top (couch), and phantom on the flat couch with coil on a coil bridge (radiotherapy). PET images were also acquired in each setup without the anterior coil. PET attenuation correction of the flat couch and coil bridge were generated using kilovoltage computed tomography (CT) images and of the anterior coil using megavoltage CT images. MR image quality was substantially affected, with MR signal to noise ratio (SNR) relative to the diagnostic setup of 89% ± 2% (mean ± standard error of the mean, couch) and 54% ± 1% (radiotherapy), likely due to the increased distance between the patient and receive coils. The reduction impacted the low-contrast detectability score: 23 ± 1 (diagnostic), 19.7 ± 0.3 (couch) and 15 ± 1 (radiotherapy). All other MR metrics agreed within one standard error. PET quantitative accuracy was also affected, with measured activity with anterior coil being different to diagnostic without anterior coil by −16.7% ± 0.2% (couch) and −17.7 ± 0.1% (radiotherapy), without attenuation correction modification. Including the couch and coil bridge attenuation correction reduced this difference to −7.5% ± 0.1%, and including the anterior coil reduced this to −2.7% ± 0.1%. This was better than the diagnostic setup with anterior coil (difference −8.3% ± 0.2%). This translated into greater PET SNR performance for the fully corrected radiotherapy setup compared to diagnostic with coil. However contrast recovery was unchanged by the modified attenuation correction, with the diagnostic setup remaining ∼2% better. Quantitative PET in the radiotherapy setup is possible if appropriate attenuation correction is used. Pelvic radiotherapy PET-MR imaging protocols will need to consider the impact on PET-MR image quality.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Combined positron emission tomography-magnetic resonance (PET-MR) scanners have great potential for improving radiotherapy with molecular PET information obtained simultaneously with functional and anatomical MR information (Thorwarth et al 2013). In particular PET-MR images may facilitate radiotherapy dose painting through identifying active tumour sub-volumes to receive radiotherapy 'boost' doses (Zamboglou et al 2018). It is important that the delineation of active tumour sub-volumes is robust and repeatable which requires accurate, quantitative imaging (Alber and Thorwarth 2014).
Radiotherapy planning images need to be acquired in the radiotherapy position for accurate treatment and registration with other planning images (Paulson et al 2015). Acquiring PET-MR images for pelvic radiotherapy planning therefore requires patients to be scanned on a radiotherapy flat couch-top which mimics the treatment machine couch, with patients in appropriate radiotherapy immobilisation devices and with the MR receive coils supported away from the patient so that the patient external contour is not deformed (Paulson et al 2015). The carbon fibre couches typically used for PET-CT imaging have low PET attenuation but produce significant MR artefacts, whereas glass fibre MR couches do not interfere with the MR signal but significantly attenuate the 511 keV photons detected in PET (Paulus et al 2014). This means dedicated PET-MR radiotherapy hardware needs to be developed that is MR-compatible and has low PET attenuation (Paulus et al 2016).
Acquiring PET-MR images in the radiotherapy position will have an impact on MR image quality (Schmidt and Payne 2015) since the receive coils will be further from the patient anatomy, reducing the coil filling factor and therefore the SNR (McJury et al 2011, Gruber et al 2018). The radiotherapy planning position will also impact on PET image quality since the flat couch-top and immobilisation devices will add additional and non-uniform PET attenuation, degrading the image quality (Paulus et al 2014). Therefore it is important to assess the impact on PET-MR image quality of dedicated PET-MR radiotherapy hardware so that: (i) MR protocols can be modified to compensate for the MR signal loss (Schmidt and Payne 2015), and (ii) software methods of correcting for the PET attenuation can be developed for accurate quantitative PET imaging (Paulus et al 2016). Previous studies have investigated the impact of PET-MR imaging in the pelvic radiotherapy position using uniform MR and PET phantoms (Paulus et al 2016, Brynolfsson et al 2018, Witoszynskyj et al 2019). However, to the best of the authors' knowledge the broader impact on PET-MR image quality relative to diagnostic image quality using standard image quality phantoms has not been assessed. Further the PET attenuation from the anterior MR receive coil for pelvis imaging has not been considered for the GE Signa PET-MR. The aim of this study was to evaluate the impact of using a flat couch top and coil bridge on PET-MR image quality and PET quantification for radiotherapy pelvis imaging.
2. Methods
2.1. Imaging
All images were acquired on a SIGNA PET/MR software version MP26 3T scanner (GE Healthcare, Waukesha, USA). Three different experimental setups were used for both the MR and PET image quality assessments: diagnostic, couch and radiotherapy (figure 1). The diagnostic setup consisted of the image quality phantom (PET or MR) placed on the soft foam overlay on the PET-MR couch with the anterior array coil placed directly on phantom. The couch setup comprised the phantom placed on the radiotherapy flat couch-top with the anterior array coil directly on phantom. The radiotherapy setup had the phantom placed on the radiotherapy flat couch-top with the anterior array coil placed on a pelvis coil bridge. The radiotherapy flat couch-top and pelvis coil bridge were produced by Knightec (Stockholm, Sweden) and were similar to those evaluated by Brynolfsson et al (2018).
Figure 1. Photographs of the diagnostic (a), couch (b) and radiotherapy (c) experimental setups with the ACR phantom and holder used for the MR image quality assessment. The same experimental setups with the NEMA phantom setup on foam blocks without the phantom holder were used for the PET image quality assessment.
Download figure:
Standard image High-resolution imageThe MR image quality assessment was carried out using the American College of Radiologists (ACR) large image quality phantom (Price et al 2015). The phantom was imaged in three different imaging sessions on separate days. Each imaging session included all three setups. The phantom was placed in an in-house manufactured holder (figure 1). The holder had three screws which enabled easy levelling of the phantom in two axes using a spirit level. For each setup the phantom was imaged using the recommended ACR sequences consisting of a sagittal localiser, an axial T1-weighted spin echo (ACR T1) and an axial double-echo T2-weighted spin echo (ACR T2) (table 1) (Price et al 2015). The second echo images in the ACR T2 series were used for all image analyses.
Table 1. The MR parameters used for the MR image quality assessment.
| Parameter | Sequence | ||
|---|---|---|---|
| Localiser | ACR T1 | ACR T2 | |
| Field of view (mm2) | 250 × 250 | 250 × 250 | 250 × 250 |
| Matrix | 256 × 256 | 256 × 256 | 256 × 256 |
| Slice and slice gap thickness (mm) | 20.0 | 5.0 | 5.0 |
| Slices/slice gaps | 1/0 | 11/10 | 11/10 |
| Echo time (ms) | 20 | 20 | 80 |
| Repetition time (ms) | 200 | 500 | 2000 |
| Bandwidth (Hz pixel−1) | 651 | 651 | 651 |
The PET image quality assessment was carried out using an International Electrotechnical Commission (IEC) 61675-1 emission phantom (PTW, Freiburg, Germany). The phantom was set up with the six spheres and the background filled with a mixture of 18 F-FDG and water, with the activity concentration within the spheres being approximately four times more than in the background, as specified by the National Electrical Manufacturers Association (NEMA) NU 2-2007 standard (National Electrical Manufacturers Association 2007). Unlike the NEMA specification all six spheres were hot compared to the background as this is more representative of a radiotherapy planning context. The lung insert was used for all measurements. For the couch and radiotherapy setups the phantom was placed on two small foam blocks (height 20 mm) to approximately centre the phantom in the scanner bore. For the diagnostic setup foam blocks with twice the height were used, to compensate for the lack of the flat couch-top. The holder shown in figure 1 was not used for the PET acquisitions. The phantom was filled on two separate imaging sessions on separate days, with the positions of the spheres within the phantom kept the same for both sessions. This was so that the same phantom attenuation correction map could be used, however it did mean that the same size sphere was closest to the anterior coil in both scans. Each imaging session consisted of six sequential acquisitions. There were two acquisitions in each of the three experimental setups, one with the anterior array coil and one without. The position of the phantom relative to the anterior array was kept the same for each setup and between the two sessions. All acquisitions consisted of one bed position with the phantom centred in the PET field of view. The first acquisition in each session used a five minute bed position with an activity concentration of 5.5 kBq ml−1 and subsequent acquisitions used longer bed positions to allow for radioactive decay, giving approximately the same number of counts in each.
For each PET acquisition and attenuation map two reconstruction algorithms were used: an ordered subset expectation maximum (OSEM) reconstruction with 16 subsets and 4 iterations and a 5.0 mm Gaussian filter, and a Bayesian penalised-likelihood iterative image reconstruction (Q.Clear) with a relative noise regularising term factor of β = 350 (Ross 2014). Both reconstructions used point spread function correction and time of flight information.
2.2. Attenuation correction
All PET reconstructions incorporated a standard attenuation map consisting of a model of the phantom and the coil components contained within the scanner bed for attenuation correction of the PET data (ACstd). Additional images were reconstructed for the couch and radiotherapy setups with a modified attenuation correction map which included a kilovoltage computed tomography (kVCT) scan of the radiotherapy couch (ACc). For the radiotherapy setup further images were also reconstructed with another modified attenuation correction map that included kVCT scans of both the radiotherapy couch and the radiotherapy coil bridge (ACcb). The kVCT scan of the coil bridge were positioned relative to the centre of the phantom using measurements of the distance from the end of the phantom to the end of the coil bridge for both superior and inferior ends. The kVCT scans of both the couch and the coil bridge were acquired using a Somatom Open scanner (Siemens, Erlangen, Germany) with a tube voltage of 140 kVp, a voxel size of 1.2 × 1.2 × 1.5 mm3 and an axial field of view of 600 × 600 mm2. The CT scan was converted into a PET attenuation map by using the PET-MR vendor supplied mapping from 140 kVp Hounsfield Units (HU) to 511 keV linear attenuation coefficients.
Finally a megavoltage (MV) CT of the coil bridge with the anterior array coil on it was acquired using a TomoHD TomoTherapy helical linear accelerator (Accuray, Sunnyvale, California, USA). MVCT images were obtained due to the high atomic number elements in the array coil creating substantial streak artefacts on kVCT imaging (Patrick et al 2017). Images were acquired with the detuned imaging beam energy (∼1 MV) and 2 mm slice thickness. Three MVCT images were acquired: with the bridge centred, laterally displaced to the right, and to the left. The left and right images were registered to each other via registration to the central image, cropped to the midpoint of the bridge and merged to produce one MVCT image containing the whole coil and bridge. A relative electron density phantom was also imaged on both the MVCT and kVCT scanners to derive relative electron density as a function of MVCT HU and kVCT HU respectively. Combining these with the vendor-supplied PET linear attenuation coefficient as a function of kVCT HU enabled a PET linear attenuation coefficient as a function of MVCT HU to be calculated. This was applied to the MVCT image of the anterior coil on the coil bridge and combined with ACc to produce a couch, bridge and anterior coil corrected attenuation map (ACcba) The four different attenuation maps can be seen in figure 2.
Figure 2. Example slices of the attenuation maps used. (a) The standard attenuation map contained a model of the phantom and the scanner bed. (b) The couch corrected map included a model of the radiotherapy couch. (c) The couch and bridge corrected map included an additional map of the bridge and (d) the couch, bridge and coil corrected map added in a map of the anterior coil.
Download figure:
Standard image High-resolution image2.3. MR image quality assessment
MR images were analysed according to the ACR recommendations by evaluating geometric accuracy, high-contrast spatial resolution, slice thickness accuracy, slice position accuracy, image intensity uniformity, percent-signal ghosting and low-contrast object detectability. In addition SNR was also assessed in the T1- and T2-weighted images.
The first six ACR tests were analysed using in-house developed Matlab software (version R2017a Mathworks, Natick, USA). This was based upon open source software (Sun et al 2015), with modifications to reduce the influence of the partial volume effect when calculating the signal from the phantom, improve the accuracy of profiles and edge detection through up-sampling and make the analysis more robust to image artefacts such as air bubbles.
The software performed the six ACR tests evaluating geometric accuracy, high-contrast spatial resolution, slice thickness accuracy, slice position accuracy, image intensity uniformity and percent-signal ghosting. The geometric test compared measured and known lengths in the phantom. The resolution test used the smallest diameter holes that could be distinguished in a horizontal or vertical array. The slice thickness test used the measured profile of two angled ramps. The slice position test used crossed 45° wedges at the inferior and superior edge of the phantom. The uniformity test used the near-maximum and near-minimum pixel values in the uniform section of the phantom. The ghosting took the ratio of mean pixel values of four ROIs against the edges of the field of view (outside the phantom) to a ∼200 cm2 ROI in the uniform section of the phantom. The seventh ACR test, low-contrast object detectability, was performed manually using RayStation (version 7, RaySearch Laboratories, Stockholm, Sweden). The low-contrast detectability score was the total number of visible 'spokes' of disks of decreasing diameter (7.0–1.5 mm) and contrast (5.1%–1.4%).
In addition the MR signal-to-noise ratio (SNR) was calculated using the methodology of McCann et al with one 20 × 20 mm2 region of interest (ROI) centred on the phantom centre and four more 20 × 20 mm2 ROIs centred at (±40, ±40) mm from the phantom centre (McCann et al 2013). This method robustly calculated SNR from a single image by: (i) smoothing the image by convolution with a square boxcar filter, (ii) subtracting the smoothed image from the original image to create a noise image, and (iii) calculating the SNR for each ROI using
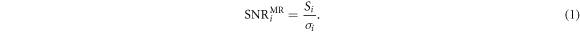
Here Si was the mean pixel value within ROI i in the original image and σi was the standard deviation of pixel values within ROI i in the noise image. The SNR for the whole image was calculated as the mean over all ROIs. The SNR analysis was carried out using MICE Toolkit (Nyholm et al 2015). All MR image quality measurements were presented as the mean over three repeats ± the standard error of the mean.
2.4. PET image quality assessment
All PET images were analysed using four metrics: background activity deviation, PET SNR, contrast recovery and background variability. Spherical ROI matching the known sphere volume were drawn on each sphere using RayStation. Twelve cylindrical ROIs with a diameter of 15 mm and a length of 5 mm were placed in the background in the central slice passing through the spheres. These background ROIs were repeated contiguously down the longitudinal length of the phantom. Within a phantom setup these ROIs were drawn on one image and copied onto all the others. The background activity deviation was calculated as the difference between known activity concentration and the reconstructed activity concentration averaged over the background ROIs as a function of longitudinal distance in the phantom. The relative difference in background deviation for all setups and reconstructions to the background deviation for the diagnostic setup without anterior coil was calculated since this was the gold standard for PET image quality. The PET SNR was determined using (Ziegler et al 2015)
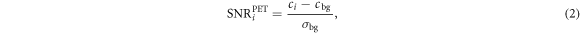
where ci , was the mean reconstructed activity concentration in sphere ROI i, cbg was the mean reconstructed activity concentration in the twelve background ROIs in the central slice only, and σbg was the standard deviation of the reconstructed activity concentration in the same background ROIs. The injected activity concentration ratio between the spheres and background was compared to the measured ratio to derive the contrast recovery, defined as Daube-Witherspoon et al (2002)
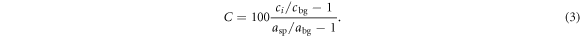
Here asp was the injected activity concentration in the spheres, abg the injected activity concentration in the background and ci and cbg as defined in equation (2). The background variability was calculated using (Daube-Witherspoon et al 2002)

where σbg and cbg were as defined in equation (2). All PET image quality measurements were reported as the mean over two repeats ± the standard error of the mean.
3. Results
3.1. MR image quality assessment
The results for all the ACR image quality metrics agreed within one standard error between the different setups (table 2) except for the low contrast detectability test, where the couch setup was lower than the diagnostic, and the radiotherapy substantially lower (figure 3). The MR SNR for the couch and radiotherapy setups was lower than for diagnostic setup, being 89% ± 2% and 54% ± 1% of the diagnostic setup respectively for the ACR T1 images (figure 3). The ACR T2 image results were similar, with the couch SNR being 91% ± 2% of the diagnostic setup and the radiotherapy SNR 56% ± 1%.
Figure 3. MR image quality assessment. (a) Low contrast detactability, measured in the number of spokes visible in the images and (b) MR SNR. The mean over three acquisitions performed on separate days is represented for each experimental setup for the T1 and T2 MR images. Error bars are one standard error of the mean.
Download figure:
Standard image High-resolution imageTable 2. MR image quality assessment: standard ACR tests. All results given as mean ± one standard error of the mean. For the geometric accuracy, spatial resolution and slice position tests with multiple measurements per image, the mean of those measurements is shown. The units for each test are displayed with the test name.
| Test | Sequence | Setup | ||
|---|---|---|---|---|
| Diagnostic | Couch | Radiotherapy | ||
| Geometric accuracy (mm) | Localiser | 146.8 ± 0.1 | 146.73 ± 0.03 | 146.8 ± 0.1 |
| T1 | 188.9 ± 0.2 | 189.8 ± 0.2 | 189.7 ± 0.2 | |
| T2 | 189.6 ± 0.2 | 190.0 ± 0.2 | 189.8 ± 0.2 | |
| Spatial resolution (mm) | T1 | 1.0 | 1.0 | 1.0 |
| T2 | 1.0 | 1.0 | 1.0 | |
| Slice thickness (mm) | T1 | 5.4 ± 0.2 | 5.1 ± 0.1 | 5.1 ± 0.3 |
| T2 | 5.0 ± 0.3 | 4.7 ± 0.1 | 5.0 ± 0.3 | |
| Slice position (mm) | T1 | 2.3 ± 0.5 | 3.4 ± 0.3 | 3.3 ± 0.6 |
| T2 | 2.3 ± 0.4 | 3.4 ± 0.3 | 3.2 ± 0.5 | |
| Image uniformity (%) | T1 | 66.5 ± 0.5 | 60.3 ± 0.9 | 67.0 ± 0.6 |
| T2 | 62 ± 2 | 54 ± 1 | 66.1 ± 0.3 | |
| Ghosting (%) | T1 | 9 ± 5 × 10−5 | 12 ± 8 × 10−5 | 7 ± 3 × 10−5 |
| T2 | 30 ± 15 × 10−5 | 28 ± 8 × 10−5 | 72 ± 20 × 10−5 | |
3.2. PET image quality assessment
The background activity deviation was approximately uniform (within 1%) along the length of the phantom for each setup acquired without the anterior array coil in place (figure 4). Using the ACstd map, the mean background deviation of the couch setup relative to the diagnostic setup without coil was −9.0% ± 0.1% and the radiotherapy −13.0% ± 0.1%. Using ACc instead reduced this to −1.0% ± 0.1% and −5.0% ± 0.1% respectively. For the radiotherapy setup, using ACcb led to a −2.0% ± 0.1% difference. The images acquired with the anterior array coil in place showed a non-uniform background activity deviation along the phantom length, with differences from the images acquired without the coil in place between −6% and −12% (figure 4). The mean difference to diagnostic setup without coil for the diagnostic setup with coil was −8.3% ± 0.2%. For the couch and radiotherapy setups using ACstd the difference was −16.7% ± 0.2% and −17.7% ± 0.1% respectively. Correcting for the radiotherapy hardware improved the performance, with ACc included reducing the activity difference to −9.7% ± 0.2% (couch setup) and −10.8% ± 0.1% (radiotherapy setup). Using ACcb in the radiotherapy setup gave similar performance to the diagnostic setup (activity difference −7.5% ± 0.1%). With ACcba, the radiotherapy setup outperformed the diagnostic setup and was only −2.7% ± 0.1% different to the diagnostic setup without anterior coil. In all setups there was no difference between the OSEM and Q.Clear reconstructions.
Figure 4. PET image quality assessment: percentage difference in background activity deviation for a given setup and attenuation map to the background activity deviation of the diagnostic setup without anterior coil with standard attenuation as a function of longitudinal distance from the largest sphere (negative indicates superior and positive inferior directions). Plots show data acquired without (a) and with (b) the anterior coil in place. Green, blue and purple lines indicate the diagnostic (diag), couch and radiotherapy (RT) setups respectively. Solid lines/circular markers show images reconstructed with the standard attenuation map (ACstd), dashed lines/downward triangular markers images incorporating the attenuation of the couch (ACc), dotted lines/upward triangular markers the couch and coil bridge (ACcb) and dash-dotted lines/diamond markers the couch, bridge and anterior coil (ACcba). Data shown used the Q.Clear reconstruction.
Download figure:
Standard image High-resolution imageThe PET SNR as a function of sphere diameter is shown in figure 5. When the anterior array coil was not in place the diagnostic setup showed the best performance, with the ACstd corrected couch and radiotherapy setups being worse (>one standard error), but similar to each other. Correcting for the couch and coil bridge improved the performances of both couch and radiotherapy setups to a similar quality to the diagnostic setup (within one standard error). Including the anterior array coil in the acquisition caused a general decrease in SNR of ∼17% across the setups with the exception of couch, coil bridge and coil corrected radiotherapy setup. The differences between the setups and corrections, with the above exception, agreed within one standard error. The couch, coil bridge and coil correction of the radiotherapy setup approached the performance of the diagnostic setup without anterior coil. The Q.Clear reconstructions performed better than the OSEM for all different setups with and without the anterior coil by approximately 5% (OSEM figure available in the supplementary material (available online at stacks.iop.org/PMB/66/035018/mmedia)).
Figure 5. PET image quality assessment: PET SNR for the different sphere diameters without (a) and with (b) the anterior coil for the Q.Clear reconstructions. Green, blue and red lines indicate the diagnostic, couch and radiotherapy setups respectively. Solid lines/circular markers show images reconstructed with the standard attenuation map (ACstd), dashed lines/downward triangular markers images incorporating the attenuation of the couch (ACc), dotted lines/upward triangular markers the couch and coil bridge (ACcb) and dash-dotted lines/diamond markers the couch, bridge and anterior coil (ACcba). Error bars indicate one standard error of the mean.
Download figure:
Standard image High-resolution imageThe contrast recovery increased as a function of sphere size due to the partial volume effect of the relatively poor PET resolution. Figure 6 shows the results for the Q.Clear reconstruction. Without the anterior array coil there was not a large difference between the setups except for the smallest sphere diameter, where the diagnostic setup performed best. The couch and coil bridge corrections did not appear to significantly change the performance of the couch and radiotherapy setups. With the anterior array coil the diagnostic setup performed better for most sphere diameters, with small differences between the other setups and corrections. Similarly to the SNR results, the Q.Clear reconstruction outperformed the OSEM reconstruction by ∼5% for all setups and corrections (OSEM results available in the supplementary material).
Figure 6. PET image quality assessment: Contrast recovery curves without (a) and with (b) the anterior coil for the Q.Clear reconstructions. Green, blue and red lines indicate the diagnostic, couch and radiotherapy setups respectively. Solid lines/circular markers show images reconstructed with the standard attenuation map (ACstd), dashed lines/downward triangular markers images incorporating the attenuation of the couch (ACc), dotted lines/upward triangular markers the couch and coil bridge (ACcb) and dash-dotted lines/diamond markers the couch, bridge and anterior coil (ACcba). Error bars indicate one standard error of the mean.
Download figure:
Standard image High-resolution imageThe background variability for all setups with the Q.Clear reconstructions is given in table 3. The OSEM reconstructions (see supplementary material) had a higher (≥1.0%) background variability for all setups and attenuation corrections. The presence of the anterior coil increased the background variability for all setups and corrections. Without the coil the background variability was lowest in the diagnostic setup and decreased in the other setups once the appropriate attenuation corrections were applied (ACc for the couch and ACcb). With the coil the effects were more variable, with the couch setup using ACstd and the radiotherapy setup using ACstd and using ACcba) giving the lowest values, although the results were within 0.8% of each other so the differences were relatively small.
Table 3. PET image quality assessment: The mean background variability for each PET image with Q.Clear reconstructions. Values reported as mean ± standard error on the mean. Coil present indicates images acquired with (yes) and without (no) the anterior coil. The standard attenuation map included the phantom and scanner table (ACstd), the couch attenuation map added the radiotherapy couch (ACc), the couch and bridge map added the coil bridge (ACcb) and the couch, bridge and coil map added the anterior array coil (ACcba).
| SetUp | Coil present | Attenuation | Background variability |
|---|---|---|---|
| Diagnostic | No | Standard | 5.8% ± 0.4% |
| Couch | No | Standard | 6.2% ± 0.1% |
| Couch | No | Couch | 5.8% ± 0.1% |
| Radiotherapy | No | Standard | 6.0% ± 0.1% |
| Radiotherapy | No | Couch | 5.9% ± 0.2% |
| Radiotherapy | No | Couch and bridge | 5.6% ± 0.2% |
| Diagnostic | Yes | Standard | 7.2% ± 0.4% |
| Couch | Yes | Standard | 6.8% ± 0.2% |
| Couch | Yes | Couch | 7.2% ± 0.3% |
| Radiotherapy | Yes | Standard | 6.6% ± 0.1% |
| Radiotherapy | Yes | Couch | 7.3% ± 0.1% |
| Radiotherapy | Yes | Couch and bridge | 6.8% ± 0.1% |
| Radiotherapy | Yes | Couch, bridge and coil | 6.0% ± 0.1% |
4. Discussion
PET-MR imaging has great potential for radiotherapy treatment planning and radiotherapy images need to be acquired in the planning position. This study has investigated the impact on both PET and MR image quality from acquiring PET-MR images in the radiotherapy planning position for treatment of pelvic cancers.
To the best of the authors' knowledge, this is the first study to investigate the impact on MR image quality of the pelvic radiotherapy hardware using an MR image quality phantom in a PET-MR scanner. The impact was substantial with the couch SNR being 91% ± 2% of the diagnostic setup and the radiotherapy SNR 56% ± 1%. This was likely due to the receive coils being at a greater distance from the phantom and so reducing the SNR (Gruber et al 2018). This consequently gave a substantial reduction in low-contrast detectability (figure 3) but not on any other of the evaluated image quality metrics. This suggests that MR parameters in radiotherapy PET-MR protocols need to be modified, for instance by increasing signal averages, to take into account the reduction in SNR in order that MR images retain sufficient quality for accurate organ delineation. Alternatively, noise reduction reconstruction techniques, such as the recently proposed deep learning reconstruction could be used to improve image quality (Label 2020). These techniques would not require compromises in acquisition time, voxel size or field of view as modifying the scan parameters would, but would require validation for the specific clinical task. Further work could assess the impact of this image quality reduction in patient images using radiotherapy sequences, including the impact of noise reduction reconstruction techniques.
Brynolfsson et al evaluated the same couch and a similar coil bridge using a large uniform MR phantom (Brynolfsson et al 2018). They reported SNRs of 74% when using the couch only and 67% when using the couch and coil bridge compared to the diagnostic setup. These results are slightly different to those reported here, which may be due to the use of a larger uniform phantom. This would increase the contribution of the spinal coil in the scanner bed relative to the anterior coil to the MR signal, thus increasing the SNR reduction due to the couch setup. It would also reduce the distance between the phantom and the anterior coil when the coil bridge was used, therefore reducing the SNR reduction from the coil bridge. Paulus et al developed a radiotherapy flat couch-top for PET-MR imaging consisting of a foam core surrounded by a plastic outer layer (Paulus et al 2014) and an adjustable pelvic coil bridge (Paulus et al 2016). Quantitative assessment of MR image quality using this setup was not carried out, but subjective image quality of three abdominal patients was reported to be similar to the diagnostic setup. This suggests the SNR reduction did not substantially reduce subjective image quality, which may be due to the adjustable coil bridge enabling the coil-patient distance to be minimised, although this is also likely to be dependant on the MR sequence and protocol used. Witoszynskyj et al developed a plastic and fibre glass radiotherapy couch for PET-MR imaging and found minimal differences in MR SNR with and without the couch using a uniform MR phantom (Witoszynskyj et al 2019). However, their images were acquired with the integrated body coil, and so the radiotherapy hardware did not change the distance of the receive coil from the phantom. The integrated body coil is not used clinically and so these results are not relevant to clinical practice.
This is also the first study to investigate the impact on standard PET image quality metrics using the NEMA phantom from PET-MR images acquired in the pelvic radiotherapy setup. The radiotherapy hardware reduced PET SNR for all spheres, but incorporating the couch and bridge attenuation correction recovered the PET SNR performance to within one standard error (figure 5). Including the coil attenuation correction resulted in the radiotherapy setup considerably outperforming the diagnostic setup with anterior coil and approached the performance of the diagnostic setup without anterior coil. However, the corrections appeared to make minimal difference to the contrast recovery curves (figure 6), with the diagnostic setup performing better but within 2% of the couch and radiotherapy setups. This suggests that qualitative image quality, of which contrast recovery is a surrogate measure, was not substantially changed by the presence of the radiotherapy hardware. Similarly, the presence of the anterior coil appears to make minimal difference to all setups, despite the significant attenuation it introduces, confirming that qualitative diagnostic imaging does not require correction for the coil (Wollenweber et al 2014). Several authors have evaluated PET image quality on PET-MR scanners acquired in the diagnostic setup (with no anterior coil). Grant et al reported contrast recovery values of [35.2, 48.9, 59.9, 68.6]% for the four smallest sphere sizes acquired on the same scanner model with similar acquisition settings, which shows reasonable agreement (within 6%) of the OSEM diagnostic setup values reported here, except for the smallest sphere size which was 11% lower (Grant et al 2016). Similarly measurements on a Siemens Biograph mMR PET-MR scanner using a different image quality phantom showed good agreement for the three smallest spheres (Øen et al 2019). For some of the setups with the anterior coil in place the smallest sphere has a larger PET SNR and contrast recovery than the second smallest sphere. This is a counter-intuitive result since the increasing sphere volume should reduce the impact of partial volume effects. Potentially this may be due to the impact of the coil since the second smallest sphere was the most anterior sphere and so would have the most lines of response passing through the coil. This would explain why the setups without the coil in place do not show the same effect. This is a potential confounding factor for this study. The impact of this could be investigated doing repeat images with the different sphere positions within the phantom, although this would require using different phantom attenuation correction maps.
The radiotherapy setup also significantly reduced PET background activity, with losses of −12.7% ± 0.5% (without the anterior coil) and −10.2% ± 0.5% (with anterior coil) compared to the diagnostic setup without and with anterior coil respectively. The smaller activity loss with the anterior coil in place was possibly due to the coil bridge raising the anterior coil away from the phantom, and so reducing the number of lines of response passing through the coil and therefore being attenuated. These results are similar to those reported by Brynolfsson et al using the same couch and coil bridge (Brynolfsson et al 2018). They also found correcting for the attenuation of the radiotherapy hardware reduced these differences to −1.5% and −0.7% for the couch and coil bridge respectively, very similar to the −1.5% and 0.8% reported in this study. These results were similar to other approaches in the literature. Paulus et al reported −3.8% and −8.5% activity differences from their couch and pelvic coil bridge respectively, which reduced to −0.6% and −1.2% when attenuation correction was used (Paulus et al 2016). Witoszynskyj et al found their couch reduced PET activity by −8.7% ± 2.1%, reducing to 1.2% ± 3.9% when CT-based attenuation map of the couch was included in the reconstruction (Witoszynskyj et al 2019).
The PET reconstruction algorithm used may also have an impact on PET image quality. This study evaluated both the clinical standard OSEM reconstruction and a more novel Bayesian penalised-likelihood iterative image reconstruction (Q.Clear). This was to evaluate the potentially higher performing reconstruction in the radiotherapy setup but also enable comparisons to the literature using the clinical standard OSEM reconstruction. The Q.Clear reconstuction outperformed the OSEM reconstruction on the PET SNR, contrast recovery and background variability metrics (OSEM data reported in supplementary material).
This study acquired repeat measurements over multiple days. This was primarily to mitigate the impact of phantom preparation and positioning. However, the small standard errors do suggest that the PET-MR scanner performance was repeatable in terms of the image quality metrics evaluated. However, the repeatability of images would need to be assessed in patients though, since it is likely to be dominated by physiological and anatomic differences between imaging sessions (differences in bladder and bowel filling, patient setup and posture and so on) rather than variability in the scanner performance.
This study has evaluated the impact on PET-MR image quality of using the radiotherapy setup for pelvic cancer patients. Radiotherapy treatment positions for other anatomical sites (e.g. breast cancer, head and neck cancer) require different patient positions, coil supports and patient immobilisation devices. Further work would be required to investigate the impact on PET-MR image quality for those other anatomical sites.
Previous investigations have not included the anterior array coil in attenuation correction since this is what is routinely done in diagnostic imaging. Primarily this is due to the variable position and flexible shape of the anterior array coil being difficult to model accurately with attenuation maps since it will vary significantly in position and shape from patient to patient and cannot be directly imaged. Therefore ignoring the anterior coil avoids errors from incorrectly positioned attenuation maps and facilitates a simple workflow. However this study has shown that the anterior coil has a substantial and variable attenuation of between 6% and 12%. This is consistent with the mean reduction in activity of −7.3% reported for the same coil by Wollenweber et al (2014). They concluded that for qualitative diagnostic imaging this was not significant. But for radiotherapy dose painting this is problematic, since it requires accurate quantitative imaging (Alber and Thorwarth 2014). On the other hand, an advantage of the radiotherapy setup compared to a diagnostic one is that the position and shape of the flexible anterior coil is the same for each patient since the coil bridge fixes the coil shape and height relative to the radiotherapy couch. This potentially means the anterior coil can be included in attenuation correction maps as long as the position of the centre of the coil is known relative to the centre of the image. This can easily be achieved by setting the scanner reference point to the centre of the coil and using the known height of the coil brdige relative to the posterior edge of the patient (the couch top). An issue with generating attenuation correction maps of MR coils containing high atomic number elements is that large streak and starvation artefacts are produced in kVCT images. This study has confirmed, as reported by Patrick et al (2017), generating attenuation correction maps for MR coils can be done simply and robustly through MVCT imaging. In this study, the radiotherapy setup, once corrected, outperformed the standard diagnostic setup by 6.2% ± 0.5% in activity measurements and its performance was within ∼ 2% of the diagnostic setup without an anterior coil (i.e. an ideal, non-clinical, PET setup). This suggests that quantitative PET-MR is possible within the radiotherapy setup as long as the anterior coil is included within the attenuation map.
5. Conclusion
Acquiring PET-MR images in the radiotherapy planning position reduced MR image quality substantially, with a loss of MR SNR of 45%. The radiotherapy position also impacted PET image quality with reductions in measured activity to the diagnostic setup without anterior coil of −17.7% ± 0.1%, which reduced to −7.5% ± 0.1% when attenuation correction map of the radiotherapy hardware was included. Contrast recovery curves were largely unchanged, suggesting qualitative PET image quality was not substantially affected. Noticeably the presence of the flexible anterior coil also had a significant and non-uniform effect on the PET attenuation. Including this coil in the attenuation correction map, which the radiotherapy setup enables, outperformed the standard diagnostic setup with anterior coil by 5.6%. The same impact was seen in PET SNR curves, where the radiotherapy setup with anterior coil corrected outperformed the diagnostic setup with anterior coil. This implies that accurate quantitative PET imaging is possible in the radiotherapy setup as long as appropriate attenuation correction is applied. The impact on PET-MR image quality will need to be considered when designing radiotherapy PET-MR imaging protocols.
Acknowledgments
We gratefully acknowledge funding for this work received from GE Healthcare. We also wish to thank the Northern Medical Physics and Clinical Engineering Mechanical Workshop at the Newcastle upon Tyne Hospitals NHS Foundation Trust for designing and building the ACR phantom holder and Mr Stephen Hedley, Northern Centre for Cancer Care, Newcastle Hospitals, for developing the in-house software for evaluating the ACR images.







