Abstract
Although Joule's experiment on the mechanical equivalent of heat is well known and discussed in many introductory physics courses and textbooks, we are not aware of any version of this experiment that can be set up by lecturers or teachers using standard laboratory equipment. To fill this gap, we present a setup quite similar to Joule's original experiment and show what kind of quantitative results can be obtained with it. It will be shown that temperature control is a major challenge, making Joule's work even more impressive.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Joule's experiment on the mechanical equivalent of heat is a famous historical milestone in physics, showing that mechanical energy can be converted into thermal energy by friction. In his 1850 publication in the Philosophical Transactions, Joule presents an experiment in which masses lose potential energy, causing an axle to rotate, which forces a set of paddles through the water in a vessel [1]. The friction between the paddles and the water increases the internal energy of the water, which can be detected by an rise in its temperature. Although this experiment is regularly discussed in lectures and textbooks on mechanics and thermodynamics, to our knowledge there is no description of how Joule's original experiment can be carried out in lecture halls or classrooms using standard laboratory equipment.
1.1. Joule's historic experiment
In the mid-19th century, James Prescott Joule worked on the connection between mechanical work and the temperature of a body [2, 3]. Carnot had already shown that heat is not a substance contained in a body that can be exhausted, but is linked to the mechanical work done on the body. Thus it was already clear that an unlimited amount of heat could be extracted when drilling cannon barrels and that it would never be exhausted. It was Joule's endeavour to find a mathematical description of the relationship between temperature and work, or potential energy, which is reflected today in specific heat capacity.
The principle of Joule's experiment is simple. Water is stirred in a apparatus and heated by friction. The apparatus is driven by strings on which masses move downwards (figure 1).
Figure 1. Two masses—the horizontal cylinders on the strings on the left and right—move downwards on strings and set water in motion in a stirring device—the container in the centre [1]. (Reproduction of the figure with permission from the Royal Society).
Download figure:
Standard image High-resolution imageThe energy balance (for one ideal circle) is as follows: in the initial state, the total energy consists of the potential energy of the masses (Epot) and the internal energy of the water. In the final state, the energy balance contains only the internal energy of the water. The work (W) has been converted into heat (Q), thus raising the temperature (T) of the water (with mass m and specific heat capacity c):
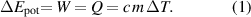
It was therefore Joule's aim to establish a relation between the elevated position of a mass, or its downward movement, and the temperature of a quantity of water by means of a constant specific factor. He determined this factor, the heat capacity of water, with a standard deviation of 1.8%, including the value we know today. It is impressive to see how Joule controlled the conditions of his experiment, particularly the temperature, and estimated unknown quantities with such accuracy. As a brewer, Joule was privileged to have access to a brewery cellar [3]. In these rooms, the air temperature is almost constant (which is crucial for the brewing process). Under these conditions, the rise in temperature can be convincingly attributed to the work done on the water using thermometers with a resolution of 0.01 °F.
It is obvious that not only the water in the vessel heats up, but also the copper of the vessel and the brass of the paddle wheel inside it, and Joule took this into account [3]. Perhaps this is the reason for his decision to use a thin-walled copper vessel rather than an insulated one: it keeps this influence small. There are also friction losses in the cables and pulleys due to the enormous load. Joule estimated this by running the apparatus without water in such a way that he used just enough mass to move the apparatus just as fast as when it was filled with water. This determined the proportion of potential energy that was converted into friction losses [3]. Joule also considered the losses due to the mass hitting the ground. From today's point of view, this is a loss of kinetic energy.
It is obvious that these advanced considerations go beyond the level of a discussion in a classroom or lecture hall. However, it must be made clear to students that only this comprehensive approach will lead to adequate results.
1.2. Joule's experiment in the classroom—status quo
Our review of websites and papers led to two types of classroom adaptations of Joule's experiments.
First, we found an experiment that shows the relationship between work—not potential energy—and heat. A metal cylinder is rotated by hand with a friction band around it 1 . The upper end of the band is attached to a spring balance and the lower end to a mass. By applying a constant force between the band and the cylinder, rotating the cylinder and counting the revolutions, the mechanical work performed can be determined. In addition, the temperature of the metal cylinder is measured, and with the specific heat capacity of the metal and the mass of the cylinder, the change in thermal energy of the cylinder can be determined. We argue that this realisation is very different from Joule's original setup, although it makes the mechanical energy and heat measurable—with losses in the order of 50%. Joule used 'falling' masses and looked at the temperature rise of water, not metal.
Secondly, there is an available replica of the complete setup of Joule's original experiment for teaching purposes 2 . In this, the potential energy of the masses is converted into the thermal energy of the water, thus raising its temperature. However, we do not have any reliable information as to whether it is true that 'the temperature of the water in the copper jug rises after two to three minutes of rotation', as promised by the manufacturer. In fact, as you will see below, we doubt it!
From this we conclude that there is no suitable experimental setup for Joule's famous experiment on the mechanical equivalent of heat that is similar to Joule's original experiment and that can be built by lecturers or teachers themselves using basic laboratory equipment. To fill this gap, we have developed a version of Joule's experiment that uses only standard equipment and reliably demonstrates the main effect.
2. Methods
In the following, we describe our setup, explain how the experiment is performed and present the measurement results.
2.1. The experimental setup
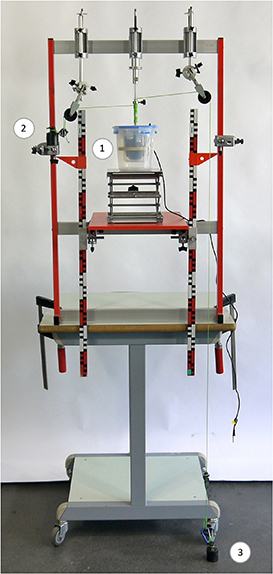
Because there is no commercial set up to buy, improvisation was needed. A toilet brush was easy to acquire and moreover attracted students' attention. Further, the use of a household object makes it transparent that no special requirements have to be fulfilled by the paddle. In our case, we used a brush with an interchangeable head. We mounted it on a ball bearing axle (laboratory stand material) by just plugging the axle in it (figure 2). A suitable household box (made of PP plastic) with a lid is used as a container for the water. Preliminary tests have shown that the temperature of the water does not rise during stirring, but falls when the water is in an open container, because water evaporates quickly at low humidity. For our setup, an amount of water of 200 ml has been proven to be effective. This causes sufficiently strong friction to make the weights fall slowly and in a controlled manner. Due to the laminar flow at the beginning, the apparatus starts smoothly, with the inset of turbulence after a short time, a constant fall speed is obtained.
Figure 2. The setup for Joule's experiment, with the head of a toilet brush (1) and the household box as the water container (2). To start the experiment, the box is filled with water, raised on a lifting platform, and the container closed. Other objects: 500 g mass (3), pulley (4), rope (5), ball bearing axle (6), coiled rope (7).
Download figure:
Standard image High-resolution imageThis is the heart of Joule's apparatus. Unlike Joule's original experiment, a second, larger household box has been placed around the first to provide further insulation, in expectation of changes in air temperature in the classroom over the course of the experiment.
Two ropes are wound onto the axle in opposite directions (figures 2 and 3). When the axle rotates, one rope is wound up while the other is unwound. The ropes run horizontally to one pulley each and run down from there. At each end of the rope there is (at all times) a small mass (50 g) attached to keep the ropes tight. The apparatus is driven by 500 g masses. One of these masses is attached to the end of a coiled rope at the upper position and sets the axle in motion while moving downward. During this period, the second mass is placed on a platform at the upper position of the apparatus in a 'waiting position'. (For an easy handling, the weights are fixed to a snap hook through which the rope runs). With the downward movement of the large mass, the other rope is wound up on the opposite side—we call this one cycle of the experiment. Every 500 g mass is always dropped at the same height (ruler with marking). Finally, it hits the ground. In this way, the momentum of the moving mass is prevented from being transferred to the apparatus. In addition, the height of fall can be easily determined. (For technical data of our setup and a comparison with Joule's experiment, see table 1) It should be noted that, at first glance, our setup looks similar to Joule's. However, in Joule's construction, the masses run down simultaneously to achieve a uniform load on the axle, but the rope has to be rewound by hand in an intermediate step.
Figure 3. The complete experimental setup in operational condition (heat shield and digital temperature acquisition not shown). Head of a toilet brush in two household boxes (1), mass on platform in upper position (2), mass on the ground (3).
Download figure:
Standard image High-resolution imageTable 1. Technical data of our setup and a comparison with Joule's experiment.
| Joule's experiment [1, 3] | Our experiment | |
|---|---|---|
| Masses | 2 × ∼13 kg | 2 × 500 g ± 2 g |
| Hight masses travel | 160 cm | 147 cm ± 1 cm (mass 1) and 151 cm ± 1 cm (mass 2) |
| Water volume | 6 l | 200 ml ± 5 ml |
| Vessel | Copper vessel with lid | Inner plastic box (55 g ± 1 g); c = 1.70 J (gK)−1 (PP plastic); outer plastic box |
| Stirring device | Brass paddle wheel | Toilet brush (51 g ± 1 g); c = 1.70 J (gK)−1 (PP plastic); |
| Location of the experiment | Cellar of a brewery | Classroom with blinds without air conditioning |
| Shielding of the experimenter | Wooden wall | Acrylic glass pane |
| Thermometer | Liquid in glass thermometer, resolution of about 0.01 °F | Digital thermometers with a precision of 0.03 °C and an accuracy of 0.2 °C at 0 °C, 0.5 °C at 100 °C |
| Number of cycles in the experiment | 20 in about 35 min | 200 in about 45 min |
| Temperature change | 0.31 K; standard deviation 1.8% | 0.84 K, standard error of regression 3.5%, R2 = 98.8% |
The small temperature change—expected to be in the range of 1.9 K with about 200 experimental runs in about one hour—requires a measurement in the range of 0.1 K. This resolution is achievable with low heat capacity, fast response digital thermometers from typical laboratory equipment manufacturers (precision: 0.03 °C). However, our instruments are only calibrated to within half a degree (±0.2 °C at 0 °C, ±0.5 °C at 100 °C). This means that temperature changes in one location are very well resolved, but temperatures in different locations, such as water temperature versus room temperature, cannot be reliably compared. We overcome this problem with our own calibration using a water bath in which all the thermometers are placed before and after the experiment, assuming that there is no drift of the thermometers over the time of the experiment.
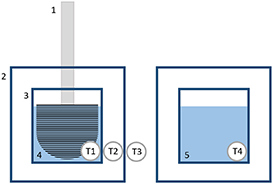
For continuous measurement of the water temperature of the sample, the thermometer is glued into a hole in the bottom of the plastic box. In addition to the temperature T1 of the water (sample), the air temperature T2 between the inner and outer box and the room air temperature T3 in the immediate vicinity of the box are also measured (figure 4). This allows conclusions to be drawn as to whether heat is being introduced into the water from the outside. Finally, a fourth temperature (T4) is measured on another 200 ml of water in the box—which remains undisturbed during the experiment—as a reference measurement (figure 4). The temperature change of this reference water can be directly compared with the temperature change of the stirred water. Joule did not use a reference vessel parallel to the measurement, but checked beforehand whether the water temperature changes without the water being stirred—for example, by people in the room.
Figure 4. Four temperatures (T1, T2, T3 and T4) were measured simultaneously during the experiment (1 brush, 2 outer box, 3 inner box, 4 water sample, 5 reference water).
Download figure:
Standard image High-resolution imageAll the thermometers in the school experiment are read by a digital data acquisition system (LabQuest Mini). The experimenter triggers a temperature measurement by a light barrier after each 'drop' of a 500 g mass. Digital data acquisition and multiple fast temperature sensors allow a large number of measurement points to be recorded at different locations. This makes it possible to detect temperature changes in the air caused by the experimenter's body heat. In a preliminary experiment we were able to show that the direct influence of body heat from a human on the apparatus could be significantly reduced by an acrylic safety shield.
Various preliminary experiments have shown that controlling the temperatures of the objects involved is a demanding challenge. At the beginning of the experiment, the water, the container and the air in the room should be at the same temperature. However, this is not easy to achieve. Because the air temperature in the room varies by up to 10 K (on a summer day), the water sample and the ambient air never really reach thermal equilibrium, but go through a non-synchronous cycle. This was determined by long-term measurements in preparation for the experiment. Consequently, our experiments were carried out during a temperature plateau in the late afternoon. To keep further disturbances to a minimum, the experiment was carried out by one person only. Windows and doors remained closed. The window panes were covered with roller blinds. The light was switched off.
Temperature sensors:
- Vernier surface temperature sensor, temperature sensor: 20 kΩ NTC thermistor, resolution: 0.03 °C (0 °C–40 °C), accuracy: ±0.2 °C at 0 °C, ±0.5 °C at 100 °C, response time (time for 90% change in reading): 50 s (in still air), 20 s (in moving air),
- Vernier go direct surface temperature sensor, resolution: n/a, accuracy: ±0.5 °C, response time (from 25 °C to 100 °C in water): 5 s,
- Vernier stainless steel temperature probe, temperature sensor: 20 kΩ NTC thermistor, resolution: 0.03 °C (0 °C–40 °C), accuracy: ±0.2 °C at 0 °C, ±0.5 °C at 100 °C, response time (time for 90% change in reading): 10 s (in water, with stirring), 400 s (in still air), 90 s (in moving air).
3. Results and discussion
3.1. Results
Figure 5 shows the temperatures for an increased number of cycles of our final measurement. (The graph includes corrections due to the calibrations of the thermometers mentioned above). As can be seen, the temperature of the water sample and the reference water are almost the same at the beginning. As the number of cycles increases, the temperature of the water sample rises from 22.3 °C to 23.4 °C. The temperature of the reference water is not constant but changes from 22.2 °C to 22.4 °C. The higher slope of the sample temperature compared to the reference temperature clearly shows the main effect of the conversion of work to heat.
Figure 5. Measured values for room temperature, water temperature, reference water temperature and air temperature between the inner and outer box for an increased number of cycles.
Download figure:
Standard image High-resolution imageIt can also be seen that the air temperature in the room is slightly higher than the temperature of the two water samples, even though they were prepared the day before. The high room temperature causes the reference water to rise and, we must assume, the stirred water to rise as well. This 'undesirable' effect must therefore be taken into account by subtracting the rise in the reference water from the rise in the stirred water.
In addition, the temperature of the air between the inner and outer capsule rises more than the room temperature, starting from the same point. This shows that heat is moving from the inner box to the outside.
We can therefore conclude that there is an effective increase in water temperature due to the work done by the mechanical system.
3.2. Discussion
Further analysis of the data leads to the following interpretations. A linear fit of the temperature of the water sample shows an increase of 5.46 mK (±0.05 mK) per cycle. This shows the tiny effect of a falling mass on the temperature of the water. Doing the same for the temperature of the reference water gives a slope of 1.30 mK (±0.01 mK) per cycle. It is clear that the number of cycles has no connection with the temperature of the reference. However, if we assume that each cycle takes the same time, it can be regarded as a time fit. Having done so, it is useful to reduce the temperature rise of the water sample by the proportion of the reference. We can therefore conclude that the average increase in water temperature caused by the mechanical work is 4.17 mK (±0.06 mK) per cycle, giving a total of 833 mK (±12 mK) for 200 cycles. The calculated uncertainty (12 mK) is smaller than the uncertainty of the thermometer used (30 mK), so we use the latter in our following calculation (and omit a phytagoretic addition for simplicity).
Thus, the work done on the water is Q = c m ΔT = 4.19 kJ (kg K)−1 ⋅ 0.2 kg ⋅ 0.83 K = 0.70 kJ (±0.05 kJ) (Q the amount of heat, c the specific heat capacity of water, m the mass of the water, ΔT the temperature rise), neglecting the heat capacity of the vessel and paddle in a first approach, as it would be done in the school.
On the other hand, we put the following work into the system W = n m g h = 200 ⋅ 0.5 kg ⋅ 9.81 m s−2 ⋅ 1.49 m = 1.46 kJ (±0.03 kJ) (n the number of cycles, m the masses of the 'falling' weights, g the earth acceleration, h the height of the 'fall').
So, about half of the mechanical energy goes into the thermal energy of the water. This shows that there is a loss of about half of the energy, which is a typical result for school experiments and leads to a discussion of possible causes.
In a next step, the theoretical model can be extended by assuming that the brush and the inner box (without lid) are heated to the same temperature as the water (overestimation): Q = (c1 m1 + c2 (m2 + m3)) ΔT = (4.19 J (g K)−1 ⋅ 200 g + 1.70 J (g K)−1 (51 g + 55 g)) 0.84 K = 0.86 kJ (Q the heat quantity, c1 the heat capacity of the water, c2 the heat capacities of the brush and the inner container, m1 the mass of the water, m2 the mass of the brush, m3 the mass of the container, ΔT the temperature difference).
However, due to the low mass and heat capacity of plastic, this only results in an increase of 0.16 kJ. This means that 41% of the energy must be 'lost' in the mechanics of the apparatus.
Estimating the losses due to friction, as Joule did, is difficult because the winding of the string leads to an uneven flow. However, a trail shows that it takes a mass of 18 g to run the machine without water, which is about 1/3 of the mass used in the experiment. We assume that the 'real' friction is higher in the case of a filled vessel, due to a higher load on the pulley (underestimation). Taking into account that there is a loss of kinetic energy due to the weights hitting the ground at a low speed will only add a few percentage points.
4. Conclusion
The experiment shows the desired effect with standard laboratory equipment: the temperature of the water rises due to mechanical work. The main challenge is to measure the temperatures. It is recommended to perform the experiment in an environment with an almost constant temperature. Finding this is an interesting task in school because heat sources can be found in almost every place in a modern building. Furthermore, because of the small changes in temperature that can be expected, the experiments require measuring equipment that is precise and accurate. Precision is relatively easy to achieve as modern digital thermometers have a resolution in the range of 0.03 °C. However, the accuracy of about 0.2 °C requires calibration before and after the measurement.
The setup uses only standard laboratory equipment (except—perhaps—the toilet brush) and takes some time to prepare, while the measurement itself takes about 45 min. Therefore, we recommend either letting the students do the experiment in a laboratory course, or demonstrating a few cycles of the experiment in a lecture and providing the students with previously measured data.
Looking back on our experience with this experiment, we pay great respect to Joule's results. He has achieved a setup with very little dissipated energy.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding information
The authors received no financial support for the research, authorship, or publication of this article.
Ethical statement
Neither humans nor animals are used as research subjects in this work.
Footnotes
- 1
Phywe (n.d.) Mechanisches Wärmeäquivalent www.phywe.de/versuche-sets/hochschulversuche/mechanisches-waermeaequivalent_10676_11607/, Pasco (n.d.) Mechanical Equivalent of Heat Apparatus Manual https://cdn.pasco.com/product_document/Mechanical-Equivalent-of-Heat-Apparatus-Manual-TD-8551A.pdf.
- 2
Japson (n.d.) Joule's Mechanical Heat Experiment Apparatus www.japson.com/products/physics-lab-products/heat/joule-s-mechanical-heat-experiment-apparatus.html.
Biographies
Franz Boczianowski is a postdoctoral researcher in physics education. His work is focused on the usage of experiments and digital media in primary and secondary school science teaching.
Burkhard Priemer is a full professor in physics education, his research is focused on measurement uncertainties and argumentation.