Abstract
Hierarchical structure and mechanics are crucial in biological systems as they allow for smaller molecules, such as proteins and sugars, to be used in the construction of large scale biological structures exhibiting properties such as structural support functionality. By exploring the fundamental principles of structure and mechanics at the macroscale, this general theme provides a clear insight into how physics can be applied to the complex questions of biology. With a focus on biopolymer networks and hydrogels, we present a series of interactive activities which cover a range of biophysical concepts at an introductory level, such as viscoelasticity, biological networks and ultimately, hierarchical biomechanics. These activities enable us to discuss multidisciplinary science with a general audience and, given the current trends of research science, this conceptualisation of science is vital for the next generation of scientists.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Across the world, the prevailing view of science appears to be that it is split into three disciplines: physics, chemistry and biology, and that these disciplines are completely separate. Beyond the so-called 'hard' sciences and into the social sciences, the perception of separation becomes even more apparent. Popular science educators appear to be attempting to change this conceptualisation by covering a wide range of overlapping scientific topics. Examples include Brian Cox's multiple 'Wonders...' series, Hannah Fry's books, podcasts, and recent 2019 Royal Institution Christmas Lectures, Neil deGrasse Tyson's reboot of 'Cosmos' and Helen Czerski's various works. Nevertheless, it cannot be overlooked that each of these educators are known primarily by their single core field of expertise. For Fry this field is mathematics and for the others, physics. The public perception has been quite accurately captured in the XKCD comic 'Purity', which can be found online [1].
At the highest level of study where pioneering research takes place, the separation between all scientific disciplines often becomes significantly less well-defined, especially in the more applied fields. Indeed, the very definitions of physics, chemistry and biology become exceedingly blurred in the field of applied liquid crystals, for example, where the optical properties of liquid crystals make them an interesting candidate for contact lenses [2, 3]. It is therefore of vital importance that the next generation of scientists develop an appreciation of the interdisciplinary nature of science.
Our field of study is protein-based hydrogels, where the number of traditionally separate disciplines required to progress our understanding is astonishing. The remainder of this paper will present a series of activities which aim to showcase interdisciplinary science by using protein hydrogels as a focus. We will give a brief introduction to the field in section 2 with a general description of the wide range of disciplines involved, introducing 'hierarchical biomechanics' as a core theme. Sections 3 and 4 will then present our designed activities, which aim to interactively guide participants through the interplay of the different fields in a logical manner. Finally, in section 5 we share our experiences of delivering these activities to two different audiences. One of these was to a group of students aiming to study physics at A-level, but likely studying the other sciences as well at GCSE level. The second was at the University of Leeds research open day 'Be Curious', an annual event which aims to bring current research, across all disciplines, to the public.
2. A brief introduction to protein hydrogels
Proteins are a diverse class of nanoscale biological machinery which are able to perform the vast array of specific mechanical and chemical functions required for biological organisms to survive. These functions include signalling, catalysis, transport, molecular synthesis and a whole range of additional tasks whose regulation directly controls the life and death of an organism [4]. To showcase this diversity, we direct the reader to an animation created by Harvard University [5], and to recent work on the network of interactions present in the cell [6].
Protein-based hydrogels on the other hand are a class of human-engineered biomaterials which aim to exploit this intrinsic protein functionality. Protein sub-units are chemically bonded, or 'cross-linked', together to form a three-dimensional connected network with useful biomechanical properties, such tunable viscous and elastic behaviour (see figure 1). In principle, we are able to create materials with an ideal combination of 'solid-like' and 'liquid-like' behaviour for a given application [7]. However, the general structure of these networks is largely unknown and remains an active field of research [8–10]. What is known is that the specific interactions between proteins and the surrounding aqueous environment couples with the network structure to induce an increase in viscosity to the entire system, thus transforming the protein solution into a gel with a protein network supporting it; a protein hydrogel.
Figure 1. A representation of the gelation process of chemically cross-linked protein hydrogels. It is likely that the structures shown do not accurately represent the true network structures of protein hydrogels; this diagram is meant as a visual aid only. (a) A protein monomer, represented as a blue sphere, can be biologically engineered to contain chemical cross-linking sites, represented in red on the surface. (b) These protein monomers can also be engineered as polymers connected by chains of amino acids, shown here as grey springs. (c) and (d) Once formed, a large batch of these sub-units can be placed in solution. (e) and (f) Chemical cross-linking can be triggered, for example, by exposure to light in a specific frequency range. This causes the proteins to connect and form a covalently bonded and system-spanning network, with bonds represented here as black bars. When immersed in water, the resulting viscoelastic material is known as a protein hydrogel.
Download figure:
Standard image High-resolution imageAs they are made almost entirely of water, with the remainder being biological material, protein hydrogels are also ideal for medical applications as they are highly biocompatible. For example, because the networks can be relatively sparse, they may have use in drug delivery. Or, due to their elastic properties, they may have use as artificial tissue scaffolds [11], akin to the fibrinogen networks which enable blood to clot [12]. In a general sense, because the proteins forming the structural backbone of the hydrogels are functional objects in and of themselves, capable of natural biological processes, the applications are far reaching [13].
In order to rationally design these gels we must understand how tuning the specific properties of each protein, using techniques from experimental biology, can modify the macroscopic properties to obtain the results we wish. This type of coupling over length- and time-scales is what we mean by the term 'hierarchical biomechanics'. In our activities we are concerned with exactly this: how the properties of the single protein sub-units and their spatial organisation translate into the macroscopic properties of the protein hydrogels after they have formed. Such an understanding is a direct application of physics to biology. The following sections will present our activities, which were designed to guide a a participant through this hierarchy of mechanical behaviour in biology, and we will describe how we communicate these interdisciplinary topics to our audiences.
3. Single proteins and viscoelasticity
When considering how proteins function from a physical perspective, it is perhaps best to think not about what the specific function of each protein is, but instead how each protein is able to retain its mechanical equilibrium and deform about this equilibrium 'folded' structure at body temperature. The chemical bonds within a protein impart the system with elasticity (energy storage), yet the surrounding water environment and internal friction give rise to viscosity (energy loss). This pairing of elastic 'solid-like' and viscous 'liquid-like' behaviour is known generally as viscoelasticity, and the specific combination of structure, energy storage and energy loss capabilities is different in each protein. In fact, the core constituent to protein physical behaviour is their viscoelasticity. Some are more solid-like, retaining a highly conserved equilibrium structure, and some are more liquid-like, going so far as to completely loose their equilibrium structure under small external forces [14]. To describe these different forms of viscoelasticity and energy storage/loss to a general audience, we can utilise simple household materials to make 'slime'.
3.1. Communicating the concept
Viscoelastic materials have already gained traction in recent years in the form of the 'slime' craze. This is great way of introducing the idea of viscoelasticity to people of all ages, as we can use a variable slime recipe to observe a smooth transition from an almost purely viscous liquid to an elastic solid, making the concept much easier to understand. From there, we can demonstrate that materials such as toothpaste, shaving foam, and even custard may not be as simple as the solid/liquid dichotomy we are introduced to when we are younger.
For our discussion, we refer to a slime made of polyvinyl acetate (PVA) glue and borax [15], although many other recipes are available. PVA is a polymer, meaning that it is formed of a series of identical sub-units connected together in a continuous chain. In isolation, PVA glue is effectively a viscous liquid comprised of a huge number of these individual polymers in solution. The polymers are able to flow past one another and, although they can briefly get stuck and tangled, they are never so entangled that the substance as a whole cannot flow. The addition of borax solution into the system causes chemical cross-links to form spontaneously between the polymers, which then begin sticking together at certain points along the chains. This additional connectivity slightly inhibits the flow of the PVA glue, thus giving rise to viscoelastic behaviour as the polymers form a connected network in a very similar manner to protein hydrogels themselves (see section 4). If we add this cross-linking agent a small amount at a time, we can demonstrate an almost continuous transition from liquid-like polymeric fluid through a range of different viscoelasticities. Once the proportion of cross-links within the system is sufficiently high, the polymers within the system can no longer flow past one another at all. Here, the material properties are dominated by the cross-links themselves, resulting in a purely elastic object. An example of this recipe is shown in figure 2. When discussing viscoelasticity with an audience, more obvious physical characteristics can be noted. For example, we observe that the purely elastic slime made with these materials (an excess of borax) is quite brittle, breaking into multiple parts when stretched. However, the intermediate phase shown in figure 2, whilst softer and easier to stretch, will not break if a similar level of force is applied. This is because the viscous (liquid-like) component of the material allows the slime to passively dissipate energy. If too much energy is dissipated too quickly, though, so much so that the material cannot support any solid structure at all, then we have a liquid. We may also want to emphasise that each different type of material would be useful in different scenarios, and indeed, there are biological examples of proteins with each type of behaviour [16].
Figure 2. Slime made from a PVA glue base and borax solution cross-linker, with three examples using different ratios of borax to PVA. The closest is pure PVA glue, forming a liquid, and the furthest uses so much borax the slime is effectively solid. The middle one is viscoelastic, behaving as a liquid when left alone, but as a solid when stretched. (a) 0 s. (b) 15 s. (c) 60 s.
Download figure:
Standard image High-resolution image4. Polymers, protein networks and hydrogels
Some proteins, such as titin in muscle tissue, are themselves naturally able to polymerise [16]. Although this polymerisation gives them unique mechanical properties [17], often by combining the capabilities of multiple types of viscoelasticity into a single object, they are still only one-dimensional objects. In recent years the development of protein hydrogels (see section 2) has seen the use of similar methods of cross-linking as in section 3 with the aim of making three-dimensional viscoelastic materials, whilst also exploiting the natural function and mechanical stability of individual proteins themselves. Aside from the protein functionality these hydrogels have complex, and potentially even fractal structures [18–20]; it is this viscoelastic complexity which is of interest to us as biophysicists.
To explore with a general audience the type of mechanical behaviour we may see in our gels, and how that behaviour may emerge from the underlying protein structures, we have designed a macroscopic two dimensional representation of a biological network. An example of the grid supporting a network is shown in figure 3.
Figure 3. Photographs of our two-dimensional grid, designed to support arbitrarily connected bead-spring networks. (a) The two-dimensional grid in the absence of a bead-spring network. On each network edge, we can see connections to pulleys and weights at the edge of the table. (b) A randomly arranged example network with 20 kg of mass applied (5 kg on each edge) supported by the two-dimensional grid. We see that while the weight is applied equally on all sides, the way in which the strain is distributed throughout the network is highly non-trivial.
Download figure:
Standard image High-resolution image4.1. Communicating the concept
In addition to the intrinsic properties of the proteins themselves, and the chemical cross-links that they form, a major determining factor in the mechanical behaviour of protein hydrogels is their network structure. These two properties combine to represent the concept of hierarchical biomechanics; the study of how mechanical properties emerge from the underlying components. Our objective here is to show how the connectivity of the protein sub-units can have an effect on the the resulting mechanical strength of the macroscopic hydrogel.
We introduce hierarchical biomechanics using a macroscopic two-dimensional grid of our own design that is able to support a network of rigid metal disks connected by springs, as shown in figure 3. With reference to protein hydrogels, the metal disks can be thought of as representing the stable folded proteins, and the springs (depending on their stiffness) may represent unstructured proteins or cross-linkers. The network itself is supported on a system of horizontal wheels, allowing free movement and expansion of the network around the grid. Weight can also be attached to the grid in a variety of directions to apply external forces to the assembled networks. Figure 3(a) shows the grid in the absence of a bead-spring network, and figure 3(b) shows the level of complexity that can be introduced.
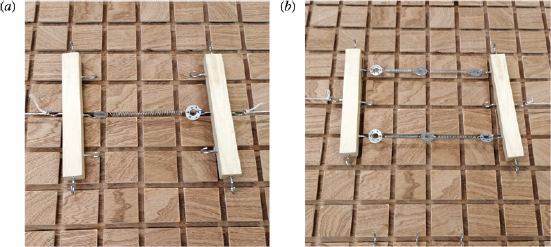
Before introducing such complexity into the network, simple bead spring systems can be constructed to better explain the importance of network structure in addition to the intrinsic properties of the individual components. Figure 4 shows two different networks, one made of a single spring, and another made of four springs. Mathematically, the effective stiffness of the entire network is identical in both systems. This can be seen in the fact that approximately the same amount of extension is seen in figures 4(a) and 4(b), where both networks have the same amount of weight attached to the left and right sides. The difference is in how the energy introduced from the applied weight is spread around the network. In figure 4(b), the energy is able to distribute equally to each spring and so each spring extends less individually. However, in figure 4(a), all of the energy is localised in a single spring. This example allows us to discuss how we might want to construct different types of networks to get different properties. By continually building in further complexity, adding new springs and beads at different angles with multiple connections, we arrive at something like figure 3(b).
Figure 4. Two different networks with related physical properties. (a) A network formed of a single spring. (b) A network formed of four identical springs, two sets of two springs in parallel, where the springs in each individual set are in series.
Download figure:
Standard image High-resolution image5. Discussion and future outlook
We have described a series of activities for communicating complex biophysical ideas to a non-expert audience, using current research into protein hydrogels as a primary focus. We delivered these activities as part of an interactive lecture on biophysics to a GCSE student group at a widening student engagement event. Following our presentation, we noted a common response from the students. As implied in section 1, from their previous studies, many students appeared to have never thought of physics and biology as being related at all, with one student saying that they did not think physics even applied to biology. Whilst we have no direct evidence, we speculate that this response was due to the separation of topics at GCSE level [21, 22]. Further anecdotal evidence suggests that the gradual increase in the complexity of our network on the grid, with continuous reference back to the underlying biological examples, allowed the presence of physics in biology to be realised in quite a generalised manner.
Our second delivery was at the 'Be Curious' event at the University of Leeds, a free and unticketed research open day with an audience of approximately 1200 people. At the event a number of different stalls were set-up, each showcasing areas of research from across the full spectrum of disciplines at the University. The hierarchical biomechanics stall incorporated a core theme of interdisciplinary science in the form of three sub-activities, two of which were those described in sections 3 and 4, and the third was a computer simulation of protein network formation projected onto a large wall behind the stall.
Here we found that the slime making activity was extremely popular with young children. While the children were making the slime, there was an opportunity to talk with them and their parents about the principles which were being explored. As the children were engaged in the making process, the parents had the time and flexibility to listen and ask questions. With time, this allowed the parents to become the instructors to their children, passing on the information they had learned. For the two-dimensional grid activity, the more complex machinery attracted older children and parents who were intrigued to learn what the grid was, and how such a clearly mechanical system was related to slime or the computer simulations behind us. After an introduction to the functionality of the grid, the public were invited to suggest new networks and to predict what the outcome of the new structure would be. This provided an opportunity for discussion whilst real live experiments were being performed.
These previous deliveries highlight the scope for continuation and expansion of our activities. On the topic of formal science education, Parthasarathy has provided a wonderful framework for how undergraduate level biophysics could be taught to non-experts, as an elective or minor subject [23]. However, he also notes that the general aversion to mathematics [24] may dissuade some students who are otherwise genuinely interested in interdisciplinary science education. The generality of the activities presented in this paper enables us to explore hierarchical biomechanics from either a conceptual standpoint, as was shown here, or with more formalised mathematics. To this end, we have developed the activity further to explore biomechancial concepts such as spring constants, elastic moduli and Poisson ratio for delivery to A-level and undergraduate foundation year physics students. This work will be the subject of a separate paper. We also note that the network showcased in section 4 could easily be adapted to include viscous components such as dashpots. This would allow the discussion of specific viscoelastic models such as the Maxwell and Kelvin–Voigt models for continuum systems [25].
Acknowledgments
We wish thank Erin McNeill for her help, advice and endless optimism with physics pedagogy and outreach at the University of Leeds. We would also like to sincerely thank Craig Goddard and Gillian Hanson, who took time out of their lives to both build the two-dimensional network system and proofread the work so that the concepts are accessible to a general audience. Finally, we would like to thank Dr Peter Hine and Angela Beddows for their patience in letting us use random pieces of undergraduate lab equipment to test our two-dimensional network grid, and Dr Geoff Wells for taking pictures of a viscoelastic slime that became liquid-like faster than Ben could grab his camera!
We are grateful to the West Yorkshire branch of the Institute of Physics who supported our hierarchical biomechanics events with a public engagement grant. We acknowledge funding from the Engineering and Physical Sciences Research Council (EP/P02288X/1) Exploiting Engineered Polyproteins in the Modular Design of Robust, Tuneable and Biofunctional Hydrogels.
Biographies

Benjamin S. Hanson earned his PhD at the University of Leeds developing novel computational frameworks to simulate the dynamics of complex biological objects. He is now a postdoctoral researcher at the University of Leeds, with research interests encompassing hierarchical biomechanics, molecular motors, and the fundamental assumptions and applications of computational physics to mesoscopic biology. He is also interested in the general public perception of science, and is an advocate for scientific thinking in all walks of life.

Christa P. Brown received a MPhys and BSc in Physics from the University of Leeds. She is a PhD student researching hydrogels, lipid vesicles and microbubbles with an interest in how these materials can be used in medicine.

Harrison Laurent received an MPhys and BSc from the University of Leeds. He is a postgraduate researcher at the University of Leeds in the Molecular and Nanoscale Physics group. His current research interests include water structure and dynamics and the responses of model beta proteins to extreme chemical environments.

Matt Hughes is a final year PhD student at the University of Leeds, working on understanding the translation of protein stability on the structural and mechanical properties of protein networks. His interests also include applying physics-based approaches to untraditional systems and communicating the importance and prevalence of interdisciplinary science to young aspiring scientists.

Lorna Dougan is a Professor of Physics in the University of Leeds. She is a physicist by training (MPhys and PhD, University of Edinburgh). Her current research interests span hierarchical biomechanics, extreme biophysics, water structure and creative approaches to science communication.