Abstract
Quaternary NbTiZr-based refractory high entropy alloys (RHEAs) have been studied in present work systematically based on empirical parameters and first principles calculations. Firstly, some simple rules based on the elemental properties are applied to screen out all possible elements in the quaternary NbTiZr-based RHEAs. Then, several commonly used empirical parameters, such as atomic size mismatch, average mixing enthalpy, the parameter Ω, are used to screen out the alloys which have a great potential of forming a single-phase solid solution from the remaining alloys. Finally, first principles method is employed to calculate the elastic properties for all screened NbTiZr-based RHEAs, and the mechanical performance of each alloy is evaluated accordingly. The results show that the quaternary NbTiZr-based RHEAs containing Al, Cr, Hf, Mo, Ta, V, W are the most likely to form a single-phase solid solution with bcc structure. Furthermore, the alloys containing W, Ta, and Mo will have high strength, while the alloys containing Al, Hf, and V will have good ductility. This work can provide guidance for the selection of the fourth element in the quaternary NbTiZr-based alloy.
Export citation and abstract BibTeX RIS
1. Introduction
With the rapid development of the aerospace industry, there is an urgent need for materials that can meet the requirements of both high strength at elevated temperatures and low density. Nickel-based superalloys, such as INCONEL 718, Haynes 230, exhibit excellent performance under conditions below 1000 K, and therefore have been widely used [1]. However, when the temperature is higher than 1000 K, the strength of nickel-based superalloys will decrease rapidly, which limits their further application. Since 2004, a brand-new alloy concept, high entropy alloy (HEA) or multi-principal-element alloy or complex concentrated alloy, which is composed by multiple principal elements (usually more than 5 principal elements, if not strictly, 4 principal elements are also recognized), was developed and investigated through an increased number of studies [2]. The HEAs based on refractory elements, called refractory high entropy alloys (RHEAs), have impressive high-temperature performance and are therefore considered to be the most likely candidates for the next generation of superalloys [3]. With the efforts of many researchers, more than 150 RHEAs have been developed over the past decades [4].
Among the many RHEAs, Nb–Ti–Zr-based alloys are the most frequently studied and some of which are with excellent mechanical properties, such as NbTiZrV [5] and NbTiZrHfTa [6]. From the perspective of research methods, most of the current researches on NbTiZr-based RHEAs are based on experiments. Senkov et al [7] produced NbTiZrV and NbTiZrVCr and found that the disordered body-centered cubic (bcc) solid solution phase is the major phase. Hui et al [8] prepared Nb25Ti25Zr25Hf25 with single bcc structure and found that the alloy has a tensile yield strength of 879 MPa and an ultimate tensile strength of 969 MPa, which are much higher than most reported face-centered cubic (fcc)-type HEAs. Stepanov et al [9] investigated the structures and mechanical properties of AlNbTiVZrx RHEAs and found that the phase structures of AlNbTiVZrx alloys with different Zr ratios may have considerable differences. In addition to these experimental studies, there are also some theoretical researches on NbTiZr-based RHEAs which are mainly based on first principles calculations. Qiu et al [5] studied the alloying effect of Al on the structure stability, mechanical properties, electronic structure, and chemical bonding of bcc NbTiZrVAl RHEAs using special quasi-random structure (SQS) method, and found that the addition of Al in NbTiZrVAl RHEAs can improve the thermodynamic stability, and the calculated elastic properties and micro-hardness increase dramatically with the increase of Al concentration. Liao et al [10] investigated the phase stability, elastic and thermodynamic properties of random NbTiVZrx RHEAs by combining virtual crystal approximation method, CALPHAD modeling and quasi-harmonic Debye–Grüneisen model, and drew some conclusions, for example, bcc is the most stable structure for NbTiVZrx when x is between 0.4 and 1.6; the addition of Nb and V can increase the stability of bcc structure, while the addition of Ti and Zr will decrease the stability; with the increase of Nb and V, the Young's modulus and shear modulus of NbTiVZr RHEAs also increase, while Ti and Zr have a counterproductive effect. Tian et al [11] studied the elastic properties of relaxed and unrelaxed NbTiZrMo using the coherent potential approximation method and SQS method, and found that the addition of Mo can increase the elastic constants by distorting the relaxed crystal lattices.
Although these remarkable studies are of great significance to the understanding and development of RHEAs, the composition of the NbTiZr-based RHEAs studied only accounts for a small proportion among the entire composition space, and there are still huge compositions for exploration. Theoretical calculations can be used to explore the composition space rapidly and systematically, and screen out potential alloy compositions, thereby helping to point out the direction for experimental design and accelerate the development of RHEAs. In present work, a systematic study on quaternary NbTiZr-based RHEAs (also denoted by NbTiZrX, where X represents the fourth element in the alloy) are performed using empirical parameters and first principles calculations. Specifically, some simple rules based on the properties of the chemical elements are used to screen out all qualified elements, which are regarded as the X elements and combined with Nb, Ti and Zr to form the NbTiZrX alloy systems. Then the commonly used empirical parameters for each alloy system are calculated to predict the corresponding phase structure and phase stability. For each NbTiZrX alloy system, four alloys with different X element ratios are studied. The alloys that are expected to be single-phase solid solutions are left. After that, first principles method are used to calculate the elastic properties of each alloy and the mechanical performance is evaluated accordingly. The paper is organized as follows: section 2 is an introduction to the computational methods, including the overall research route, empirical parameters, elastic properties and the details of first principles calculations, section 3 presents the obtained results and the corresponding discussions, and section 4 is a short summary on the main conclusions.
2. Methodology
2.1. Overall research route
The overall research route of present work is shown in figure 1. Firstly, the X element in the quaternary NbTiZrX alloy system is determined according to some simple rules based on the properties of the element, such as the boiling point, the ratio of melting point to the density. Secondly, some commonly used empirical parameters, such as the atomic size mismatch, the average mixing enthalpy and Ω, are employed to predict the phase structure and stability of each alloy system. The alloy systems with single-phase solid solutions are left for the follow-up study. Thirdly, first principles calculations based on SQS method are used to research the elastic properties of all NbTiZrX alloy systems screened out from previous step. By analyzing these elastic properties, the stability and mechanical performance of each NbTiZrX alloy can be evaluated. Finally, the quaternary NbTiZrX alloy systems with stable single-phase solid solution structures and excellent mechanical properties are obtained, which can be regard as potential RHEAs for further investigation. A more detailed introduction to the theories or methods used in present work is given below.
Figure 1. Schematic research route in present work. The blue boxes indicate the research purposes or obtained results and the yellow boxes indicate the corresponding research methods.
Download figure:
Standard image High-resolution image2.2. Empirical parameters
According to the definition of HEA, there is no provision for the phase structure of the alloy, which means that the corresponding phase structure can be either a single-phase solid solution, or an intermetallic compound, or a combination of the two. Nevertheless, most HEAs reported in the literatures are single-phase solid solutions, or it can be said that researchers expect to obtain single-phase solid solution alloys. The main reason for such phenomenon is that HEAs with a single-phase solid solution generally have better comprehensive mechanical properties. In addition, single-phase solid solution HEAs are more convenient to study. Hence, it's particularly important to predict the phase structure of HEA based on its composition. In the past few decades, many methods have been proposed to solve this problem, such as CALPHAD method [12–16], empirical parameter method [17, 18] and machine learning method [19]. Among them, the simplest and most commonly used method is the empirical parameter method, which is also used in present work.
Researchers have proposed many different empirical parameters according to different properties such as atomic radius, melting point, electronegativity, mixing entropy, etc. In present work, the most commonly used empirical parameters are chosen as the criteria for predicting the phase structure or stability of the quaternary NbTiZr-based RHEAs, including the atomic size mismatch (δ), the mixing entropy (ΔSmix), the average mixing enthalpy (ΔHmix), the average melting point (Tm), Ω and the average valence electron concentration (VEC) [20]. To maintain the integrity of present paper, the definitions of these parameters are given directly below:






where xi
, ri
, Tmi
and VECi
are the atomic percent, the atomic radius, the melting point and the valence electron number of the ith component, respectively, and a and Hij
are the average atomic radius ( ) and the mixing enthalpy of the corresponding binary alloy, respectively. In present work, the average mixing enthalpies of all alloys were calculated using Miedema model [21, 22], and the data of binary enthalpy was acquired from the work of Takeuchi et al [23].
) and the mixing enthalpy of the corresponding binary alloy, respectively. In present work, the average mixing enthalpies of all alloys were calculated using Miedema model [21, 22], and the data of binary enthalpy was acquired from the work of Takeuchi et al [23].
The atomic size mismatch δ, describing the distortion degree of the solid solution alloy structure, has a significant effect on the phase structure and stability of the corresponding alloy. Empirically, δ should be less than 6.6% if a single-phase solid solution alloy is to be formed [24]. According to the second law of thermodynamics, the Gibbs free energy, which can reflect the phase stability of the alloy, is determined by its mixing entropy and mixing enthalpy at a specific temperature. Therefore, both the entropy and mixing enthalpy can be used to characterize the stability of the alloy structure. In terms of mixing entropy, as the temperature increases, it will play an increasingly important role in stabilizing the alloy. As for the mixing enthalpy, it is suggested that ΔHmix should between −15 KJ mol−1 and 5 KJ mol−1 so as to form a single-phase solid solution HEA [24]. Besides, the parameter Ω, which is a combination of ΔSmix, ΔHmix, and Tm, was proposed to predict the phase structure of HEAs [20, 25, 26]. To form single-phase solid solution HEAs, Ω must be greater than 1. It is found that these empirical parameters are not always consistent in predicting the phase structure of HEAs. Therefore, it should be more reliable to apply these parameters comprehensively.
For single-phase solid solution HEAs, the crystal structure has a crucial influence on its mechanical properties. Generally speaking, alloys with bcc structure have higher strength, while alloys with fcc or hexagonal close-packaged (hcp) structure have better ductility. It is shown that the parameter VEC plays a vital role in predicting the crystal structure of single-phase solid solution alloys. According to experience, when VEC is less than 3.0, the structure of the HEA will be hcp; when VEC is greater than 3.0 and less than 7.5, the structure will be bcc; when VEC is greater than 7.5 and less than 7.8, the structure will be a combination of bcc and fcc; when VEC is greater than 7.8, the structure will be fcc.
2.3. Elastic properties
Elastic properties are the basic properties of alloys, which cannot only characterize the stability of alloys, but also reflect the corresponding mechanical performance to a certain extent. In present work, various elastic properties of quaternary NbTiZr-based RHEAs were investigated, including elastic constants, Born stability criteria, elastic moduli, Poisson's ratio and Pugh ratio, elastic anisotropy indices, etc. The relevant calculation methods of these properties are as follows.
Elastic constants, which are the most basic elastic properties and can be used to derive many elastic properties, were obtained by first principles calculations in present work. Considering that the atomic distribution in the relatively small unit cell may lead to an anisotropic chemical environment, an averaging scheme [2] was adopted to obtain the final elastic constants:



where Cij are single crystal elastic constants.
Based on the elastic constants, Born stability parameters [27], which include C44 , C11 − C12 , C12 + 2C12 , and can be used to measure the mechanical stability of a crystal lattice, can be calculated accordingly. For a cubic crystalline lattice to be stable, the following inequalities must be satisfied: C44 > 0, C11 − C12 > 0, C12 + 2C12 > 0, which can be called Born stability criteria. Generally speaking, the HEAs satisfying these criteria can be considered mechanically stable.
An elastic modulus is a quantity that measures an object or substance's resistance to being deformed elastically when a stress is applied to it. Specifying how stress and strain are to be measured, allows for many types of elastic moduli to be defined. The three primary ones are Young's modulus (E), shear modulus (G) and bulk modulus (B). Based on the obtained elastic constants, elastic moduli can be calculated by Voigt–Reuss–Hill method [5]. For cubic structure, the bulk and shear moduli in Voigt and Reuss approximation are as follows:


where BV and GV are the bulk and shear moduli calculated using Voigt model, while BR, GR are those calculated using Reuss model. They can be obtained using the following formulas:


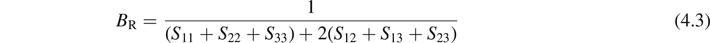
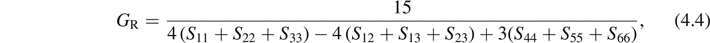
where Sij are the elastic compliances, and the matrix composed by Sij is the inverse of the elastic matrix composed by Cij . Then the Young's modulus E is given by:

To estimate the ductility or brittleness of materials, Pugh introduces the B/G ratio (sometimes G/B ratio is used) [27]. Generally speaking, the larger the B/G ratio is or the smaller the G/B ratio is, the better the ductility of a material is. Quantitatively, if the B/G ratio is larger than 1.75 or G/B is less than 0.57, the ductile behavior can be predicted, and otherwise the material behaves in brittle manner. Besides, Poisson's ratio (ν) [27] can also be used to measure the ductility or brittleness of a material, which is given by:

According to experience, the material with a Poisson's ratio greater than 0.31 are ductile and the larger the value is, the more ductile the system is. In fact, there is an inherent correlation between the Poisson's ratio and the Pugh ratio, which can be seen from their respective formulas clearly. In addition to these two ratios, Cauchy pressure (C12 − C44 ) [28] can also be applied to evaluate the ductility of a material. For a material with good ductility, positive Cauchy pressure can usually be observed, while negative Cauchy pressure usually indicates brittleness.
Elastic anisotropy of alloys is important since it correlates with the possibility to induce micro-cracks in materials. In present work, two different indices [29, 30] Zener anisotropy index AZ and Chung–Buessem anisotropy index AVR are used to assess the elastic anisotropy of RHEAs, for isotropic materials, the values of AZ and AVR will be close to 1 and 0, respectively and can be calculated using the following formulas:


2.4. First principles calculations
As mentioned above, first principles method was employed to calculate the elastic constants of each quaternary NbTiZr-based RHEA. To compare with NbTiZrX alloys, the elastic constants of ternary NbTiZr alloy were also calculated. In the process of calculation, a reasonable crystal model is crucial to the reliability of the final results. In present work, the SQS method based on the cluster expansion formalism [31] was chosen to construct the crystal structures of both NbTiZr and NbTiZrX alloys. According to the literatures and the predicted results by empirical parameters (see below), if the NbTiZrX alloy can form a single-phase solid solution, it tends to be a bcc structure. Hence, bcc was used when constructing the crystal structure of NbTiZrX alloy. Besides, supercells were constructed for both NbTiZr alloy and NbTiZrX alloy to simulate the bulk phase. Specifically, a 3 × 3 × 3 supercell with 54 atoms was constructed for NbTiZr alloy, while a 4 × 2 × 2 supercell with 32 atoms was constructed for NbTiZrX alloy. Besides, the lattice constant for each quaternary NbTiZrX alloy was set according to Vegard's Law [32], which can be expressed as:
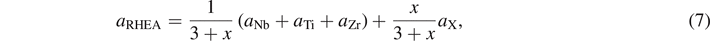
where x is the molar ratio of undetermined element X, and aNb, aTi, aZr, and aX are the equilibrium lattice constants of the elements Nb, Ti, Zr and X in bcc structure, respectively. Figure 2 shows the unrelaxed supercell models of the ternary NbTiZr alloy and the quaternary NbTiZrX alloy constructed using the SQS method.
Figure 2. The unrelaxed supercell models of (a) the ternary NbTiZr alloy and (b) the quaternary NbTiZrX alloy constructed using the SQS method.
Download figure:
Standard image High-resolution imageIn terms of computational detail, Vienna ab initio simulation package (VASP) [33] was adopted to perform all the first principles calculations. Exchange and correlation were treated at the Perdew–Burke–Erzernhof functional level [34]. The plane ware energy cutoff was set 400 eV. Brillouin-zone sampling was performed using the Monkhorst–Pack scheme [35] with a 3 × 5 × 5 k-point mesh. Before calculating the elastic constants, the crystal structures were relaxed to minimize the atomic forces until the force on each atom is less than 10−2 eV Å−1.
3. Results and discussions
3.1. Determination of the element X in NbTiZrX alloy
As mentioned above, the first step in present work is to determine the elemental composition of the NbTiZrX alloy, that is, to determine the fourth principal alloying element X in the alloy in addition to Nb, Ti and Zr. In order to reduce the subsequent calculations, some basic rules were applied to screen all the 118 elements in the periodic table of chemical elements.
Firstly, the three elements (Nb, Ti, Zr) that already exist in the NbTiZrX alloy can be excluded, leaving 115 elements. Although non-metallic elements can appear in HEAs, they are mainly in the form of minor elements rather than major elements. Therefore, the second rule is that the element X in the NbTiZrX alloy must be a metal element. According to this rule, the range of candidate elements is reduced to 91 metal elements. The third rule is that the element X must be non-radioactive, which narrows the candidate range to 59 non-radioactive metal elements. The fourth rule is that the boiling point of the element X mush be greater than the maximum melting point of the three elements Nb, Ti and Zr, so as to avoid the element X from burning out during the alloy forming process. The melting point of Nb is the largest among the three elements of Nb, Ti and Zr, reaching 2750 K, so the boiling point of the fourth element X in the NbTiZrX alloy should be greater than this value. According to this rule, 25 elements can be excluded, leaving 34 elements. The fifth and final rule is based on the melting point and density of the element. For RHEAs, high melting point and low density are two requirements that need to be considered comprehensively to meet the application in aerospace field. Therefore, the elements in RHEAs should meet the two requirements as much as possible. For this reason, the ratio of melting point to density, or called specific melting point, was created as a criterion to screen the remaining elements. Specifically, the specific melting point of Hf was employed as the threshold value, and the elements whose specific melting point is greater than the threshold value were retained. Based on this rule, 13 more elements were excluded, leaving 21 elements, which are Al, Co, Cr, Dy, Er, Fe, Gd, Ge, Hf, Ho, La, Lu, Mo, Ni, Ru, Sc, Ta, Tb, V, W, Y. Table 1 lists all the elements that were screened out and the corresponding melting points, densities and the specific melting points. By observing these elements, it can be found that the most frequently researched elements in quaternary NbTiZrX RHEAs are all included, such as Cr, Hf, Mo, Ta, V and W, which reflects the rationality of the screening rules used in present work. These screened elements would be regarded as element X and combined with Nb, Ti, Zr to form quaternary NbTiZrX alloy systems, and subsequent investigations would be carried out on these alloy systems.
Table 1. Candidates for the fourth alloying element X in quaternary NbTiZrX alloy screened using some simple rules and corresponding physical properties, including melting point, density and specific melting point. The units for Tm, ρ and Tm/ρ are K, g cm−3 and K cm3 g−1, respectively.
| Element | Sc | Y | V | Al | Cr | Mo | Fe |
|---|---|---|---|---|---|---|---|
| Tm | 1812 | 1799 | 2175 | 933 | 2130 | 2890 | 1808 |
| ρ | 2.989 | 4.470 | 6.000 | 2.700 | 7.150 | 10.280 | 7.874 |
| Tm/ρ | 606.223 | 402.461 | 362.500 | 345.704 | 297.902 | 281.128 | 229.616 |
| Element | Ge | Ru | Gd | Ta | Co | Er | Ho |
|---|---|---|---|---|---|---|---|
| Tm | 1211.4 | 2520 | 1585 | 3269 | 1768 | 1795 | 1740 |
| ρ | 5.380 | 12.410 | 7.900 | 16.654 | 8.900 | 9.066 | 8.790 |
| Tm/ρ | 286.286 | 203.062 | 200.633 | 196.289 | 198.652 | 197.992 | 197.952 |
| Element | Tb | Dy | Lu | Ni | La | W | Hf |
|---|---|---|---|---|---|---|---|
| Tm | 1630 | 1680 | 1936 | 1726 | 1190 | 3680 | 2500 |
| ρ | 8.230 | 8.540 | 9.841 | 8.902 | 6.162 | 19.250 | 13.310 |
| Tm/ρ | 198.056 | 196.728 | 196.728 | 193.889 | 193.119 | 191.169 | 187.829 |
3.2. Prediction of single-phase solid solution for NbTiZrX alloys
To predict the phase structure and stability of all involved NbTiZrX RHEAs, the empirical parameters introduced in section 2.2 were calculated for each alloy system, which includes the atomic size mismatch (δ), the mixing entropy (ΔSmix), the average mixing enthalpy (ΔHmix), the average melting point (Tm), the parameter Ω and the average VEC. Specifically, four alloys with different contents of element X, including NbTiZrX0.5, NbTiZrX1.0, NbTiZrX1.5, NbTiZrX2.0, were considered for each alloy system. Besides, the empirical parameters of the ternary NbTiZr alloy were also calculated as a comparison. According to the calculated value of mixing enthalpy ΔHmix, all 21 NbTiZrX alloy systems screened out in the previous step can be divided into two categories, one of which meets the requirements of forming a single-phase solid solution, including Al, Cr, Hf, Mo, Ta, V, W, and the other does not meet the requirements, including Co, Dy, Er, Fe, Gd, Ge, Ho, La, Lu, Ni, Ru, Sc, Tb, Y. The calculated empirical parameters of the former category are listed in table 2.
Table 2. Calculated empirical parameters for ternary NbTiZr alloy and quaternary NbTiZrX RHEAs with different contents of X. The X element here can be Al, Cr, Hf, Mo, Ta, V, W. The units for δ, ΔSmix, ΔHmix and Tm are %, KJ mol−1 K−1, KJ mol−1 and K, respectively. The parameter Ω and VEC have no units.
| Composition | δ | ΔSmix | ΔHmix | Tm | Ω | VEC |
|---|---|---|---|---|---|---|
| NbTiZr | 4.292 | 0.009 | 2.667 | 2264.333 | 7.759 | 4.333 |
| NbTiZrAl0.5 | 4.418 | 0.011 | −7.184 | 2075.057 | 3.246 | 4.143 |
| NbTiZrAl1.0 | 4.426 | 0.012 | −12.500 | 1932.350 | 1.782 | 4.000 |
| NbTiZrAl1.5 | 4.381 | 0.011 | −15.407 | 1821.356 | 1.345 | 3.889 |
| NbTiZrAl2.0 | 4.309 | 0.011 | −16.960 | 1732.560 | 1.131 | 3.800 |
| NbTiZrCr0.5 | 6.994 | 0.011 | −2.286 | 2246.000 | 11.043 | 4.571 |
| NbTiZrCr1.0 | 8.146 | 0.012 | −5.000 | 2231.500 | 5.144 | 4.750 |
| NbTiZrCr1.5 | 8.734 | 0.011 | −6.519 | 2220.222 | 3.876 | 4.889 |
| NbTiZrCr2.0 | 9.041 | 0.011 | −7.360 | 2211.200 | 3.328 | 5.000 |
| NbTiZrHf0.5 | 4.352 | 0.011 | 2.612 | 2298.857 | 9.890 | 4.286 |
| NbTiZrHf1.0 | 4.311 | 0.011 | 2.500 | 2324.000 | 10.714 | 4.250 |
| NbTiZrHf1.5 | 4.229 | 0.012 | 2.370 | 2343.556 | 11.252 | 4.222 |
| NbTiZrHf2.0 | 4.130 | 0.011 | 2.240 | 2359.200 | 11.665 | 4.200 |
| NbTiZrMo0.5 | 4.771 | 0.011 | −0.653 | 2354.571 | 40.521 | 4.571 |
| NbTiZrMo1.0 | 4.965 | 0.012 | −2.500 | 2421.500 | 11.163 | 4.750 |
| NbTiZrMo1.5 | 5.029 | 0.011 | −3.556 | 2473.556 | 7.918 | 4.889 |
| NbTiZrMo2.0 | 5.024 | 0.011 | −4.160 | 2515.200 | 6.697 | 5.000 |
| NbTiZrTa0.5 | 4.045 | 0.011 | 2.286 | 2408.714 | 11.843 | 4.428 |
| NbTiZrTa1.0 | 3.834 | 0.012 | 2.000 | 2516.250 | 14.501 | 4.500 |
| NbTiZrTa1.5 | 3.651 | 0.011 | 1.778 | 2599.889 | 16.644 | 4.556 |
| NbTiZrTa2.0 | 3.492 | 0.011 | 1.600 | 2666.800 | 18.460 | 4.600 |
| NbTiZrV0.5 | 5.518 | 0.011 | 0.816 | 2255.429 | 31.010 | 4.429 |
| NbTiZrV1.0 | 6.605 | 0.012 | −0.250 | 2242.750 | 103.397 | 4.500 |
| NbTiZrV1.5 | 6.325 | 0.011 | −0.889 | 2235.222 | 28.620 | 4.556 |
| NbTiZrV2.0 | 6.437 | 0.011 | −1.280 | 2229.200 | 19.289 | 4.600 |
| NbTiZrW0.5 | 4.644 | 0.011 | −1.796 | 2428.429 | 15.197 | 4.571 |
| NbTiZrW1.0 | 4.774 | 0.012 | −4.250 | 2550.750 | 6.917 | 4.750 |
| NbTiZrW1.5 | 4.799 | 0.011 | −5.630 | 2645.889 | 5.349 | 4.889 |
| NbTiZrW2.0 | 4.772 | 0.011 | −6.400 | 2722.000 | 4.711 | 5.000 |
For Al, as can be seen from table 2, with the increase of Al in NbTiZrAlx , the atomic size mismatch δ and the average mixing enthalpy ΔHmix both decrease. The decrease of δ will make it easier for the alloy to form a single-phase solid solution, while the decrease of ΔHmix will make it easier to form an intermetallic compound. Combined with the parameter Ω, it can be determined that when the molar ratio of Al is relatively low, the NbTiZrAlx alloy will form a single-phase solid solution, and when the molar ratio of Al is greater than 1.5, the value of ΔHmix no longer satisfies the requirement of forming a single-phase solid solution for HEAs. This result is consistent with the conclusions reported by Stepanov et al [9, 36] and Senkov et al [37, 38], that is, when the content of Al is very low, it serves as a stabilizer for the bcc/B2 phase, and when the content of Al gets higher, it tends to form short-range ordered structures with Hf and Zr in the alloy, which promotes the formation of hcp phase.
For Cr, referring to the calculated values of ΔHmix and Ω, it can be determined that the NbTiZrCrx should be able to form a single-phase solid solution. However, the values of δ have exceeded the corresponding threshold of 6.6%. Therefore, the NbTiZrCrx alloys are not necessarily able to form a single-phase solid solution. Moreover, with the increase of Cr, the possibility of forming a single-phase solid solution decreases. Besides, since the ratio of the atomic radius of Cr to that of Zr is between 1.1 and 1.6, it is likely to form Laves phases by combining with Zr in NbTiZrCrx alloy. Miracle and Senkov [39] used Al to replace Cr when studying RHEAs so as to avoid the formation of brittle, topologically tightly packed Laves phases, thereby improving the ductility at room temperature, which is also consistent with the conclusions on Cr in present study.
For Hf, Ta and W, the corresponding NbTiZrX alloys are predicted to be single-phase solid solutions no matter which empirical parameter is used. Furthermore, it can be observed that, for NbTiZrHfx and NbTiZrTax alloy systems, the calculated empirical parameters differ greatly with their respective thresholds, and the values of the parameter Ω increase with the increase of Hf and Ta, indicating that a single-phase solid solution structure can still be maintained for alloys with higher content of Hf or Ta. As for NbTiZrWx alloy systems, the values of the parameter Ω are only slightly larger than the corresponding threshold 1.0, and decrease with the increase of W, indicating that the content of W is approaching the limit value of being able to form a single-phase solid solution.
For Mo and V, all involved NbTiZrMox and NbTiZrVx alloys are predicted to be single-phase solid solutions according to the calculated empirical parameters listed in table 2. However, when the molar ratio of V increases from 1.0 to 2.0, the value of the parameter Ω for corresponding alloy decreases dramatically from 103.397 to 19.289, which suggests that when the molar ratio of V is larger than 1.0, the NbTiZrVx alloy tends to become unstable with the increase of V. For NbTiZrMox , similar tendency can be observed, indicating that alloys with higher content of Mo are likely to be unstable. Such conclusions agree well with those reported by Zhang et al [40] and Hui et al [41], that is, the addition of V can promote the phase separation of bcc, and the addition of Mo can enhance the tendency of phase decomposition. Nevertheless, for NbTiZrX alloys with a molar ratio of Mo or V less than 2.0, their stability of single-phase solid solution is very good.
As for the remaining 14 elements, they can be further divided into two sub-categories, one is transition metals (including post-transition metals) and the other is lanthanide metals. The former sub-category includes 6 transition metals (Co, Fe, Ni, Ru, Sc, Y) and one post-transition metal (Ge), and the latter sub-category includes 7 lanthanide metals (Dy, Er, Gd, Ho, La, Lu, Tb). For NbTiZrX alloy systems where the X element belongs to these two sub-categories, the calculated empirical parameters are listed in tables S1 and S2 (https://stacks.iop.org/MSMS/29/075002/mmedia) of the supplementary information file, respectively. For transition metals, it can be seen from table S1, although the atomic size mismatch δ of NbTiZrX alloys for some X elements, such as Sc and Ge, can meet the requirements of forming a single-phase solid solution, the average mixing enthalpy ΔHmix of all NbTiZrX alloys do not meet the requirements. As for lanthanide elements, as can be seen from table S2, none of their NbTiZrX alloys can form a single-phase solid solution whether it is based on the atomic size mismatch δ, or the average mixing enthalpy ΔHmix, or the parameter Ω.
In summary, according to the calculated empirical parameters, among the 21 elements screened out in the previous step, only 7 elements, namely Al, Cr, Hf, Mo, Ta, V, W, can form a quaternary NbTiZr-based RHEA with a single-phase solid solution structure. Furthermore, according to the value of VEC, it can be easily determined that all the NbTiZr-based alloys listed in table 2 are predicted to be bcc structure.
By observing these remaining elements screened out by empirical parameters, it can be found that all elements except Al are around the three elements Nb, Ti, and Zr in the periodic table of chemical elements, that is, transition metal elements from groups IVB to VIB. Generally, the closeness of elements in the periodic table means that some of their basic properties, such as atomic size, electronegativity, etc, are relatively close, which can ensure their chemical compatibility with each other [42]. In addition, from the perspective of crystal structure, all the remaining elements except Al (fcc) and Hf (hcp) have a bcc structure, which are the same as the final crystal structure of the single-phase solid solution of NbTiZr-based RHEA. Conversely, the crystal structures of those elements excluded during the screening process are mostly hcp, except Ge (diamond), Ni (fcc) and Fe (bcc). Therefore, a simple, slightly rough rule to determine whether an NbTiZr-based RHEA can form a single-phase solid solution can be concluded, that is, the added element should be around the basic elements of the alloy in the periodic table and the corresponding crystal structure should be the same as that of the final single-phase solid solution.
3.3. Calculation of elastic properties for NbTiZrX alloys
Based on the assumption of ideal mixing, MacDonald et al [43] proposed that the atomic composition with equimolar ratio can maximize the configurational entropy of corresponding HEA. Therefore, the elastic properties of equimolar NbTiZrX RHEAs were investigated, where the X element is among the 7 elements screened out by the empirical parameters in the previous step.
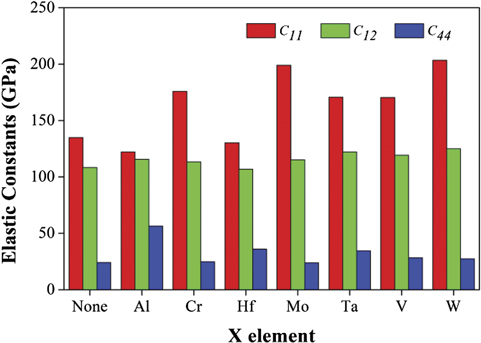
The elastic constants for both NbTiZr and NbTiZrX alloys (X = Al, Cr, Hf, Mo, Ta, V, W) with bcc structure were calculated using first principles method. Table 3 lists the calculated results of the elastic constants C11 , C12 and C44 for all involved alloys and more detailed results of C11 ∼ C66 can be found in table S3 of the supplementary information file. To facilitate the comparison of the calculated elastic constants for all involved alloys, figure 3 is drawn according to the relevant data. In the figure, the label none denotes the ternary NbTiZr alloy (same below). It can be seen that C11 of NbTiZrW alloy is the largest, followed by NbTiZrMo alloy, and that of NbTiZrAl alloy is the lowest. C12 of all alloys are almost the same. C44 of NbTiZrAl alloy is the largest, followed by NbTiZrHf alloy and NbTiZrTa alloy, and the rest are almost the same.
Table 3. Calculated elastic properties for NbTiZrX, where the X element is among Al, Cr, Hf, Mo, Ta, V, W. The units for elastic constants (C11 , C12 , C44 ) and moduli (G, B, E) are GPa. All ratios have no units.
| Properties | NbTiZr | NbTiZrAl | NbTiZrCr | NbTiZrHf | NbTiZrMo | NbTiZrTa | NbTiZrV | NbTiZrW |
|---|---|---|---|---|---|---|---|---|
| C11 | 134.978 | 122.116 | 175.783 | 130.277 | 198.982 | 170.644 | 170.373 | 203.387 |
| C12 | 108.368 | 115.747 | 113.410 | 106.932 | 115.168 | 122.152 | 119.231 | 124.954 |
| C44 | 24.103 | 56.518 | 24.892 | 36.002 | 23.884 | 34.450 | 28.412 | 27.360 |
| C11 − C12 | 26.609 | 6.369 | 62.373 | 23.344 | 83.814 | 48.492 | 51.142 | 78.433 |
| C11 + 2C12 | 351.714 | 353.610 | 402.604 | 344.140 | 429.318 | 414.947 | 408.836 | 453.294 |
| G | 18.990 | 21.263 | 27.244 | 22.952 | 29.970 | 29.927 | 27.239 | 31.613 |
| B | 117.208 | 118.307 | 134.201 | 114.713 | 143.093 | 138.276 | 136.260 | 151.078 |
| E | 54.050 | 60.182 | 76.551 | 64.550 | 84.042 | 83.740 | 76.613 | 88.656 |
| B/G | 6.172 | 5.564 | 4.926 | 4.998 | 4.775 | 4.621 | 5.002 | 4.779 |
| G/B | 0.162 | 0.180 | 0.203 | 0.200 | 0.209 | 0.216 | 0.200 | 0.209 |
| ν | 0.423 | 0.415 | 0.405 | 0.406 | 0.402 | 0.399 | 0.406 | 0.402 |
| C12 − C44 | 84.265 | 59.229 | 88.159 | 70.930 | 91.284 | 87.702 | 90.819 | 97.594 |
| AZ | 1.811 | 17.749 | 0.798 | 3.084 | 0.570 | 1.421 | 1.111 | 0.698 |
| AVR | 0.042 | 0.655 | 0.006 | 0.145 | 0.037 | 0.015 | 0.001 | 0.015 |
Figure 3. Calculated elastic constants (C11 , C12 , C44 ) of NbTiZr and NbTiZrX (X = Al, Cr, Hf, Mo, Ta, V, W).
Download figure:
Standard image High-resolution imageBased on the obtained elastic constants of these NbTiZrX alloys, other elastic properties, which includes Born stability parameters (C44 , C11 − C12 , C12 + 2C12 ), elastic moduli (shear modulus G, bulk modulus B and Young's modulus E), Pugh ratio (B/G), Poisson's ratio (ν), Cauchy pressure (C12 − C44 ), elastic anisotropy indices (Zener index AZ and Chung–Buessem index AVR), can be calculated by referring to the formulas introduced in section 2.3. The corresponding results are listed in table 3. To show these results clearly, figure 4 with four sub-figures are plotted.
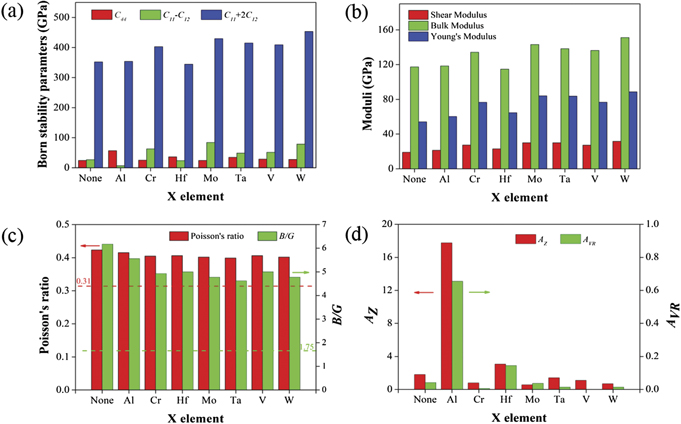
Figure 4. Calculated elastic properties for NbTiZr and NbTiZrX (X = Al, Cr, Hf, Mo, Ta, V, W). The sub-figures represent (a) the Born stability parameters, including C44 , C11 − C12 , C11 + 2C12 , (b) the elastic moduli, including shear modulus G, bulk modulus B and Young's modulus E, (c) Poisson's ratio ν and Pugh ratio B/G, and (e) the elastic anisotropy indices, including AZ and AVR, repectively.
Download figure:
Standard image High-resolution imageFigure 4(a) shows the calculated Born stability parameters for both the ternary NbTiZr alloy and the 7 quaternary NbTiZrX alloys. It can be seen easily that all these values are larger than 0, which means that all these involved alloys are predicted to be mechanically stable according to the Born stability criteria. What should be mentioned, although the C11 − C12 values of NbTiZrAl alloy and NbTiZrHf alloy are greater than 0, they are closer to 0 compared with other alloys, indicating that the mechanical stability of these two alloys is not as good as other alloys. The main reason for such result is that the crystal structure of Al (fcc) and Hf (hcp) is different from the corresponding NbTiZr-based alloy (bcc), which makes the structure of the alloy more distorted and less stable.
Figure 4(b) displays the calculated elastic moduli, including shear modulus, bulk modulus and Young's modulus, for ternary NbTiZr alloy and quaternary NbTiZrX alloys. Combined with table 3, it can be seen that each modulus of any quaternary NbTiZrX alloy is greater than that of the ternary NbTiZr alloy, except for the bulk modulus of NbTiZrHf alloy, which indicates that the addition of the fourth element to NbTiZr alloy can strengthen the alloy. Secondly, although the three moduli of different alloys can be quite different, the order of each modulus among these involved alloys is almost the same, that is, the alloy with a larger shear modulus will also have a larger bulk modulus or Young's modulus, which reflects the intrinsic relationship among these three moduli to some extent. Quantitatively, the three moduli of NbTiZrW alloy are the largest among all alloys, which indicates that the W element has the best strengthening effect on the ternary NbTiZr alloy. NbTiZrMo alloy and NbTiZrTa alloy also have good performance in these three moduli. In contrast, the moduli of NbTiZrAl and NbTiZrHf alloys are relatively low.
From the perspective of Cauchy pressure, it can be seen from table 3 that all the calculated results of NbTiZr and NbTiZrX alloys are positive, which demonstrates that all alloys involved are ductile. Nevertheless, the ductility of these alloys remains different, which can be observed from the calculated values of Poisson's ratio and Pugh ratio, as drawn in figure 4(c). From the figure, it can be seen that the Poisson's ratio and Pugh ratio of quaternary NbTiZrX alloys are smaller than those of ternary NbTiZr alloy, which indicates that the addition of the fourth alloying element to the NbTiZr alloy will reduce the ductility of the resulting alloy. This is exactly the opposite of the influence of alloying elements on the strength of the alloy mentioned above, which demonstrates again that strength and ductility are a pair of mutually exclusive properties to some extent. What can be found next is that the Poisson's ratio and the Pugh ratio are consistent in changing trends for all alloys. Specifically, the NbTiZrX alloys with Ta, Mo and W as the X element has relatively small Poisson's ratio and Pugh ratio, which means that the ductility of these three alloys is not as good as other alloys. On the contrary, NbTiZrAl alloy has relatively good ductility, followed by NbTiZrV and NbTiZrHf.
The calculated results of anisotropic indices AZ and AVR for NbTiZr and NbTiZrX alloys are drawn in figure 4(d). It can be seen clearly that the AZ value and AVR value of most alloys are close to 1 and 0, respectively, except those of NbTiZrAl alloy. Therefore, it can be inferred that most NbTiZrX alloys here are elastically isotropic. As for NbTiZrAl alloy, from table 3, the AZ value and AVR value of NbTiZrAl alloy have reached 17.749 and 0.655, exceeding their respective thresholds, which indicates that the alloy has obvious anisotropy. In addition, the NbTiZrHf alloy also shows a certain degree of anisotropy. By analyzing the crystal structure of these elements and the corresponding NbTiZr-based RHEA, it can be inferred that the anisotropy of the alloy is closely related to the crystal structure of the added element. If the crystal structure of the added element is consistent with that of the final alloy, the anisotropy of the alloy will be weaker, and vice versa.
3.4. Discussion on the properties of NbTiZrX alloys
From the introduction of the above sub-sections, it can be seen that the quaternary NbTiZrX alloy, with the X element in Al, Cr, Hf, Mo, Ta, V, W, has relatively outstanding performance compared with other alloys. For a clear comparison, the various properties of these alloys in terms of relative degrees, such as good, average, fair, etc., are listed in table 4.
Table 4. Various properties of the quaternary NbTiZrX (X = Al, Cr, Hf, Mo, Ta, V, W) alloys.
| Property | X element in NbTiZrX alloy | ||||||
|---|---|---|---|---|---|---|---|
| Al | Cr | Hf | Mo | Ta | V | W | |
| Density | X-low | Low | High | Fair | High | Low | X-high |
| Melting point | Low | Fair | High | High | High | Fair | X-high |
| Single-phase solid | |||||||
| solution | Fair | Fair | Good | Average | Good | Average | Good |
| Mechanical stability | Fair | Good | Good | Good | Good | Good | Good |
| Strength | Fair | Average | Fair | Good | Good | Average | Good |
| Ductility | Excellent | Average | Good | Fair | Fair | Good | Fair |
| Isotropy | Bad | Good | Average | Good | Good | Good | Good |
As a whole, the NbTiZrX alloys listed in table 4 can form a stable single-phase solid solution structure, although the stability can be different for different alloys. From the perspective of strength and ductility, no alloy can have the best performance in both properties. That's to say, the alloys with higher strength are generally less ductile than other alloys. Specifically, the alloys containing W, Ta, and Mo have higher strength, while the alloys containing Al, Hf, and V have better ductility. It should be noted that although the strength of some alloys is not as good as other alloys, it does not mean that these alloys are not competent for the environments where high strength is required. On the one hand, the properties shown here are intrinsic properties, while the actual properties are determined by many factors, such as the fabrication process. On the other hand, the properties listed in table 3 are relative, not absolute. Therefore, NbTiZrHf alloy may also be able to meet the requirements of high strength. In addition to the above, it is necessary to consider the density or melting point sometimes. For example, for those occasions that require high temperature but not too much density, NbTiZrW alloy should be a good choice, and if there is a requirement for low density, NbTiZrMo or NbTiZrV should be a better choice.
4. Conclusions
In present work, the quaternary NbTiZr-based alloys are systematically studied using empirical parameters and first principles calculations, and several prospective and research-worthy alloys with a stable single-phase solid solution structure and excellent mechanical properties are screened out. Specifically, some simple rules based on the elemental properties, such as the boiling point, the ratio of melting point to the density, were applied to filter out all possible X elements in NbTiZrX alloy. As a result, 21 elements were selected, which includes Al, Co, Cr, Dy, Er, Fe, Gd, Ge, Hf, Ho, La, Lu, Mo, Ni, Ru, Sc, Ta, Tb, V, W, Y. Then, some commonly used empirical parameters, such as the atomic size mismatch, the average mixing enthalpy and the parameter Ω, were employed to predict the phase structure of each NbTiZrX alloy system, where the X element belongs to one of the 21 elements. Besides, four alloys with different contents of element X, including NbTiZrX0.5, NbTiZrX1.0, NbTiZrX1.5, NbTiZrX2.0, were considered for each alloy system. According to the calculated results, only 7 elements, including Al, Cr, Hf, Mo, Ta, V, W, can form a quaternary NbTiZrX alloy with a single-phase solid solution structure. Finally, first principles calculations based on SQS method are used to research the elastic constants of all the 7 equimolar NbTiZrX alloys. Based on the obtained elastic constants of these NbTiZrX alloys, other elastic properties, which includes Born stability parameters (C44 , C11 − C12 , C12 + 2C12 ), elastic moduli (shear modulus G, bulk modulus B and Young's modulus E), Pugh ratio (B/G), Poisson's ratio (ν), Cauchy pressure (C12 − C44 ), elastic anisotropy indices (Zener index AZ and Chung–Buessem index AVR). By analyzing the calculated results, it is found that the NbTiZrX alloys containing W, Ta, and Mo have higher strength, while the alloys containing Al, Hf, and V have better ductility. In summary, this work can provide guidance for the selection of the fourth element in the NbTiZr-based quaternary alloy.
Acknowledgments
This work is supported by the Hubei Key Laboratory of Mechanical Transmission and Manufacturing Engineering (MTMEOF2020B01), Science Foundation of Wuhan Institute of Technology (K2021039) and Key Laboratory of Advanced Reactor Engineering and Safety, Ministry of Education (ARES-2018-04).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.