Abstract
Nuclear medicine (NM) procedures for diagnosis and treatment of disease are performed routinely in hospitals throughout the world. These involve preparation and administration to patients of pharmaceuticals labelled with radioactive material. The International Atomic Energy Agency (IAEA) and the World Health Organisation highlighted the need for improvement in prevention of medical radiation incidents and accidents in the Bonn Call-for-Action in 2012. An IAEA Technical Meeting was held on prevention of unintended exposures and accidents in NM in 2018 to address the issue. Exposures can take place at any time when radioactive material is being produced and used, and the risk continues after procedures have been completed. Thus there is potential for staff or members of the general public to be exposed, as well as patients. This paper sets out guidelines for incident prevention based on presentations and discussions at the meeting, and review of reports from the literature. It deals with potential incidents in in-house radionuclide production, radiopharmaceutical preparation, administration to patients, and following a procedure, as well as aspects in management of radioactive materials. Special attention has been paid to therapeutic procedures, as these have the potential to cause more harm to patients from erroneous administrations, including tissue reactions from extravasation of radiopharmaceutical, and could lead to significant contamination events. Administration of NM therapy is generally contraindicated in pregnancy. Identification of any patient who may be pregnant is crucial and it might be necessary to verify this with a pregnancy test for patients within the age band considered to be fertile. Inclusion of NM therapy incidents in the IAEA automated reporting system SAFRON is recommended. In summary, the paper aims to highlight errors that could occur during different phases of NM procedures in order to aid prevention of incidents. The value of periodic audit in evaluating systems in place on a regular basis is emphasised. Approaches to incident investigation and follow-up are described, and the need to ensure corrective action is taken to address any deficiencies stressed.
Export citation and abstract BibTeX RIS
1. Introduction
Nuclear medicine (NM) is the diagnosis and treatment of disease through the administration to patients of radiopharmaceuticals. Procedures are performed routinely in hospitals throughout the world. Although the level of safety is generally high, because radioactive materials are handled directly and administered to patients there is the potential for a variety of incidents to occur, involving unintended exposures of patients or staff members (Martin 2005). The International Atomic Energy Agency (IAEA) and the World Health Organisation (WHO) have called for methods for prevention of incidents and accidents in medical uses of ionizing radiation to be strengthened. This is one of ten actions highlighted at a conference held in Bonn in 2012 that resulted in the Bonn Call-for-Action (IAEA/WHO 2012). Action 7, entitled 'Improve prevention of medical radiation incidents and accidents', encouraged stakeholders to 'implement and support voluntary educational safety reporting systems for the purpose of learning from the return of experience of safety related events in medical uses of radiation'. The first priority for action was therapeutic applications and the statement specifically mentioned therapeutic NM, but called for organizations to work towards inclusion of all modalities of medical usage of ionizing radiation in voluntary safety reporting. These recommendations are linked to Action 8 'Strengthen radiation safety culture in health care'. These requirements are further emphasised in the International Basic Safety Standards (BSS—GSR Part 3) (IAEA et al 2014) and IAEA Member States are encouraged to include requirements for both minimizing the likelihood of such exposures and investigating them when they occur in regulations.
GSR Part 3 defines an incident as 'any unintended event,—the consequences or potential consequences of which are not negligible from the point of view of protection and safety'. The European Directive 2013/59/EURATOM (European Council EC (2014)) defines an 'unintended exposure' for medical exposures of patients as 'significantly different from the exposure intended for a given purpose'. GSR Part 3 requires implementation of an appropriate system for record keeping and analysis of events involving or potentially involving accidental or unintended medical exposures, commensurate with the radiological risk posed by the practice. Moreover, it requires reporting of significant events to the regulatory body and to the relevant health authority if appropriate, with the European Directive also requiring timely dissemination of information about lessons learned from significant events involving medical exposures. Guidelines on implementation of the requirements of GSR Part 3 in medicine, including prevention, investigation, reporting of unintended and accidental exposure, and implementing corrective actions, are provided in the IAEA Specific Safety Guide SSG-46 'Radiation Protection and Safety in Medical Uses of Ionizing Radiation (IAEA 2018a).
The IAEA organised a technical meeting in 2017 to consider unintended and accidental medical radiation exposures in radiology, but did not include NM (Martin et al 2017). NM is a technique for metabolic and functional uptake of radiopharmaceuticals, and has different characteristics from radiology in that the result of an examination or therapeutic procedure and the associated risk for the patient are not determined primarily by operation of the imaging equipment, but by the administration of radiopharmaceuticals and subsequent patient management and patient conditions (age, gender, pregnancy, breastfeeding etc). There is also a greater potential for exposure of staff and others in NM.
A safety culture requires procedures and standards that include features such as acknowledgment of the high-risk nature of what is done, commitment to achieving operations that are consistently safe, a blame-free environment, encouragement of collaboration and teamwork, recognition of expertise, commitment of the organization at all levels, and resilience. This paper aims to identify issues that can potentially result in radiation incidents and brings together advice based on expertise and experience from professional groups to provide guidelines for implementing systems within healthcare facilities to assist in preventing unintended and accidental exposures in NM. The paper does not discuss the reporting outside the healthcare facility of patient exposures that are substantially greater than intended, as this will be the subject of separate guidelines.
2. Methods
2.1. Collation of information on incidents and their prevention
A Technical Meeting on 'Preventing Unintended and Accidental Exposures in NM' was held at IAEA Headquarters, Vienna on 16–18 May 2018. It was attended by 45 delegates from 33 Member States including NM physicians, medical physicists, technologists, radiopharmacists, regulators and equipment manufacturers. There were representatives from the World Health Organization (WHO), International Commission on Radiological Protection (ICRP), United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), International Society of Radiographers and Radiological Technologist (ISRRT), Global Diagnostic Imaging, Healthcare IT and Radiation Therapy Trade Association (DITTA), Heads of the European Radiological Protection Competent Authorities (HERCA), European Association of NM (EANM), European Federation of Organisations For Medical Physics (EFOMP), European Society of Radiology (ESR), the American Association of Physicists in Medicine (AAPM), Conference of Radiation Control Program Directors (CRCPD), as well as a range of national regulatory agencies.
The meeting reviewed unintended and accidental exposures that had occurred, discussed the causes and factors contributing, and considered others that could occur in all stages of the NM process. It was apparent incidents involving therapy patients could have significant consequences and the need for guidance was highlighted by the meeting's participants. Experiences in reporting of incidents within healthcare facilities, methods for investigating to determine root causes, and implementation of changes to reduce the risk of similar events occurring in the future were presented. These include regular analysis and review of procedures to look for vulnerabilities, as part of the development of a safety culture within NM departments, with action taken to modify and improve arrangements to reduce risks of system failure. Incidents in radionuclide production, radiopharmaceutical preparation, the administration to patients for diagnosis or therapeutic purposes, and potential exposures that could occur once patients had been discharged from hospital were discussed, as well as incidents resulting from failures in management of radioactive materials.
Information from the meeting has been collated and is presented here in the form of guidance. The range of incidents that may occur is wide, and potential causes and actions that might prevent them occurring are linked closely to the type of incident. Therefore potential consequences and methods for prevention are described within sections for each category, namely the different stages that can contribute to patient exposures, staff exposures including contamination events, exposure of members of the public, and the management of radioactive materials, which if not controlled effectively can lead to persons being exposed unknowingly. The investigation and follow-up of incidents when they occur is considered in the final section, together with the role of the regulators in highlighting and disseminating good practice. Short descriptions of incidents either from presentations at the meeting based on personal experiences, or reports in the literature, are included as examples of things that can go wrong.
2.2. Terminology
A similar approach in incident terminology to that used in a paper on unintended and accidental radiation exposures in radiology has been adopted (Martin et al 2017). The term 'incident' is used to encompass all 'unintended events' that might lead to exposures of staff or the public and 'accidental or unintended medical exposures' of patients.
For patient exposures, the terms maladministration and misadministration have been used widely to describe errors in NM procedures (IAEA 2006a). However, it became apparent during the meeting that their use varies between countries and different groups used them in the opposing sense. Therefore alternative terminology is used here.
2.2.1. Error in procedure
- failure in preparation chemistry
- administration of the wrong radiopharmaceutical
- administration of correct radiopharmaceutical but with the wrong activity
- administration of radiopharmaceuticals containing radionuclidic or radiochemical impurities in excess of acceptable levels
- administration of radiopharmaceutical to the wrong patient
- failure to carry out a procedure after administration of a diagnostic radiopharmaceutical
- administration to pregnant or breast-feeding woman, without having information about pregnancy or breastfeeding status.
2.2.2. Failure in administration
- Extravasation of injection: radiopharmaceutical injected into tissue rather than vein
- Injection into the wrong blood vessel or outside the synovial membrane
- Injection via a catheter, with possible stasis, adhesion to tube walls, or leakage decreasing the activity injected into the body, leading to poor counting statistics and low-quality images in diagnostic examinations, or reduction of the prescribed absorbed dose in therapeutic applications
- Error in oral administration (e.g. failure of patient to consume iodine therapy capsules or liquid).
2.3. Follow-up and prevention of incidents
When an incident occurs, any corrective medical actions that are required to minimise the effects should be undertaken immediately. When required, counselling of any patient involved in a medical exposure incident should be undertaken by an individual with appropriate experience and clinical knowledge. The reporting, investigation, and follow-up of incidents is a crucial component in their management, in building the capacity to prevent incidents, and enabling staff to react appropriately when an incident does occur (Anderson et al 2013, Pham et al 2013, Leistikow et al 2017). This will be discussed in more detail later in the paper.
General strategies for addressing problems that could lead to an accidental exposure are identified in IAEA Safety Guide SSG-46 (IAEA 2018a) and include, the regular maintenance of medical radiological equipment and software, a comprehensive programme of quality assurance (QA), continuing education and training of staff, and the promotion of a safety culture. The IAEA Safety Guide SSG-46 defines the following measures to minimise the likelihood of unintended and accidental medical exposure:
- (a)The introduction of safety measures at identified critical points in the process, with specific quality control checks at these points.
- (b)Actively encouraging a culture of always working with awareness and alertness.
- (c)Providing detailed protocols and procedures for each process.
- (d)Providing sufficient staff who are educated and trained to the appropriate level, and an effective organization, ensuring reasonable patient throughput.
- (e)Continuous professional development and practical training, and training in applications for all staff involved in providing NM services (ICRP 2009).
- (f)Clear definitions of the roles, responsibilities and functions of staff in the NM facility that are understood by all staff.
Preventive measures should include checking the robustness of the safety system of the facility against reported incidents (retrospective risk analysis), as well as applying a prospective risk management strategy.
3. Results and discussion
3.1. Incidents: causes and prevention
NM is a complex process requiring specialised facilities and using many different skills, so comprehensive training in all tasks that individuals perform is essential for minimizing the risk of incidents. It involves not only handling and use of radionuclides, but also aseptic techniques, and QA and quality control (QC) of radiopharmaceuticals (IAEA 2006a, IAEA 2018a). Factors that influence the frequency and severity of accidental exposures are identified in the Safety Guide (IAEA 2018a) and these include insufficient training and expertise of the medical and radiological professionals involved, which, coupled with other deficiencies such as poorly written procedures, a lack of operating documents, limited understanding of displays and software messages, and inconsistent use of different quantities and units provide many potential causes of error. Training should be confirmed periodically, and staff returning after a long absence, should undergo re-training or confirmation of competence. In addition, staffing levels should be reassessed following installation of new equipment or increases in workload.
Although the types of human error and factors within the environment that contribute to different categories of incident may be similar, the nature of the incidents themselves, their potential effects, and methods for prevention are specific to each type of incident. The following sub-sections consider in turn exposures of patients, staff and the public, followed by incidents involving management of radioactive materials. Types of error that can occur are pointed out and recommendations made on safety measures through which the risk of incidents may be minimised.
3.2. Patient exposures
In NM unintended exposures may occur due to errors during the preparation or administration of radiopharmaceuticals, and for diagnostic procedures also during the imaging or data recording phase. Radiopharmaceuticals are administered to patients either at the start of treatment, or at a specified time before an image is acquired or a measurement is recorded for diagnostic purposes.
Many aspects are similar for therapeutic and diagnostic procedures, so these will be discussed at the same time. The effects of unintended exposures from diagnostic NM on adults are likely to be limited, although absorbed doses may be substantial with some radiopharmaceuticals (e.g. with relatively long half-life's, such as 201Tl and 67Ga), but special consideration is required for paediatric patients, and female patients who are pregnant or lactating, particularly for therapeutic administrations. However, absorbed doses to critical organs such as the bone marrow, kidneys, and thyroid from unintended therapeutic administrations can be significant, and the extravasation of therapeutic injections can cause tissue reactions at the injection site.
3.2.1. Preparation of radiopharmaceuticals
NM procedures are complex, structured processes with many components, all of which influence the outcome of a procedure. The process starts with preparation of the radiopharmaceutical in the radiopharmacy. Failings in the radiochemistry can result in poor uptake of activity, while use of the wrong radiopharmaceutical could induce a variety of conditions, including cardiovascular effects (such as the adrenergic effect of metaiodobenzylguanidine, a sympathetic agent labelled with radioiodine for cardiac and neuro-endocrine imaging), antibody-induced immune response, or allergic reactions; failings in the pharmaceutical and microbiological safety aspects of the process can lead to severe consequences (Fleury et al 2003, Patel et al 2006, Bombardieri et al 2010).
Problem areas in different components of the process that may lead to an error in a procedure are summarised in table 1. There is a global movement to improve quality standards in the preparation of radiopharmaceuticals (IAEA 2008, Lange et al 2015), driven partly by the need to comply with regulatory requirements that apply to all 'conventional' pharmaceuticals, but also to improve safety, efficacy, and quality of radiopharmaceuticals. The improvements aim to reduce microbiological contamination to a negligible level, and to maintain sterility and apyrogenicity in all phases of the process.
Table 1. Errors that may contribute to incidents during the preparation of radiopharmaceuticals.
| Component | Possible errors |
|---|---|
| Storage of precursors, kits, cassettes etc | • Wrong environmental conditions may alter the products |
| • A product may have expired and not be taken out of use | |
| • Poor demarcation of storage areas, leading to the selection of the wrong agent | |
| Biological contamination during synthesis | • A module or vial may not have been sealed adequately |
| • Aseptic conditions in a hot cell or laboratory may not be adequate, resulting in microbial contamination of the product | |
| Labelling of kits/Synthesis of radiopharmaceuticals | • Incorrect set-up of 18F synthesis module, e.g. failure to seal module effectively or wrong loading of reagents/cassette |
| • Incorrect preparation of kits, e.g. exposure to air of MDP or wrong heating cycle for Sestamibi, resulting in low radiochemical purity | |
| Quality control (QC) of the | • Errors in laboratory procedures |
| radiopharmaceutical | • Inaccurate calibrations or equipment with poor sensitivity |
| • Components of the QC tests omitted | |
| Dispensing of the radiopharmaceutical | • Poor procedures or environmental conditions that may contaminate the product |
| • Inaccurate activity or mixing up of different products resulting from simultaneous dispensing of many vials before their administration | |
| • Missing, inaccurate, or ambiguous labels or colour coding for vials and syringes | |
| • Lack or poor compliance in use of protective shields | |
| Receipt and control of vials containing radiopharmaceutical | • Poor system for checking orders at the facility where the radiopharmaceutical is administered to confirm that the contents of each vial that has been delivered are correct. |
Success of a NM procedure in providing images of adequate quality for diagnosis or delivering the prescribed activity for therapy is linked to the use of the correct radionuclide activity, so activity levels should be measured shortly before administration. Inaccuracies in radionuclide activity meters (frequently, and improperly termed 'dose calibrators') can occur if the device itself is not calibrated and checked periodically within a QA/QC programme (IAEA2006b). The implementation of a QA/QC programme under the supervision of a medical physicist to ensure accurate and consistent results in activity measurements is therefore of paramount importance (IAEA et al 2014). The calibration of these instruments needs to be traceable to a national or international standard (Zimmerman and Judge 2007, Bergeron et al 2014), and it should be in terms of the activity of radionuclides used in clinical practice. Calibration with a traceable source of 137Cs, for example, is useful for a constancy check, but in itself it only confirms that the instrument can measure this radionuclide, but does not ensure that the radionuclide activity meter is accurately measuring activities of all radionuclides typically administered to patients, such as 99mTc or 131I. However, calibration services for radionuclides commonly used in NM are not available in all countries.
A QA/QC programme for a radionuclide activity meter begins with its selection and acceptance testing, to establish whether the functionality and initial performance conform to the manufacturer's specifications and the intended use. This is followed by reference tests, against which future performance should be compared, and routine tests, normally divided into operational tests (performed daily) and periodic tests performed at appropriate intervals (e.g. weekly, monthly, quarterly, etc). Lists of recommended QC tests on radionuclide activity meters can be found in various guidance documents (IAEA 2006b, National Physical Laboratory NPL (2006), (AAPM (2012)). Many require the use of sealed long lived radioactive sources of γ-emitting radionuclides covering a wide range of photon energies, such as 137Cs, 57Co and 133Ba, that act as surrogates for the radionuclides used in clinical practice. When the activity meter has been adequately calibrated, its accuracy should be tested periodically, but the most critical test for prevention of unintended radiation overexposures in NM is probably the daily check of its reproducibility with a reference source. An example of an error, which lead to an incident, was measurement of 99mTc activities with the radionuclide selected on the activity meter left as 137Cs, which had been used for the daily constancy test. The hospital changed the procedure for daily QC tests, requiring the constancy test to be performed with 99mTc selected on the activity meter, as only the reproducibility needed to be confirmed.
In therapeutic procedures the calibration for new radionuclides, and the proper radiation protection skills to handle and accurately measure the administered activity are important aspects requiring direct involvement of a medical physicist. An unintended event is defined in para. 3.180 of GSR Part 3 (IAEA et al 2014) as an absorbed dose administered in radiopharmaceutical therapy differing substantially from (over or under) the prescribed activity. However, there is a lack of consensus recommendations on the difference in activity level that would be considered substantial. The Safety Guide SSG-46 (IAEA 2018a) recommends as a pragmatic approach for use by NM facilities, the specification for therapeutic procedures of deviations greater than ±10% as being substantially different. A system with clear procedures should be put in place for identifying when this type of event occurs. For general operational considerations the Safety Guide SSG.46 (IAEA 2018a) gives a challenging recommendation on accuracy of the activity to be dispensed and administered for radionuclide therapies, stating that (paragraph 4.185) the total activity does not deviate significantly from the prescribed administered activity (e.g. < 5% deviation), and the measured value should be recorded.
Radiopharmacies sometimes prepare and dispense a variety of diagnostic radiopharmaceuticals for a number of NM departments at the same time. This has to be done in advance, so strict, carefully controlled systems, with accurate labeling are required to avoid errors, with additional checks being made at the NM department on receipt. Colour coding and pictorial identifiers on labels are useful in aiding correct identification, but systems must be rigorous.
The potential risks for patients following an error in the early stages of preparation are:
- a possible adverse reaction from a product of inadequate quality
- an unjustified exposure, if the radiopharmaceutical is not adequate for producing an image of acceptable quality, or delivering the desired treatment outcome
- delay or postponement of treatment if image quality is too poor to provide the diagnostic information required.
More attention is now paid to traceability in structured processes, such as NM, whereby the status of a specific, individual process (e.g. the examination of a given patient) is controlled and can be identified at any point. A radiopharmacy laboratory technologist should be able to check the identification information for a patient, whether she/he has undergone the necessary preparation before the administration, the timing of the administration for the activity, the prescription for the radiopharmaceutical, and the activity to be administered. Information should be available on the batch number of the radiopharmaceutical kit and the radionuclide generator for 99mTc, as well as the activity dispensed, and the calibration time, and linked to data on QC of the product and its approval by the qualified person. These are now standard features of traceability software packages that can be integrated with the hospital/radiology information systems of the health institution (e.g. ASTRIM Nuclear Medicine, [ASTRIM srl, Milan, Italy], BioDose/NMIS by ec2 [Software Solutions, Somerset, NJ, USA], IBC packages [Comecer, Castelbolognese, Italy], SYNtrac [CardinalHealth, Dublin, OH, USA]).
Proper control of the process applies whether vials or syringes containing activities specific to individual patients or a standard activity are prepared in-house or by an external radiopharmacy. The radiopharmaceuticals and activities delivered should be checked carefully to ensure they correspond to the order. This may not be trivial, since commercial radiopharmacies tend to deliver 'standard' levels of activity that have to be adapted to specific patients (paediatric, lightweight, overweight, etc).
Examples reported at the meeting of the wrong radiopharmaceutical preparation being injected that highlight the importance of checking labels and measuring activity levels are given below:
- 1.Two 131I therapy capsules (4 GBq and 400 MBq) for separate hospitals, were delivered to the wrong hospitals. This resulted in the 4 GBq capsule being administered to the wrong patient without the activity being measured. See, as an example, a similar scenario described in Al Aamri et al (2016).
- 2.Two vials were delivered together to a NM department, vial 1 containing 131I and vial 2 18F. Normally 131I would have been delivered at a different time of day. The technologist, assuming both vials contained 18F labelled FDG, measured the activity levels with the 18F window setting on the radionuclide activity meter as 20.3 MBq and 1402 MBq. He injected the first patient with radiopharmaceutical aspirated from vial 1 and made up the required 18F activity with part of the contents of vial 2, resulting in an unnecessary thyroid dose of 131I. The error resulted from a lack of attention to the vial label, but the differences in the weight of the lead pot should have alerted the staff member to the difference. The availability of modern radiation protection instruments with gamma-spectrometric functions could also be helpful here.
- 3.Kits of 99mTc-MDP and 99mTc-DMSA were delivered by a local supplier in colour coded vials. The NM department used a different system of colour coding and did not put labels on the outside of the lead pots. A DMSA kit was placed in the wrong colour pot on a busy day exacerbated by low staffing levels, and the vial was not re-checked. As a result a 10 year old patient was injected with the wrong radiopharmaceutical.
A significant example of rare anaphylactic reaction following an IV administration of a radiopharmaceutical is given in a report from South Korea by Lee et al (2013). In terms of prevention, limited specific action is possible: the informative documentation given to a patient prior to any procedure, verbal information and queries, together with a well-organised capacity to react to unexpected events (including up-to-date training of the staff in Basic Life Support, readily available and properly functioning resuscitation equipment, easy communication with emergency departments), should contribute to minimizing consequences.
3.2.2. Patient preparation and administration of the radiopharmaceutical
Factors that can contribute to errors in the preparation of the patient and administration of radiopharmaceutical are listed in table 2. The importance of patient preparation can be illustrated by processes involved in an oncological PET-CT scan. 18F-Fluorodeoxyglucose (FDG) is used in staging and control of therapy for many types of tumour. FDG is not a specific tumour tracer, but an analogue of glucose and accumulates in many organs of the human body. The success of the technique depends on the whole process, including patient preparation (the patient should fast for at least 6 h). Following the injection the patient should rest in a comfortable environment and avoid any exercise while waiting (otherwise active muscles will take up the radiopharmaceutical), and for brain studies minimise stimulation (no reading, talking, or television), coupled with appropriate timing of image acquisition (the patient should be imaged about one hour after administration). Thus, all the processes involved in the procedure, including the radiopharmaceutical preparation, patient preparation, injection, the imaging protocol used, the setup and performance of the scanner, the acquisition and reconstruction parameters, and the interpretation of the result, are relevant in determining the quality of the imaging procedure. An example of an event affected by preparation was when a patient was asked to wait in an environment that was too cold. This caused activation of brown adipose tissue leading to accumulation of 18F-FDG that could obscure metastatic disease (Kiefer 2017).
Table 2. Factors that could contribute to an incident during patient preparation and administration.
| Component of the process | Possible errors |
|---|---|
| Patient preparation | • Incomplete instructions given to the patient or instructions not communicated accurately |
| • Fasting condition not checked before administration of radiopharmaceuticals | |
| • Pregnancy or lactation not verified before administration of radiopharmaceuticals | |
| • Biochemical tests omitted (e.g. glucose level) | |
| Stress testing/pharmacological | • Errors in the procedure. |
| stimulation | • Errors in timing. |
| Patient identification | • Identification not confirmed |
| • Lack of physical tools for identification (e.g. wristband) | |
| • Poor management of patients with same name | |
| Administration of radiopharmaceutical | • Errors in the procedure |
| • Wrong radiopharmaceutical/activity administered | |
| • Extravasation of injection (section 3.2.3) | |
| • Contraindications not checked | |
| • Wrong iodine therapy capsules or liquid administered, failure to check patient has consumed all capsules or liquid prescribed, or administration of two prescriptions to the same patient | |
Patient preparation may also involve administration of other pharmaceuticals. When radioiodine is administered as mIBG or other labelled compounds, the radiation dose to the thyroid through uptake of radioiodine can be substantial. Therefore the patient is given stable iodine prior to the radiopharmaceutical administration in order to prevent uptake of free radioiodine by the thyroid (Bombardieri et al 2010). This is particularly important for therapy administrations and for examinations of paediatric patients, as the thyroid is many times more sensitive to radiation during childhood and adolescence (ICRP 2007). Since iodine is bitter, children may be resistant to taking it, so diligence is required to ensure the stable iodine is swallowed in order to avoid giving an unnecessary radiation dose to the thyroid.
Imaging the wrong patient has been a recurring problem. Several examples of such incidents were reported in which patients had simply been asked to respond to their name. Staff should always obtain final confirmation of identification before administration of radiopharmaceutical to a patient, by asking a question requiring a positive response (e.g. asking them to give their date of birth or address). Many centres now use a three point identification method that requires the patient's name, date of birth, and address. Other methods that can assist in identification are numbered tickets, wristbands carrying the name of the patient or a bar code, use of photographic identification, and using technology similar to that adopted in passport control (Hakala et al 2012). Although the positive response approach should avoid administration of radiopharmaceutical in error to a patient with the same name, this has been a perennial problem, and some centres avoid booking patients with the same name on the same day; using a software rule in the radiology information system, making it impossible to add a second patient with the same name to the worklist for that day.
Examples of incidents during the administration phase of radionuclide therapy reported at the meeting, all of which should have been prevented if the necessary checks had been carried out, included the following.
- 1.An 131I capsule sticking to the vial and not being taken by the patient
- 2.A second radiopharmaceutical injection being administered in error to the same patient
- 3.A patient, who did not speak the local language, being given the wrong wrist band
- 4.Delivery of an activity lower than that prescribed because of a connector leak in the infusion device.
3.2.3. Extravasation of radiopharmaceutical
Extravasation of radiopharmaceutical in tissues adjacent to the injection site, may result in a significant unintended radiation dose to localised tissues. Extravasation involves diffusion and retention of a non-negligible amount of radiopharmaceutical in tissues localised around the injection site, giving them an unintended radiation dose the magnitude of which will depend on the type and energy of radiation emitted.
In diagnostic imaging procedures, extravasation is in general not expected to produce tissue reactions, but it is a pitfall that will affect image quality and quantitative assessment of uptake and may lead to image mis-interpretation (Sonoda et al 2012). The vast majority of events will not require any intervention, although some effects have been reported following extravasation of 201Tl that required follow-up (van der Pol et al 2017).
Tissue damage is rare but can potentially be severe where therapeutic radiopharmaceuticals are involved causing skin desquamation and necrosis (Williams et al 2006, Mouridsen et al 2007, Siebeneck 2008, Bonta et al 2011, van der Pol et al 2017) and effects have on occasions required surgical intervention (Baus et al 2018). Summaries of case reports were presented involving several different radionuclides during the meeting. These involved intravenous therapeutic administrations into the patients' arms via catheters, causing initial swelling followed by erythema several days or weeks later, and a few examples are given here:
- 1.A slow injection of 1.2 GBq of 90Y-Zevalin (ibritumomab tiuxetan) was given during treatment of a non-Hodgkin's lymphoma. Extravasation was noticed at the end of the procedure, and was thought to have occurred during the flushing procedure at the needle-puncture site. Erythema reported on day 2 extended over an area of 100 cm2 and the likely dose that would be accrued was estimated as about 50 Gy. Following the development of dermatitis on the patient's elbow during subsequent weeks, analgesics, and non-steroidal and anti-inflammatory drugs were used to relieve pain. Five months later there was extensive cutaneous necrosis and plastic surgery was performed using an antero-lateral thigh free flap to cover the wound after debridement (Baus et al 2018).
- 2.An intravenous infusion of 11.1 GBq of 131I-metaiodobenzylguanidine was given for treatment of metastatic cancer. Although swelling was noted at the time the injection catheter was removed, this was attributed to an allergic reaction and not identified as being induced by radiation until 4 weeks later when the patient reported a rash on his arm measuring 50 cm2. The amount of activity in the affected arm was estimated as 5 MBq one week after the injection, giving an estimated dose to the tissue of 12–16 Gy. The patient was followed up until the tissue damage resolved. In this case difficulty had been experienced in obtaining venous access, and the authors suggest that in such circumstance the access site should not be used to administer radionuclide therapy, and that a test be performed to ensure the absence of leakage at the site (Bonta et al 2011).
- 3.Another example was linked to an injection of 7.4 GBq of 177Lu-Lutathera (oxodotreotide). Taking account of the effective half-time in the extravasated volume (3.2 h), which was estimated to be 450 cm3 by imaging, the absorbed dose was assessed as 8±4 Gy and the patient was monitored during subsequent weeks.
- 4.There is an example in the literature of extravasation occurring during treatment with 223Ra dichloride for bone metastases from a primary prostate cancer. The patient noted extravasation of the radiopharmaceutical in his left dorsal hand during the first of three treatments via intravenous catheters, which resulted in local pain, erythema, and oedema. However, no imaging was performed. Four months later, a rapidly growing lesion developed at the injection site. Biopsy revealed an aggressive acantholytic squamous cell carcinoma and the patient then underwent micrographic surgery to treat the carcinoma. The patient had no previous history of skin cancer (Benjegerdes et al 2017).
The reports demonstrated that there are real risks of tissue reactions in patients from extravasation during therapeutic NM involving β-emitters and possible skin carcinoma where α-emitters are administered. In such cases in vivo imaging or radiation monitoring, depending on the type of radiation emissions, can play an important role after the administration, to confirm bio-distribution and/or exclude extravasation (Wright et al 2015). Skin cancers on the hands and forearms, where intravenous catheters are usually sited, are common. If physicians are not aware that extravasation of an injection has occurred they may not connect a lesion to such an event. Where extravasation of an injection of an α-emitter does take place, it may be appropriate for the patient to be monitored by a dermatologist for the possible development of skin cancer (Benjegerdes et al 2017).
The options after a severe extravasation are limited, even if approaches already in use in the case of extravasation of chemotherapeutics can be attempted (Mouridsen et al 2007, Langer et al 2009, Barrè et al 2013). Therefore steps for prevention should be taken, involving the choice of good quality needles and cannulas for intravenous administration, extensive training of staff in the injection technique, and the importance of stopping the administration immediately when a problem is recognised. Injections via an intravenous catheter can be preceded by a check of patency by flushing with saline solution, visually inspecting for swelling, and withdrawal of some blood (van der Pol et al 2017). For patients with difficult venous access a check could be made by injecting 99mTc-pertechnetate while imaging the patient's thorax to visually confirm systemic spread. Once an event has been identified, staff should endeavour to reduce the patient's exposure by enhancing clearance and dispersing the extravasated radiopharmaceutical as soon as possible. Methods that have been used include warming and massaging the extravasated region, and elevating the arm used for the injection (van der Pol et al 2017). Pharmaceuticals that may aid diffusion are available, although their value is debatable. Treatment should be provided for the tissue affected and the dose to localised tissues should be assessed by a qualified medical physicist. This will require counting and/or imaging of the region at different times as the patient is followed up in order to assess the amount of radionuclide that has accumulated locally, based on which the tissue dose and the extent of the damage can be assessed (Bonta et al 2011). Surgical intervention should be considered in more severe cases (Barré et al 2013, Baus et al 2018).
3.2.4. The imaging procedure in diagnostic NM
In order to achieve maximum benefit from NM imaging procedures, the image acquisition should be optimised. All technical parameters should be controlled through a QA/QC programme (IAEA 2009a and 2009b). Some of the factors that could be set incorrectly are listed in table 3. Omissions may or may not lead to an incident, but will lead to suboptimal procedures.
Table 3. Factors that could be neglected or set incorrectly during imaging.
| Component of the process | Possible errors |
|---|---|
| Gamma camera setup | • Wrong collimator/selection of radionuclide/scan duration/matrix size or other acquisition parameters. |
| • Omitted or inadequate daily QC tests may result in sub-optimal performance of the system (e.g. poor uniformity) or missed detection of faults (e.g. a photomultiplier tube not working). | |
| PET scanner setup | • Incorrect daily QC procedure (e.g. detector block is not working) |
| Multi-modality scans | • Wrong or poorly optimised CT protocol selected |
| In all NM imaging modes | • Sub-optimal balance between acquisition time and administered activity that ultimately determine image noise levels (count statistics). |
| System calibration | • Omitted or outdated calibrations of a SPECT system (e.g. energy, linearity, uniformity, centre of rotation) may result in poor image quality |
| • Omitted or outdated calibrations of a PET system (e.g. uniformity, cross-calibration between the activity meter and the PET scanner) may result in poor image quality or inaccurate standardised uptake value (SUV) results. | |
| • Omitted or outdated calibrations of gamma cameras and in vivo counting systems, e.g. thyroid uptake counters. | |
| Mechanical safety | • Patient has not been secured to the imaging bed. |
| • Moving components of the scanner have not been checked. | |
| • Tools, furniture or other objects lie in the trajectory of motion. | |
Optimisation is a matter of achieving a correct balance between patient dose, quality of the image achievable, and the needs of the patient, but inappropriate choices could be regarded as incidents. Choices include collimator selection, activity administered to the patient and acquisition time. The CT component of multi-modality scans (SPECT-CT and PET-CT) should make use of automatic tube current modulation and iterative reconstruction where available. CT scan optimisation is a crucial element in the training of NM technologists (European Association of Nuclear Medicine EANM (2017), Gilmore et al 2013, IAEA 2019, Popilock et al 2008, Swanson et al 2018, Waterstram-Rich et al (2011)) and should be included in all NM training courses in the future.
Calibration of all equipment connected with patient exposures should be a fundamental component of the QA program performed under the direction of a medical physicist (IAEA et al 2014). This includes uniformity, sensitivity calibration for different radionuclides (e.g. for 131I uptake measurements) and collimators, and for SPECT the centre of rotation and alignment of detectors. In PET imaging, the sensitivity calibration is a fundamental component of protocols in which quantitative or semi-quantitative indicators (SUV) are used (Kaalep et al 2018). Failure of the imaging equipment or loss of image data can lead to unnecessary radiation doses to patients. Steps that can reduce risks of failure include comprehensive acceptance testing when equipment is installed and effective QA/QC programmes to ensure performance is maintained, coupled with maintenance as recommended by the manufacturer and an equipment replacement programme.
3.2.5. Assessment of doses and risks from incidents in NM
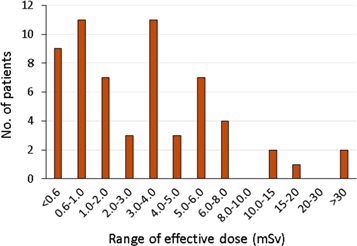
Incidents in therapeutic NM where radionuclide activities greater than intended are administered have the potential to result in significant exposures to the target organs, and doses to these organs and others that take up the radionuclide should be evaluated following such events. Coefficients to enable computed mean absorbed doses to individual organs from a wide range of radiopharmaceuticals for adults and children of various ages are available (ICRP 2015). For diagnostic NM examinations, effective doses from most procedural errors are in the range applied in normal diagnostic tests, amounting to a few mSv effective dose (figure 1). Staff working in NM are familiar with the activity levels for the radionuclides used, as they are dealing with these on a daily basis, so a knowledge of the activity administered incorrectly or spilt in a contamination incident will usually give a meaningful indication of the risk for staff on the ground, but reporting of radiation doses in terms of organ or effective doses for patient exposures is used in many countries, particularly when these are reported outside the department (Martin 2011). Absorbed doses to critical organs and effective doses can be estimated by multiplying the administered activity by the relevant coefficients (ICRP 2015) or use of software such as the NM Radiation Dose Tool developed by the Society of Nuclear Medicine and Molecular Imaging (Society of Nuclear Medicine and Molecular Imaging SNMMI (2018)) that has phantom models including paediatric patients of different age and pregnant patients at different stages of gestation. The effective dose applies to a reference person and provides a method for giving an indication of detriment from small exposures, but it is derived from risks averaged over a whole population. When the excess effective dose is much greater than 10 mSv a full evaluation using doses for individual radiosensitive organs and tissues may be appropriate. Local/regional agreement will be required on the level at which determination of organ doses is necessary. General terminology can be used to describe risks of cancer incidence, linked to the dose level (Martin et al 2017). Special attention is necessary when 131I is involved, as this gives a highly unbalanced bio-distribution, with strong uptake in the thyroid, and here the dose to the thyroid should be assessed.
Figure 1. Histogram showing the distribution of effective doses from 60 patient exposure incidents reported by 15 NM departments over a period of 13 years in the West of Scotland.
Download figure:
Standard image High-resolution image3.2.6. Paediatric patients
Paediatric applications of NM provide invaluable information in the diagnosis and follow-up of many disorders. However, there is limited information regarding the potential risk of adverse health effects from ionizing radiation in children at the dose levels most pertinent to diagnostic NM (Ernst et al 1998). Therefore, care must be taken to ensure that paediatric NM studies are performed appropriately. Radiopharmaceutical activities administered to children can vary widely despite the international efforts toward standardisation. Generally, it is considered prudent and good practice to perform paediatric studies using the lowest amount of radioactivity that will ensure the procedure has diagnostic value. The 'Pediatric Radiopharmaceutical Administration: Harmonization Guidelines', issued from expert consensus symposia (Lassmann and Treves 2014), reflect the appropriate application of NM in children, regarding the current understanding of radiation dosimetry in these patients. Appropriate selection of the administered radiopharmaceutical activity should relate to the patient population, available equipment, specific clinical requirements, and physician's judgment. Deviation from the administered activities listed in these guidelines should be considered appropriate when clinically indicated. Individual practitioners may use a lower administered activity if their equipment or software permits them to do so (Sheehy et al 2009, Stansfield et al 2010, Hsiao et al 2011). Under special circumstances, higher administered activities may be required in certain patients under the direction of the NM physician (Treves et al 2011). There is a scarcity of data on biokinetics and dosimetry for radiopharmaceuticals in paediatric nuclear medicine, so more reporting of results from paediatric studies in the literature is required to enable recommendations on administered activities to be further improved (Eberlein et al 2011, Sgouros et al 2011). This evolved approach should hopefully lead to a broad optimisation of paediatric radiopharmaceutical administered activities. Application of the guidelines should allow many paediatric NM patients to receive radiopharmaceutical doses lower than those traditionally given, resulting in an overall reduction of radiation exposure in these patients.
3.2.7. Pregnant patients
There is considered to be a finite risk of childhood cancer from foetal doses of the order of 10 mGy, so a higher level of clinical justification is required for examinations using ionising radiation of patients who are pregnant (ICRP 2000, ICRP 2003). Administration of NM therapy is generally contraindicated in pregnancy. Identification of any patient who may be pregnant is crucial, especially when 131I is to be administered, as this is taken up avidly by the foetal thyroid from 8–10 weeks gestation, and potential doses to the foetal thyroid of over 100 mGy can readily result from a maternal administration. In this case it might be necessary to confirm that female patients within the age band considered to be fertile are not pregnant with a pregnancy test. However, pregnancy is not a contraindication for a well justified diagnostic NM procedure, but pregnancy status should be known in order that an appropriate judgement can be made and risk to the unborn child and mother are properly evaluated and minimised. Generally, if the patient is known to be pregnant, administration of a radiopharmaceutical will not be performed, but an example of an exception is the case of suspected pulmonary embolism, which can occur in emergency situations, and in which the NM scan can provide vital information.
An inadvertent exposure of an embryo or foetus occurs from time to time and is of concern, due to the higher radiosensitivity (Russell et al 1997a, Berg et al 2008, Tran et al 2010, Stabin 2017, Zanotti-Fregonara and Hindie 2017, Stabin 2018). Notices in appropriate languages are displayed in patient waiting areas asking female patients to inform staff if they could be pregnant to help in reducing this risk (ICRP 2000, IAEA 2018a), but this does not eliminate the problem. An exposure may occur because either the patient did not know she was pregnant, or reported incorrectly about her status to the staff, for a variety of reasons, including familial/social issues and religious constraints. Performing a pregnancy test on every woman of childbearing age who is to receive a medical examination involving ionizing radiation is unreasonable, as benefits do not exceed the costs and inconvenience to the patient (Mossman 1985). In NM, pregnancy status should be ascertained for all radiopharmaceutical therapies, and it is advisable for all diagnostic procedures, in particular for those radiopharmaceuticals that are known to cross the placental barrier (IAEA 2018a). There should be agreed procedures for screening female patients and confirming the date of their last menstrual period prior to examination, and flow charts can provide a useful aid in the decision making process. In case of doubt, a pregnancy test or a determination of hormone levels to assess menopausal status can be carried out.
In the event of an exposure of the embryo/foetus, a qualified medical physicist should estimate the foetal dose to provide guidance to the mother and for medico-legal reasons. Tabulated data on foetal doses from radionuclide intakes by the mother (ICRP 2001), as well as web resources (SNMMI 2018), are available. By applying the data on foetal doses from radiopharmaceuticals in common use shown in table 4, it is apparent that doses to the foetus from activities normally administered for diagnostic tests vary according to the stage of pregnancy, but are all in the range 1–10 mGy. It is almost impossible for a diagnostic study to result in a foetal dose above the known thresholds for acute foetal damage (Russel et al 1997a, 1997b, Stabin 2017).
Table 4. Doses to the embryo/foetus per unit activity from administration of a selection of commonly used radiopharmaceuticals during different stages of pregnancy (Russel et al 1997a, 1997b, Stabin 2017).
| Stage of pregnancy | Early | 3 Month | 6 Month | 9 Month |
|---|---|---|---|---|
| Radiopharmaceutical | mGy/MBq | mGy/MBq | mGy/MBq | mGy/MBq |
| F-18 FDG | 2.7E-02 | 1.7E-02 | 9.4E-03 | 8.1E-03 |
| I-131 Sodium Iodide | 7.2E-02 | 6.8E-02 | 2.3E-01 | 2.7E-01 |
| Tc-99m DMSA | 5.1E-03 | 4.7E-03 | 4.0E-03 | 3.4E-03 |
| Tc-99m HEDP | 7.2E-03 | 5.2E-03 | 2.7E-03 | 2.4E-03 |
| Tc-99m MAA | 2.8E-03 | 4.0E-03 | 5.0E-03 | 4.0E-03 |
| Tc-99m MAG3 | 1.8E-02 | 1.4E-02 | 5.5E-03 | 5.2E-03 |
| Tc-99m MDP | 6.1E-03 | 5.4E-03 | 2.7E-03 | 2.4E-03 |
| Tc-99m MIBI-rest | 1.5E-02 | 1.2E-02 | 8.4E-03 | 5.4E-03 |
| Tc-99m MIBI-stress | 1.2E-02 | 9.5E-03 | 6.9E-03 | 4.4E-03 |
| Tc-99m Pertechnetate | 1.1E-02 | 2.2E-02 | 1.4E-02 | 9.3E-03 |
3.2.8. Precautions for patients during breast feeding
Precautions are necessary for female patients who are breast feeding and NM facilities should have in place a means for ensuring that breast feeding status is known (ICRP 2004b, IAEA 2018a). The first approach is through posting of clear signs, in languages that can be understood by the range of people using the NM facility. Such signs should be posted widely in the facility, including waiting rooms and cubicles. The second approach is simply asking the question 'Are you breast feeding?'. Female patients should be advised that breast feeding is generally contraindicated after therapeutic administration of some radiopharmaceuticals, because of both the external irradiation of the suckling baby and the potential excretion of radioactivity through the breast milk. In diagnostic procedures, depending on the radiopharmaceutical and the administered activity, breast feeding may need to be interrupted for a period or even stopped following the administration. A temporary cessation in breast feeding coupled with storage of milk expressed prior to the examination and discarding of expressed milk for a few hours or days afterwards is sufficient precaution in most cases. (Stabin and Breitz 2000, Leide-Svegborn 2010, Leide-Svegborn et al 2016). Recommended approaches for a range of diagnostic procedures are given in appendix III of SSG-46 (IAEA 2018a). Cessation of breast feeding will be required for the administrations of most 131I and 123I-labeled radiopharmaceuticals for protection of both the infant and of the mother's breast tissue (ICRP 2004b, 2015). An interruption time of 4 h is recommended for radiopharmaceuticals from which the level of free pertechnetate in breast milk is not negligible (IAEA 2018a). For radiopharmaceuticals not included in the recommendations, the period of interruption of breast feeding should continue until the radiopharmaceutical is no longer secreted in an amount estimated to give an effective dose >1 mSv to the child.
3.3. Staff exposures, including contamination events
NM staff may receive radiation doses through a variety of routes involving either or both external and internal exposures. Moreover, since the introduction of PET imaging and use of hospital-based cyclotrons for producing radionuclides, potential incidents linked to operation of cyclotrons need to be considered.
3.3.1. Cyclotron operation
The range of incidents that could occur with cyclotrons for the production of PET radionuclides is broad and might involve the operators or the environment, but the topic can only be treated superficially in the present paper. The high level of sophistication of cyclotrons implies that engineering controls are fundamental in reducing the risks. As an example, the risk of starting an irradiation with an operator trapped within the vault (bunker) should be reduced to very low levels of probability, since dose rates may be greater than 10 Sv h−1 for a non-self-shielded system. To satisfy this requirement, the engineered safety systems should be designed according to international standards and include appropriate redundancy, to minimise the probability of any fault in components, such as micro-switches and interlocks, sensors, radiation resistant video chambers, etc, and achieve the desired safety integrity level (IEC 2010, 2019). Among the incidents that may involve a release of radioactivity into the environment, the scenario of a 'target rupture', which is the simultaneous breaking of both target foils, is not in reality a significant issue, since the architecture of cyclotrons, with targets fitted in the high vacuum chamber of the accelerator, means that there is substantial containment of the radioactivity inside the vacuum chamber itself. However, minor leakages of limited but significant quantities of radioactivity may arise for incomplete sealing of valves and tubing used in the delivery of the radioactivity from the target to the laboratory. This issue cannot be solved in a simplistic way (e.g. filtering), since several compounds cannot be trapped efficiently. Preventive measures, such as periodic leakage tests, delay lines for the release of effluents, or containment systems have proven to be effective (Marengo 2008). Planned releases of 41Ar, due to neutron activation of natural 40Ar contained in air during irradiation, are not radiologically relevant (Infantino et al (2015), Cicoria et al (2017)).
3.3.2. External exposure from radioactive materials
Radiopharmaceutical preparation is a complex process, particularly in PET centres, and close co-operation between radiopharmacists and medical physicists/radiation protection experts is necessary to plan the operation of a PET facility and ensure safety is optimal (Sutton et al 2012). Poor planning of patient workflow and work procedures that do not make sufficient use of protection systems can increase staff doses considerably. Contingency plans should be agreed beforehand for proper mitigation if any failure occurs.
The fingers are irradiated when radiopharmaceuticals are injected and doses can be significant if syringes are not shielded (Whitby and Martin 2005) or fingers are placed on the needle during an injection (Martin et al 2019). Doses from administration of β-emitting radionuclides such as 90Y and 32P and even 18F can reach the level where dose limits are exceeded (Vanhavere et al (2012)) or even cause tissue reactions (Cremonesi et al 2006). Automated modules should be used for dispensing and drawing up radiopharmaceuticals wherever possible, especially with PET radiopharmaceuticals for which the benefits have been widely demonstrated (Wrzesień and Napolska 2015). In the case of semi-automatic or manual separation-injection systems, the syringes with Luer lock are preferable. Precautions to minimise the risk of high doses where personnel need to handle radiopharmaceuticals are listed below.
- Keep vials in pots with appropriate protection during withdrawal of radiopharmaceutical
- Use handling tongs or forceps to pick up vials and other sources
- Use vial and syringe shields of appropriate material [for γ-emitters: W or Pb, for β-emitters: PMMA, followed by Pb to reduce bremsstrahlung radiation, for α-emitters such as 223Ra with a mixture of emissions including γ-rays, standard shields of W or Pb are again appropriate (Amin et al 2014)] and thickness for drawing up and giving injections
- Use good quality needles and cannulas to allow insertion prior to injection of radiopharmaceutical
- Remove contaminated gloves or any skin contamination as quickly as possible.
The potential doses to the fingers and the need for finger dose monitoring should be considered. Dosemeters should always be used when handling the β-emitting radionuclide 90Y and considered for other therapy radionuclides (Martin et al 2019).
3.3.3. External exposure from patients
Extensive training of staff concerned with patients undergoing radionuclide therapy is important in order to minimise their doses. Technical staff should explain procedures to patients prior to administration of radiopharmaceutical and nursing staff persuaded of the importance of maintaining a safe distance from patients whenever practicable. All personnel should be given training in the procedures to be followed in situations where there is an equipment failure that could lead to further exposure.
Staff members in other departments may also receive unnecessary radiation doses, if procedures are not planned effectively. Patients injected with radiopharmaceuticals become radiation sources, so they should not be sent to other departments during the period when they present a risk to others. For example, if practicable avoid sending a patient for an ultrasound scan during the period before NM imaging when they are waiting for radiopharmaceutical distribution to be established, because the sonographer will be in close contact with the patient.
3.3.4. Contamination with radiopharmaceuticals
A risk from handling unsealed radionuclides is possible contamination and intake of radiopharmaceutical into the body. The risks can be minimised by taking basic precautions such as only handling radionuclides in facilities built for the purpose, wearing appropriate protective clothing, and not leaving vials containing radioactive liquids in locations where they can easily be knocked over. Vials should preferably be closed, i.e. have a rubber and metal closure, to avoid spills if knocked over. In addition a spill kit should be available to deal with any event that does occur. Information about good laboratory practices that should be followed is contained in IAEA (2018a).
Most radiopharmaceuticals for diagnostic applications are administered by intravenous injection. Any blockage in the syringe could result in radiopharmaceutical being sprayed over the operator or patient. Risks that a connection has not been made properly may be higher where a needle and cannula or butterfly are used. In addition, there is a risk of needle-stick injury if an attempt is made to recap a syringe. Basic good practice following the steps set out below can reduce risks:
- Physicians, technologists and nurses, as appropriate, should have been trained in injecting radiopharmaceuticals and assessing the patient's venous vascularity
- Ensure that connections between syringes and needles are secure
- Do not exert excess pressure if the resistance is high during an injection
- Flush needles and cannulas with saline before starting an injection
- Dispose of the syringe and needle assembly in a sharps container after use
- Seal sharps containers as soon as they are full and arrange for immediate disposal
- Use safe recap devices if measurement of residual activity in the syringe is required.
The recommended steps listed above are particularly important when radionuclide therapy activities are injected. The preference for oral administration of 131I therapy activities is to use capsules when possible. Liquid may be split or patients may cough when drinking solutions, but they may vomit when swallowing a capsule, so the choice of method should take the patient's condition into account. There is also a cost differential involved in the choice, which will be significant for some countries. Possible steps to limit the risk of a contamination incident include: ascertaining the patient's capacity to swallow and training him/her by carrying out a test 'mock' administration.
Methods that can be used for clean-up of spills and decontamination, together with items that should be contained in the spill kit are described in other texts (e.g. IAEA 2006a, Martin 2014, IAEA 2018a, 2018b). If the skin becomes contaminated from routine procedures or during any decontamination following a spill, absorbed dose rates at the skin surface can be significant. If this is the case, an evaluation of the skin dose in terms of Hp(0.07) should be undertaken (Covens et al 2013, Martin 2014).
Aerial contamination is a potential problem with iodine radionuclides that can readily vaporise (Brudeck et al 2018). Significant quantities of radioactive iodine should be handled within laminar flow cabinets. Aerial contamination may also result from apparatus delivering gaseous radiopharmaceuticals if it is not used effectively by a patient. Procedures for lung ventilation studies should include checks to ensure that the mask fits the patient and that they are able to tolerate wearing it for the duration of the procedure. Low levels of personnel contamination have been reported (Leners et al 2011) in cases of inadequate patient collaboration. In addition to the risk of release to the environment, such incidents will lead to unreliable ventilation study results.
3.3.5. Contamination from body fluids
The body fluids of patients to whom a radiopharmaceutical has been administered will be radioactive and represent another source of potential contamination. The period over which there is a risk will depend on the physical half-life of the radionuclide and the biological half-life of the radiopharmaceutical involved. The risk from most diagnostic procedures, especially those involving 99mTc, will be relatively small and trivial after the first day, but that for therapy administrations may persist for several days or longer.
Oral 131I therapy administrations are potentially more serious, as vomit will be highly radioactive during the period immediately following administration. If a patient were to fail to swallow the radio-iodine or were to regurgitate it in an area of public access, this could create a significant incident, requiring the area to be cordoned off for decontamination. It is good practice to have a dedicated radiopharmaceutical administration room, with a sealed floor and coving, wash-basin/sink, drip trays, shields, injection materials, and a spill kit, so that if an accident occurs, only this room is contaminated and the contamination can be removed in a controlled manner without interfering with other activities. Such a room can be used for administering both injected and oral activities, and if any 131I therapy patients are considered to present a risk following administration, they can be asked to remain within the room for 15–20 min before being discharged, or returning to their ward, depending whether they are out- or in-patients.
There is potential for contamination associated with treatment of incontinent patients and a risk assessment should be carried out, when considering how a therapy is to be undertaken. Sanitary pads may be adequate for patients undergoing diagnostic NM tests, but are not desirable for therapy patients. Catheterisation should be considered, but this has associated problems, so carers need to be aware of the necessary precautions. Leakage can occur from around a catheter, from a split urine bag, or from inadvertent opening of the tap on the bag. Care is needed in protecting urinary bags when patients are moved. Frequent emptying of the bag and basic biologic protection measures normally provide adequate safety, but both staff and carers need to be aware of the precautions to be taken and the potential risks.
3.4. Exposure of the public
3.4.1. Exposure from contact with patients
Particular attention needs to be paid to limiting exposures of the public, since they will be less aware of potential risks and unfamiliar with precautions required when dealing with radiation sources. The criteria for hospitalisation and release of therapy patients should aim to ensure that effective doses to members of the public who may come into contact with the patient are less than the public dose limit of 1 mSv/year and preferably less than a dose constraint of perhaps 0.3 mSv. The decision to hospitalise or release a patient after therapy should be made on an individual basis considering factors such as the residual activity in the patient, the patient's wishes, family considerations (particularly the presence of children), and environmental factors. Particular care is required for 131I therapy where the patient may come into contact with children (ICRP 2004a, IAEA 2009c, Han et al 2014), since the uptake of iodine in thyroids of children is high and risks of radiation induced thyroid cancer induction are greater in children and adolescents (Biological Effects of Ionizing Radiation BEIR (2006), ICRP 2007). Another specific case is the treatment of paediatric patients (e.g. 131I-mIBG for neuroblastoma), as the parents assisting the young patient can be significantly exposed. Carers and comforters who may be in close contact with an individual patient represent a special case to whom a source-related dose constraint of a few mSv per episode may be applied, with the consent of the person involved (IAEA et al 2014). Recommendations should be given on the delay before the patient returns to work, which should take into consideration the patient's work environment. For example a person working largely in isolation, e.g. a truck driver, can return to work earlier than someone working in close proximity to others, e.g. on a production line. Specific written instructions giving radiation protection precautions to be taken should be provided to the patient and their family.
3.4.2. Exposure from release of radionuclides to the environment
Solid or liquid wastes released from therapy patients, either during hospitalisation or later at home, have the potential to induce the occurrence of 'innocent accidents/incidents' through unforeseen accumulation of radioactivity. An example of such an exposure is that of workers at sewage treatment plants through accumulation of radioactivity in components of the waste. For diagnostic NM, there is no need for the collection of patient excreta, such as urine containing 131I, and ordinary toilets can be used. For therapy patients, policies vary for different States, but in principle the approaches used follow the dilution or decay methodologies. Dilution is achieved by designing the outflow from the radionuclide therapy suite so that it passes into the hospital drainage system as near as possible to the point where this enters the sewage system, where it is diluted by the large volume of effluent coming from the rest of the hospital, while decay might involve collecting and storing excreta through designing facilities with drainpipes terminating in a delay tank from which the effluent can be released at a later date (ICRP 2004a, IAEA 2009c, IAEA 2013). Granting of hospital licences for disposal of liquid radioactive waste should take account of an assessment of the radiological impact on the environment. This is usually performed by evaluating doses that representative members of the critical groups outside the facility might receive through their work, leisure activities, or food intake from the disposals using models adapted to the local situation. These would normally aim to keep doses to all members of the public substantially lower than any dose constraint. In most situations, it is better to dilute and disperse the waste activity in a continuous sewerage system, rather than to concentrate and store excreta to decay before disposal. Some precautions may be required where sewerage systems allow rapid processing of effluent with subsequent mixing with river water or usage for irrigation of land used for growing vegetables (ICRP 2004a, IAEA 2009c, IAEA 2013).
3.5. Management of radioactive materials
This section deals with the management of radioactive material, and processes and facilities for disposal of radioactive waste.
3.5.1. Loss of radioactive material
The holding, use, and disposal of radioactive material are regulated because of the potential hazard to the public (IAEA et al 2014). Control within a facility requires systems for ordering the radioactivity, receiving and documenting when it is delivered, and ensuring that it is transferred efficiently to secure, dedicated storage. Persons should be identified who take responsibility for all aspects of the process and ensure records of radionuclide activities are maintained. They should be authorised to carry the tasks out, so that there can be no unauthorised entry of radioactivity into the facility. This includes hospitals where radioactive materials are not used routinely, but may perform occasional tests with low activity sources, e.g. Schilling Tests with a few tens of kBq of 57Co. Staff must also be aware of special arrangements for disposal, so that radioactive waste is not inadvertently placed in the clinical waste stream. The following precautions may aid in reducing the risks of such incidents.
- Robust operational systems for transfer of radioactive material to secure storage with appropriate signage on entry to a department.
- Storage of radioactive waste in clearly labelled containers and agreed systems for its removal.
- Responsibilities relating to ordering, delivery, storage and accounting for radioactive sources clearly stated and understood by staff involved with clear lines of responsibility.
- Identification of staff members with trained deputies, who ensure that procedures are followed and take responsibility for all aspects of radionuclide accounting.
- Training given to all staff who deal with any aspect involving radioactive material, including procedures for nursing patients, and dealing with waste and laundry.
- All training should be updated periodically and included in induction training.
3.5.2. Long half-life radionuclide impurities
Long half-life radionuclide impurities in both diagnostic and therapeutic radiopharmaceuticals, especially in newly introduced ones, raise a series of issues in safety and radiation protection. This has been considered by the NM community with respect to radiation protection of the patient, but it also has implications from a radioactive waste view-point. The expansion in the usage of PET has led to an increase in the number of radiopharmacies synthesising radiopharmaceuticals. PET radionuclides have very short half-lives, but may contain long lived impurities from the production process. For example, small amounts of the radionuclides 57Co, 54Mn and others are produced during the production of 18F by the interaction of the proton beam with the metal foils used to seal the targets (Marengo et al 2007). These radionuclides are partially transferred to the liquid target material (enriched H218O) and removed during synthesis of the radiopharmaceuticals. The chromatographic columns and cassettes used in the purification process become contaminated and need to be managed accordingly, taking account of the longer half-lives of the radionuclides involved. Similar considerations apply to the breakthrough of 68Ge in 68Ga eluates (Belosi et al 2013) and the impurity 177mLu in 177Lu radiopharmaceuticals.
3.5.3. Disposal of liquid radioactive waste
The bulk of the radioactive waste from patients to whom radiopharmaceuticals have been administered takes the form of liquid waste disposed through the drainage system. Precautions should be taken to minimise public exposure from radionuclides in the environment, discussed in section 3.4.2, but there is also a need to reduce exposures to staff within the facility from disposals. NM departments should provide patient toilets near the waiting area, and separate toilet facilities allocated for patients undergoing therapies. Smaller amounts of waste will arise from preparation of radiopharmaceuticals, in vitro procedures or unused patient administrations, and these are usually discharged into sinks designated for the purpose. Such sinks should be connected directly to the sewer system, wherever possible, and use of traps avoided. A problem encountered linked to the mis-disposal of paper towels in therapy wards was blockage of toilets, which was overcome with installation of air-driers.
Persons can be exposed from radionuclide waste carried down pipes, although dose rates are unlikely to be high enough to require special precautions unless liquid is allowed to accumulate. Routing of pipework for liquid waste should be planned at installation to minimise such risks and arrangements checked before a procedure is undertaken for the first time. Leakage of a pipe could result in an incident and it may be appropriate for some pipes carrying radioactive waste to be checked more frequently for leakage. A radiation exposure incident could result from maintenance carried out by a plumber, who does not take necessary precautions because he/she is unaware that a pipe carries radioactive waste. Large radionuclide therapy departments have considered the installation of radioactive waste tanks to delay discharge of radioactive waste into the public sewer, thereby reducing radionuclide activity levels (section 3.4.2). However, the radiation doses delivered to tank maintenance workers coupled with risks of leakage or other accidents means that such steps are seldom justified (IAEA 2013).
Additional precautions should be taken when considering disposal of longer half-life radionuclides in liquid form, and pipe runs checked for leakage prior to the disposal. The problems resulting from disposal of contaminated building material and consequent doses to personnel if a leak occurred should be considered in the risk assessment. Checks and controls relating to liquid waste disposal carried out at installation should include the following.
- Drainage pipes carrying liquid radioactive waste should have a steady downward gradient, without sections of horizontal pipe where liquid waste could collect. This can be checked by tracking movement of a bolus of 99mTc using a contamination monitor.
- The sink designated for disposal of radioactive waste should be the lowest in the pipe run to avoid any regurgitation of radioactive liquid in the event of a blockage.
- Easy access should be provided to junctions where leaks could occur from pipes with a shallow gradient, in order to allow periodic inspection.
- All sections of pipes carrying liquid radioactive waste should be labelled with the radiation sign.
- A radiation protection expert should be contacted before a plumber carries out maintenance on pipes used to carry liquid radioactive waste.
4. The investigation and follow-up of incidents
4.1. Procedures for reporting and investigation
Radiation safety policies of hospitals providing NM services should define responsibilities for every aspect of the NM process within a department, and this should include arrangements for dealing with incidents and near-misses. Hospitals and health institutions should encourage the reporting of incidents and near misses, and investigate them promptly looking for causes. Various methods and platforms are available and many hospitals use software tools for online reporting, which facilitate efficient data collection. Despite these efforts, there is still resistance to reporting in some organisations (Lawton and Parker 2002), as health professionals sometimes feel that such information may be used in criticizing their competence.
When an incident or near miss is reported, local staff who have responsibility for radiation protection should carry out an investigation as soon as possible and prepare a preliminary report. The service manager (registrant or licensee, according to GSR Part 3 (IAEA et al 2014)) with advice from the qualified medical physicist should then decide whether a more detailed investigation is necessary with the aim of analyzing the causes of the unplanned event. Depending on the type and severity of the incident this may involve setting up a multidisciplinary working group. The procedures that should be followed in the investigation are similar to those described by Martin et al (2017) for medical incidents in radiology. The investigation has three main purposes.
- 1.To assess consequences for the patient(s), staff, or others involved, and provide any additional healthcare or advice required. This should include an estimate of the doses received and the dose distribution within the patient or person(s) involved. The NM physician should inform the referring medical practitioner and the patient or the patient's legal authorised representative of any unintended or accidental medical exposure.
- 2.To identify the cause of the incident, indicate corrective actions required to prevent recurrence, and make changes to correct any deficiencies in the system to minimise the likelihood of similar incidents occurring in the future.
- 3.
If an incident results from an error made by staff or other failing within a department, the persons carrying out the investigation should conduct face-to-face interviews with those involved, to determine the root causes and identify contributory factors. A 'no blame' environment should be created while balancing safety and accountability. A written report of the results of the investigation should then be prepared that includes details of what occurred, why it happened, and any deficiencies in procedures or staff training that contributed to the incident. Any improvements that could be made to minimise the risk of a similar incident occurring should be identified. The service manager should be given the responsibility of ensuring that remedial actions required are undertaken to prevent recurrence.
4.2. Follow-up and learning lessons from incidents
Active factors that lead to an incident may be due to decisions linked to poor staff alertness and awareness. However, there are often underlying latent factors within a department that contribute to human errors, such as poor communication, too many interruptions, staff shortages, lack of clearly defined responsibilities, inadequate storage facilities, or unsatisfactory product labelling, so efforts should be made to identify these. Learning from errors that do occur is a key factor in reducing the risk of repetition of similar mistakes, or at least decreasing their severity and maintaining and improving the quality of healthcare. So, when factors that have contributed to an incident are recognised, changes should be introduced to address deficiencies and a manager given responsibility for this within departmental procedures, with a requirement to ensure that agreed modifications are implemented. Changes may include remodelling procedures, additional training of staff, or introduction of extra checks, all aimed at trying to prevent occurrence of a similar incident in the future. There may also be adjustments to the organisation of the service to improve clarity in responsibilities and all relevant stakeholders should collaborate in the process. On completion of the investigation experiences should be shared among staff through safety meetings and reviews, so that the whole department is aware of any changes that have been made to procedures and the reasons behind them.
In addition to the internal reporting within the NM department, a large scale gathering of experiences from events is beneficial in looking for trends that extend beyond a facility and so helping to improve safety culture. The Bonn Call-for-Action (IAEA/WHO 2012) asked stake holders to 'implement and support voluntary educational safety reporting systems for the purpose of learning from the return of experience of safety related events in medical uses of radiation'. There are national voluntary reporting schemes in some countries as well as mandatory arrangements fulfilling requirements of national regulations. The Meeting recommended upgrade of the IAEA voluntary reporting and learning system SAFRON (IAEA 2017) to include incidents in radionuclide therapy which can have more serious consequences. Effort is required to develop the systems to the required level, but given the variety of professionals working in NM with physicians, clinical scientists, technologists and other health professionals, NM can be one of the driving disciplines in a process of increasing awareness and proficient use of state of the art technologies to develop a culture of sharing lessons learned and building a safer environment for patients.
4.3. The benefits of audit
Comprehensive clinical audits provide a unique opportunity for NM services to improve the quality of their practices and ensure the safe and effective use of radiopharmaceuticals. The local radiation protection service or NM staff from another facility might carry out audits on behalf of hospital management. Regular and independent clinical audits of the programme of QA/QC for medical exposures are a requirement of national and international standards (IAEA et al 2014, European Council EC (2014)). A clinical audit is an independent external assessment of how the actual clinical practice compares with good practice. By being an unbiased peer review evaluation, areas for improvement can be more easily identified with a view to bringing services to the internationally accepted level.
Clinical audits review the quality of all elements of a NM service, and involve evaluation of data, documents, procedures and resources to check performance against standards. Their purpose is to improve the quality of patient care, promote the effective use of resources, enhance the provision and organisation of clinical services, and further professional education and training. Due to the multidisciplinary nature of NM practice, comprehensive clinical audits include clinical components, as well as aspects related to medical physics, radiopharmaceutical preparation, and equipment performance. Aspects of radiation safety and patient protection are an integral part of the process.
The IAEA have developed a structured peer-review process called Quality Management Audits in NM Practices (QUANUM) and published a guidance document describing this methodology (IAEA 2015). NM services can use this publication as a tool for carrying out self-assessments aiming to promote good clinical practices by identifying improvements that can be implemented using their own resources. It contains a series of checklists covering different aspects of a NM service, including radiation regulations and safety, patient radiation protection, and QA/QC of equipment. These can be used in verifying whether all the necessary procedures to prevent incidents are available and adequate, and any underlying deficiencies can be identified in the feedback. Several IAEA Member States and NM services have benefited from this voluntary QUANUM audits process, organised in the frame of the IAEA Technical Cooperation programme. QUANUM audits are carried out by a multidisciplinary team that includes a NM physician, medical physicist, radiopharmacist and NM technologist. The routine practice of auditing, according to a standardised methodology, taking account of experience from previous incidents, has proven to be helpful both in the prevention of incidents, and ensuring that there is a response when one occurs (Dondi et al 2017a, 2017b and 2018).
4.4. The role of the regulators
The regulatory body has the responsibility to establish requirements for safety assessments as part of the licensing or authorisation process for a medical radiation facility. This should include considerations of occupational, public and medical exposure, and the possibility of any potential exposure, including unintended or accidental medical exposures (IAEA et al 2014). The regulatory body ensures that a system is put in place and all practical measures are taken to prevent such exposures, and, if such an exposure does occur, that it is properly investigated and corrective actions are taken. The regulatory body should provide guidance on which events should be reported to them, and should be proactive and encourage medical radiation facilities to participate in relevant international or national anonymous and voluntary safety reporting and learning systems. The national regulatory body, the health authority, and the relevant professional bodies should communicate and cooperate in working towards establishing the infrastructure necessary for preventing incidents and improving radiation protection and safety, and for effectively integrating it into the overall management system of healthcare organisations. Patients' representatives also need to be involved (IAEA 2018a).
Regulators must be attuned to clinical practice to be effective in their regulatory functions. Furthermore medical radiation practitioners expect their regulators to be trustworthy and show a high level of integrity for them to feel comfortable in the regulatory world to which sometimes they are not accustomed. This trust opens up more robust communication channels that allow discussions about incidents, near misses, and lessons learnt to occur on a regular basis. Regulators should provide sound guidance to clinical facilities especially where physics support may be minimal. These facilities should not fear that they would be prosecuted for any unintended or accidental radiation exposures to patients, staff or public, as this could lead to a reluctance to acknowledge and report incidents and will affect the implementation of prevention measures. Regulators should assist in determining the root causes of incidents and help in providing a course of action to prevent further occurrences in the future.
5. Conclusions
There is a wide range of radiation incidents that can occur within NM, mainly but not limited to radiation aspects. Furthermore, since patients have radioactive material inside their bodies the radiation risk, related to external exposure and/or contamination, continues after completion of the medical procedure. Therefore there is also a potential for members of staff or the general public to be exposed. An IAEA Technical Meeting was held on prevention of unintended exposures and accidents in NM, which highlighted the need for guidance on how incidents should be tackled and aid in their prevention. This paper was prepared based on topics covered during the meeting, and considered incidents in radiopharmaceutical preparation, administration to patients, and potential exposures following a procedure, as well as aspects involved in management of radioactive materials. An attempt has been made to identify errors that could occur during different phases of NM procedures and to highlight things that should be considered when trying to prevent incidents occurring. Special attention has been paid to therapeutic NM procedures, as these have the potential to cause more serious effects in patients. Approaches to reporting, investigation, and follow-up of incidents are described, together with the need to ensure corrective action is taken to address any deficiencies, and the value of periodic audit in evaluating systems in place. The Meeting also recommended that the IAEA voluntary reporting and learning system SAFRON be upgraded to include incidents in radionuclide therapy which can have more serious consequences.
Acknowledgments
The authors wish to acknowledge the contributions from Klaus Bacher, Aurele Desbrée, Alesandra Karoussou-Schreiner, Tadeu Takao Almodovar Kubo, Willem Oyen, Ivo Rausch, Jelena Subina, Bruce Thomadsen, Jorge Isidoro and others attending the IAEA Technical Meeting in providing examples of incidents, which have been included in this paper.