Abstract
The Penning-trap mass spectrometer ISOLTRAP has received ISOLDE beams for the past 30 years! Following the move of ISOLDE to the proton-synchrotron booster site, ISOLTRAP has pioneered almost all of the techniques now associated with on-line ion trapping and manipulation for precision measurements of atomic masses. After an introduction on physics motivation, a brief history and description of the ISOLTRAP spectrometer is given, followed by an overview of the numerous developments and scientific results achieved since the previous ISOLDE Laboratory Portrait.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction: mass measurements and Penning traps
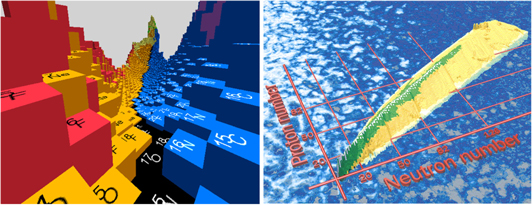
Mass measurements are almost as old as nuclear physics itself. The binding energy—determined from the mass via the famous relation E = mc2—reflects features that contribute greatly to the understanding of the nuclear structure such as nucleon pairing and deformation. Masses gave us the first clue that nucleons occupied atom-like quantized orbitals giving certain nuclides enhanced stability when the orbitals are filled with so-called 'magic' numbers. One of the central pursuits of today's nuclear-physics experiments is to probe the extent to which these magic configurations remain so in the most exotic nuclides. Figure 1 (left) shows the binding energy per nucleon over the nuclear chart, which illustrates the most important metaphor in nuclear physics: the valley of stability. The binding energy defines the very limits of nuclear stability—the so-called drip lines. Figure 1 (right) shows the two-neutron-separation energies evoking a sea-to-sky metaphor where the stable species represent majestic mountains with a beach defining a drip line before an unbound sea.
Figure 1. The mass surface formed using (left) experimental binding energy per nucleon and (right) two-neutron separation energies (experimental values in green).
Download figure:
Standard image High-resolution imageSince the binding energy is the net effect of the interactions at work within the nucleus, the different structural contributions must be disentangled using theoretical models. Despite the diversity of theoretical approaches, no model has yet satisfactorily predicted masses of the most exotic species known. Measurements therefore continue to play a decisive role in guiding and refining theory.
The history of mass measurements dates to the same epoch (and laboratory) as the discovery of the nucleus. Aston's discovery of the binding energy by mass spectrometry allowed his astrophysicist contemporary Eddington to identify the source of energy in stars, quelling a debate about the age of the Sun and the Earth that raged at the time. Since the mass determines the amount of energy available for a reaction or decay, it plays a key role in the synthesis of the elements in stars.
The binding energy is a mere percentage of the total mass of the nucleus and hence, very hard to measure. Complicating the job at hand, exotic nuclides are very short-lived and difficult to synthesize. Indeed, we have reached the drip line only for light species (green points in figure 1, right). After Aston's work with stable species, the first large data sets that included unstable isotopes came from reactions and decay spectroscopy. These techniques were associated with the invention of particle accelerators and provided all mass data until the advent of on-line mass spectrometry, pioneered by the Orsay group of Robert Klapisch through the 1970s and 1980s, at the ISOLDE facility where the low energy and superior optical beam properties were well suited to precision measurements.
Since its development that led to the 1989 Nobel Prize in physics, the Penning-trap mass spectrometer has emerged as the tool of choice for mass measurements. ISOLTRAP, located at the CERN-ISOLDE facility, is the pioneering instrument for mass measurements of exotic species online. The impact of ISOLTRAP—and the many Penning traps that have followed for mass measurements at other facilities—has been documented through reports to the ENAM and later ARIS conference series (Lunney 2015).
Mass measurements require superior precision, high sensitivity, and the particularly challenging step of purifying the isobaric beams produced from unselective nuclear reactions that add overwhelming contamination. A very recent highlight in the field of mass spectrometry is the integration of a time-of-flight mass separator into the ISOLTRAP setup and in the course of this work, a new application was developed by coupling the device with the ISOLDE laser ionization facility RILIS to determine nuclear charge radii (detailed in section 4).
2. ISOLTRAP and its illustrious history at ISOLDE
The authoritative source for the early days of ISOLTRAP is the review by Kluge (2013), who initiated the use of Penning traps in nuclear physics by starting the ISOLTRAP mass program at ISOLDE. The first Penning-trap mass measurement of a radionuclide was performed by ISOLTRAP in 1987, not long after its installation at the ISOLDE II facility (Bollen et al 1987). After its move to the PSB facility, ISOLTRAP began a long series of pioneering improvements that has never flagged. The first of those—the addition of a superconducting cooler trap for isobaric cleaning—was described in the previous ISOLDE Laboratory Portrait (Lunney and Bollen 2000). That work concluded by mentioning the next important step: the addition of a linear Paul trap that enabled the collection of all elements. Before taking up that exciting story, a few general words about how a Penning trap works would seem to be in order.
2.1. Penning-trap mass measurements: the underlying principles
The Penning trap offers the possibility of storing an ion for long periods essentially at rest in an unperturbed environment. Consequently, the measurements performed with Penning traps (as well as the related Paul trap) are of such high accuracy that the 1989 Nobel Prize was awarded to the inventors of these instruments (Dehmelt 1990, Paul 1990). Consisting of a quadrupole electric field formed by a set of electrodes placed along the axis of a static magnetic field B, the Penning trap confines ion movement in three dimensions. The magnetic field provides radial confinement and the electric field ensures longitudinal confinement. The mass is measured via the cyclotron frequency fc = qB/2πm, with B determined from fc of a reference ion with a well-known mass. The static quadrupole field also defocuses in the radial plane, slightly slowing the cyclotron frequency to f+ in addition to introducing a second, very slow radial eigenmode (called magnetron motion) with frequency f−. While slightly complicating the ion motion (by the addition of a radial mode), the use of a quadrupole field simplifies the description as in this case: fc = f+ + f−. A detailed description of the ion dynamics in Penning traps is given by Brown and Gabrielse (1986) and a review on mass spectrometry with stored ions is given by Blaum (2006). The cyclotron frequency is determined from an azimuthal quadrupolar excitation (the duration of which is proportional to the linewidth required—typically a 1 s excitation results in m/Δm of about 900 000 for A = 100 singly charged ions) and measuring the time of flight of the ions after ejection from the trap. This time-of-flight ion-cyclotron resonance (ToF-ICR) technique is the workhorse of Penning-trap mass measurements. The lineshape is derived in Koenig et al (1995) with the so-called 'Mike' fit referring to the first name of the first author of that landmark publication.
2.2. Original ISOLTRAP setup (1985)
The original setup consisted of two Penning traps: one for the collection of the ISOLDE beam and the other for the cyclotron-frequency excitation. While the latter, precision trap was mounted in a 6 T superconducting magnet, the collection trap was mounted in an electromagnet. To collect the beam, a foil on one endcap was used, which was then rotated to face the center of the trap and heated, releasing surface ionized species. The details of this setup and the operating principles are explained in marvelous detail in Bollen et al (1996) and the various events associated with the understanding and development of this sophisticated instrument are colorfully recounted in Kluge (2013).
2.3. Isobaric separation using a large-volume Penning (cooler) trap (1995)
If the first on-line Penning trap measurement of a radioactive nuclide was a landmark event, it only spurred the desire for more. While ISOLTRAP was still limited to elements that had to be surface ionized from a foil, the region of rare-earth nuclides was ripe for plundering. However, so many adjacent chemically favorable species meant prodigious isobaric contamination, requiring a mass resolving power of over 50 000. ISOLTRAP's development of powerful mass-selective ion manipulation with dipolar and quadrupolar excitations combined with buffer-gas cooling (Savard et al 1991) led to the construction of a new cooler trap with particularly large capacity (Raimbault-Hartmann et al 1997). The new trap performed brilliantly, allowing an impressive harvest of mass measurements over six isotopic chains in the region of 146Gd (Beck et al 2000).
2.4. The universal ion beam collector (1996–2000)
Going beyond surface-ionizable species required the use of an accumulator in which ions were captured in flight, without the use of a surface. Developments for this had been underway at McGill University since the mid 1980s (in the group of Moore, who had been on sabbatical leave with the Kluge group in Mainz). The first ion accumulator was a buffer-gas filled cylindrical Paul trap. After tests with a nominal-sized trap (Moore and Rouleau 1992) a scaled-up device was designed with the hope of accumulating larger ion clouds. This device, thought to be the largest Paul trap ever built (see figure 2), was installed at ISOLDE and enabled measurements of neutron-deficient Hg isotopes, the first for non-surface-ionizable species (Schwarz et al 2001).
Figure 2. Evolution of beam collection by ISOLTRAP following the initial foil scheme. The photograph shows what is believed to be the largest Paul trap ever built (explanation for the scale indication can be seen in figure 3) and first step in the development of a universal beam collector while in front of ISOLTRAP from 1996–1998. (Right) Ion trajectories showing the effect of the RF field of the cylindrical trap (top) where strong defocussing is present and in the linear trap (bottom) where the effects are attenuated due to the use of the DC axial field (Schwarz et al 2001).
Download figure:
Standard image High-resolution imageThe drawback of the three-dimensional Paul trap was the high RF fields traversed by the ions entering (and exiting) the trapping volume, which caused heavy losses. Through collaboration with chemists working in industry, the idea of using a gas-filled linear Paul trap arose (Douglas and French 1992). Here the transverse RF fields had minimal longitudinal effect and the ions were cooled and dragged to the end of the device where they could be accumulated. After ameliorations of the deceleration optics and introduction of the segmented axial field (Kellerbauer et al 2001), the RFQ cooler buncher in its present form (see figure 3) was installed (Herfurth et al 2001).
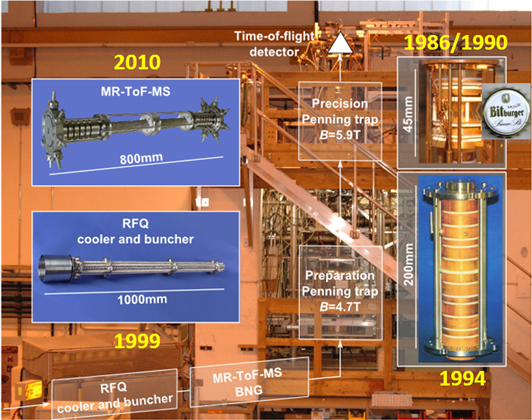
Figure 3. Photographs of the current ISOLTRAP spectrometer and components in the ISOLDE experimental hall. See text for more detailed description.
Download figure:
Standard image High-resolution image3. Pioneering technical developments and scientific communications arising
ISOLTRAP has pursued science in the idiom of the 18th century figure Lichtenberg, who coined the adage: 'to see something new, you must build something new'. ISOLTRAP's major technical highlights are mentioned in the following sub-sections, along with the (mostly) resulting science. The most recent upgrade, the multi-reflection time-of-flight isobar separator (MR-ToF), is described in section 4.
3.1. The linear paul trap (2000)
With the successful commissioning of the linear Paul trap ion collector, ISOLTRAP was now capable of measuring any chemical species with good efficiency, allowing forays into the unexplored territories of exotic nuclides. As such the publication rate took off (see figure 7 in Kluge 2013). As explained above, the cylindrical Paul trap brought the initial Hg results before being replaced by the linear Paul trap, extending the harvest from 185Hg out to 182Hg (Schwarz et al 2001). This work also allowed the highest resolving power ever attained with ISOLTRAP: over 3000 000 (using an 8 s excitation) for the reference isotope 208Pb, used for the first measurement of isomeric energies by mass spectrometry. The linear cooler proved its worth brilliantly by accumulating the noble gas species argon for precision measurements, reaching 33Ar with a half-life below 1 s in the process (Herfurth et al 2001a). Requiring ionization in a plasma, 32Ar was contaminated by 16O16O, but by judicious use of mechanical slits with the ISOLDE HRS dipole magnet, it was eventually measured (Blaum et al 2003). Another important result was on the neutron-deficient xenon isotopes down to 114Xe (Dilling et al 2004). The linear buncher opened a new vista and almost twenty variants of these devices now exist at different radioactive beam facilities worldwide.
3.2. The use of carbon clusters for systematic accuracy evaluation (2002)
The atomic mass unit is defined as one twelfth of the mass of 12C. As such, all masses are related. Indeed, decay spectroscopy and reactions give the total energy (or so-called Q value) and even Penning traps publish frequency ratios with respect to a well-known reference mass. Typically, the alkali elements are used due to their ease of production (by surface ionization): 23Na, 39K, 85Rb, 133Cs. Systematic errors accumulate the farther the reference mass is from that being measured. Carbon clusters give a reference mass every twelve mass units. The use of clusters also allowed an exceptionally comprehensive study of the systematic effects present in the apparatus (Kellerbauer et al 2003). Such work is a prerequisite for evaluating the ultimate accuracy, which determines the physics that can be addressed. While the salient effects of nuclear structure (e.g. shell closures) can be probed with accuracies of 1 ppm, effects related to weak-interaction physics requires reaching into the 10 ppt realm.
The carbon-cluster studies spawned a renaissance in the study of super-allowed beta decay partners for evaluating the conserved-vector-current hypothesis for the weak interaction and the associated unitary test of the Cabbibo–Kobayashi–Maskawa quark-mixing matrix. The first direct mass measurement of the super-allowed A = 74 decay-energy value using a Penning trap was also a record for the shortest lived species for 74Rb at 65 ms (Kellerbauer et al 2004). Another example of where high accuracy is important comes from studies of isospin symmetry via the isobaric multiplet mass equation, explored by ISOLTRAP in the cases of 32-33Ar mentioned above as well as 35K (Yazidjian et al 2007).
One road to higher accuracy is to increase the cyclotron frequency of the trapped ions. This can be done with a higher magnetic field however ISOLTRAP investigated the more tractable use of ions in higher charge states, which can be delivered by ISOLDE using a plasma ion source (Herlert et al 2006). (The Penning-trap spectrometer TITAN at the TRIUMF-ISAC facility exploits this technique (Gallant et al 2012).) Another way is the use of octupole excitation, applied at twice the frequency of the azimuthal quadrupole field that is commonly used (Rosenbusch et al 2012b). (Octupole excitation was first studied with LEBIT, at NSCL (Ringle et al 2007) and SHIPTRAP, at GSI (Eliseev et al 2007).
3.3. Resolving isomeric states (2004)
A key ingredient for high accuracy is increased resolving power. Here the Penning trap has demonstrated this superiority by resolving isomeric states. This was highlighted in the first use of ISOLTRAP, with the surface-ionized beams of 78Rb, for which the 110 keV isomer was resolved (Bollen et al 1992). This technique was later refined and ISOLTRAP's record for isomeric separation was reached for 187mPb with an excitation energy of only 33 keV (Weber et al 2005).
Using the nuclear-spin-dependent capability of laser ionization, ISOLTRAP demonstrated the controlled delivery of a purified 68Cu isomeric state (Blaum et al 2004). This work spurred the judicious combination of precision mass spectrometry with decay spectroscopy and laser ionization to study the beta-decaying 70Cu nuclide, produced with two relatively long-lived beta-decaying isomeric states that rendered spectroscopy intractable. Here mass spectrometry teamed with the selective laser ionization to 'weigh' the species purified by selecting one hyperfine ionization peak (Van Roosbroeck et al 2004).
3.4. Trapped recoil-ion spectrometry (2005)
Holding radioactive species in a trap offers the possibility of waiting for the decay. This can be useful for decay kinematics but also for populating different nuclear states by trapping the recoil species—especially for one not readily produced by an ISOL facility. The first demonstration of trapped-ion transmutation was performed by ISOLTRAP using 37K, which decayed to 37Ar (Herlert et al 2005). Later, the technique was used with trapped manganese ions that yielded iron, a species never before produced at ISOLDE (Herlert et al 2012). The trapped-ion transmutation technique has another interest since recoil nuclides may populate excited states not produced in the target. For example, the beta decay of 34Mg exclusively populates an isomeric 1+ state in 34Al. ISOLTRAP succeeded in trapping this recoiling daughter and will attempt to measure its mass to determine the excitation energy of the isomer.
3.5. The use of separated oscillatory fields—the Ramsey scheme (2007)
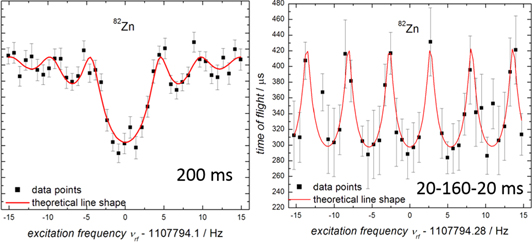
Ion storage lends itself to periodic interrogation. It is an interesting coincidence that the Nobel Prize won by Dehmelt and Paul for ion traps was shared with Norman Ramsey, for his invention of the use of separated oscillatory fields (Ramsey 1990). After an initial excitation, the ion cyclotron motion of the trapped ion is left to evolve and after a suitable number of cycles the excitation is applied again. The phase at which the excitation is applied is extremely sensitive and provides another way of attaining higher resolving power. The Ramsey technique was tested early in ISOLTRAP's history (Bollen et al 1992) but the correct theoretical description of the resulting line shape was achieved later (Kretzschmar 2007). The first measurement published using this technique was for 38Ca (George et al 2007) followed by 39Ca and 26Al (George et al 2008). It is frequently used to achieve comparable precision with the ToF-ICR scheme since it requires far fewer statistics (but the repeating pattern of Ramsey 'fringes' necessitates an initial ToF-ICR scan in order to ascertain the center frequency). An example (for 82Zn) is shown in figure 4.
Figure 4. ToF-ICR quadrupole excitation spectra for 82Zn illustrating (left) a single 200-ms pulse and (right) the Ramsey separated-field scheme with two 20-ms pulses separated by 160 ms. The total excitation time is the same, as is the resulting measurement precision (about 0.1 ppm) however the eight-times higher statistics (580 counts) necessary for the ToF-ICR resonance required over 3 h of accumulation compared to only about half an hour for the Ramsey scheme (see Wolf et al 2013 and Kreim et al 2013).
Download figure:
Standard image High-resolution image3.6. The awesome breadth of ISOLTRAP science: from the 17Ne proton halo to the discovery of 229Rn (2008 to 2011)
The mass embodies the net result of the forces at work in the atomic nucleus (even in the atomic system). As such, the mass plays a decisive role in nuclear structure and since the mass determines the amount of energy for reactions and decays, there is important impact in associated fields, such as nuclear astrophysics. This section reviews the astonishing breadth of physics addressed by ISOLTRAP.
The limits of nuclear stability are defined by the binding energy of the last nucleon. At the drip lines, barely bound neutrons or protons form extended halos, the size of which are determined by the binding energy. Masses of neon isotopes were performed out to 17Ne, a proton halo candidate (Geithner et al 2008) for which the separation energy is a key input parameter for their theoretical description.
Nuclear shell structure, originally discovered using binding energy data, continues to be a strong focus of mass measurements. The original 'island of inversion' responsible for the disappearance of the N = 20 shell was also discovered from mass measurements. All the shell closures have since been examined for robustness. In 2008, ISOLTRAP published results on proton-magic tin isotopes, discovering a 'restoration' of the N = 82 shell closure that appeared to have been quenched from a mass value derived from decay spectroscopy (Dworschak 2008). Attempts were made at probing the strength of the N = 82 south towards the neutron drip line for Ag and Cd (Breitenfeldt et al 2010) and although new masses were measured, isobaric contamination limited the extent. The N = 32 subshell was probed by measurements of chromium isotopes (Guénaut 2005). Nuclides with like numbers of protons and neutrons also possess an extra source of binding (related to pairing) often referred to as the Wigner effect. In addition to the case of 74Rb mentioned above, ISOLTRAP has measured 76Sr, the heaviest N = Z nuclide investigated by a Penning trap so far (Sikler et al 2005).
An attempt to examine the N = 126 shell closure near proton-magic lead brought a lot of mass data that was also linked via alpha decay. The precision of the ISOLTRAP measurements tidied up the mass surface significantly, also highlighting trends in the pairing energies, which were correlated with interesting trends in nuclear charge radii (Weber et al 2008). Complementary measurements enhanced by isobaric cleaning helped to extend mass knowledge in the same region (Kowalska et al 2009 and Boehm et al 2014). This work also involved the integration of a tape-transport system for nuclear spectroscopy (Kowalska et al 2012). The trans-lead nuclides out to 233Fr and 234Ra were also measured, allowing a detailed examination of the pairing and its effect on deformation moving northeast of the closed-shell 208Pb (Kreim et al 2014).
Magic nuclides are not the only ones of interest for nuclear structure. Venturing away from these spherical cases leads to deformation and changes of shape, reflected by the binding energy. Establishing the boundaries where shape transitions occur is important for nuclear models and helps guide the decay spectroscopy required for the explanation of the effects revealed by the mass surface (see figure 5). There are two neutron-rich regions that show particularly impressive manifestations of shape transitions. ISOLTRAP has measured masses near both: xenon, near A = 150 (Neidherr et al 2009a) and krypton, near A = 100 (Naimi et al 2010), continuing that chain from a study of the N = 56 sub-shell (Delahaye et al 2006). The masses of 98–100Rb, delineating the border of this deformed region, were also measured (Manea et al 2013).
Figure 5. Different mass surfaces and their illustrations of nuclear structure. (left, top) Two-neutron-separation energy values for the A = 100 area, illustrating the N = 50 magic number and the region of shape change around N = 60 with the Kr isotopes (Naimi et al 2010) forming the transition below Rb (Manea et al 2013). (Right, bottom) The two-neutron-separation energies for calcium and potassium, highlighting the N = 20 and 28 shell closures as well as the new N = 32 (sub)shell (Wienholtz et al 2013, Rosenbusch et al 2015). (right, top) The 3-point and 5-point (line only) pairing differences that clearly show the shell effects at N = 20, 28 and 32 while (bottom left) the empirical shell gap plots for N = 28 and N = 32 highlight the relative strengths for calcium (Z = 20). Note the energy scales associated with the mass differences, which illustrates the need for high measurement precision.
Download figure:
Standard image High-resolution imageThe ISOLTRAP mass measurement of 97Kr contradicted a result from nuclear spectroscopy implying deformation in 96Kr and was confirmed from later Coulomb excitation results from MINIBALL at ISOLDE. It was also the first use of the isobaric separator (cooler) trap for mass spectrometry where a new line shape, called the double-Wood-Saxon, was elaborated (Naimi et al 2011). This work with the cooler trap also spawned the idea of mass separation without the use of buffer gas (so troublesome for the noble gases) and led to the development of a technique using simultaneous excitations, called SIMCO (Rosenbusch et al 2012a).
The N = 20 island of inversion showed that deformation can overwhelm the effect of an erstwhile magic number. It might not be a surprise that this phenomenon seems to occur elsewhere and ISOLTRAP has contributed to the investigation of another island, near N = 40, with masses of nickel, copper and gallium isotopes (Guénaut et al 2007), manganese (Naimi et al 2012) and soon-to-be-published chromium (Mougeot 2017).
The synthesis of the elements in stars is one of the most brightly burning questions in science and one for which mass measurements of exotic species has a high impact. Since hydrostatic fusion reactions are no longer endothermic after nickel and iron, heavy elements are synthesized by neutron capture. This happens either slowly along stability or rapidly in an explosive process involving exotic nuclides, either in supernovae and/or neutron-star mergers. Of particular importance are the masses at shell closures, which divert the r-process path and have an effect of the isotopic abundances. This question was explored in work on zinc isotopes (Baruah et al 2008). Additional ISOLTRAP mass measurements of relevance for the r process have been made with the upgraded apparatus (see next section).
An analog process of proton capture could explain the existence of certain proton-rich species. The so-called rapid proton-capture (rp) process requires masses with high precision due to the exponential dependence of the proton-capture rates. ISOLTRAP has measured masses of relevance for the rp process, in particular the waiting point 72Kr (Rodriguez et al 2004) as well as for cadmium (Breitenfeldt et al 2009) and several elements above germanium (Herfurth et al 2011b). Although the energy associated with novae explosions is derived from proton-capture reactions involving species closer to stability, deviations in mass values led to conflicting results associated with the 21Na proton-capture producing 22Mg. A 15-ppt mass measurement of both these nuclides by ISOLTRAP resolved the ambiguity (Mukherjee et al 2004).
New isotopes are usually the fruit of fragmentation facilities but the development of a new discharge ion source with particularly good efficiency enabled the discovery of the nuclide 229Rn (Neidherr et al 2009b) in addition to new masses in the radon chain. These are some of the heaviest isotopes ever examined in a Penning trap.
Nuclear decays are also governed by mass differences and since beta decay involves neutrinos, there are many mass differences of great relevance for neutrino physics. A complementary route to the neutrino mass is through electron capture. ISOLTRAP has investigated this with mass measurements of the decay partners 194Hg and 194Au (Eliseev et al 2010). Neutrinoless double-beta decay would indicate that neutrinos are their own antiparticles (Majorana particles) with far-reaching consequences in fundamental particle physics. Again, decay partner masses are measured to obtain the Q value, which constrains the lifetime of the process and determines if it can be observed with current ultra-low-level detection techniques. ISOLTRAP addressed the case of 110Pd (Fink et al 2012).
4. A new era of mass spectrometry: fast isobar separation with ISOLTRAP's MR-ToF
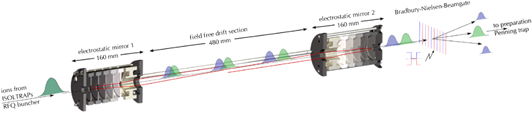
Time-of-flight separation due to the different masses is proportional to the length of the overall time of flight, a fact exploited by storage rings. Following work at the Weizmann Institute (Zajfman et al 1997), the Berkeley Human Genome Center (Benner 1997) and by a RIKEN—Giessen University collaboration (Ishida et al 2004), the University of Greifswald group took up the challenge of improving isobar separation for ISOLTRAP by developing what they have called an MR-ToF mass spectrometer (Wolf et al 2012). Illustrated in figure 6, the MR-ToF can achieve resolving powers in excess of 300 000 (Wienholtz et al 2015) in 30 ms: roughly one tenth of the time required by the lengthier centering and quadrupole excitation in the gas-filled Penning trap. A main ingredient for success is the formation of very short ion bunches, for which the ISOLTRAP RFQ cooler was retrofit (Wolf et al 2013a).
Figure 6. Schematic illustration of the MR-ToF MS showing the gradual separation of different ion species as they oscillate between the two sets of electrostatic mirrors. After ejection a Bradbury–Nielson-type beam gate is used to transmit only the desired species to the downstream Penning trap.
Download figure:
Standard image High-resolution imageAfter design and initial testing at Greifswald (Wolf et al 2011, 2012), the MR-ToF was installed at CERN after the RFQ buncher and pulsed drift tube and commissioned in 2011. The results of the first on-line tests and measurements were published shortly thereafter (Wolf et al 2013b). In addition to the desirable feature of quickly achieving high resolving power, the MR-ToF not only selects the species of interest but also allows identification of accompanying isobars in the same spectrum. This feature can be used for monitoring the ISOLDE production-target and ion-source behavior (Kreim et al 2013 and Gottberg et al 2014) and best of all, provides reference isotopes for mass measurements using the MR-ToF itself.
The first experiment aimed at determining the strength of the N = 50 shell closure by measuring the mass of 82Zn, since the earlier attempts (Baruah 2008) had been thwarted by surface-ionized gallium. On the ISOLDE side, all the stops were pulled out for this experiment: laser ionization, a neutron convertor, a quartz transfer line; but still, 6000 ions per second of 82Rb were delivered along with the few ions of 82Zn. Here, the MR-ToF performed brilliantly. A mere 2.5-ms wait (about 100 revolutions in the MR-ToF, corresponding to a resolving power of 6000) was sufficient to separate the isobars and the rest is history with the 82Zn mass used to constrain the crustal composition of neutron stars (Wolf et al 2013b). Important work was also performed to decouple the fast-cycling buncher and MR-ToF from the slower, accumulating Penning traps (Rosenbusch et al 2014).
As mentioned above, isobaric 'contaminants' can be a blessing in disguise. Such was the case during an experiment on calcium masses in which stable chromium (having a well-known mass) was present in the laser-ionized ISOLDE A = 53 and 54 beams. Avoiding transmission and decay losses that would have resulted from taking 53,54Ca ions to the Penning traps, the MR-ToF was used to measure these masses with the stable chromium isobars as references (periodic injections of stable 39K during the experiment were also used as calibrations). Thus a new era of mass spectrometry began. To top it off, the mass of 52Ca was measured using both the MR-ToF and the Penning Trap with perfectly consistent results. The masses of 52,54Ca revealed a large shell gap indicating a rather 'magic' sub-shell and a high-impact publication of these results (Wienholtz et al 2013). An extension of this work to the 51,53K nuclides at N = 32 starts the probing the strength of this shell towards the drip line (Rosenbusch et al 2015).
The MR-ToF is now used to measure the mass of the most exotic nuclide that can be detected during a run since the measurement is faster than using the Penning trap (and requires fewer ions). Such was the case for 131Cd, which was produced at a rate of only a few per proton pulse. This measurement allowed the first evaluation of the N = 82 shell gap below 132Sn and since 130Cd is a classic r-process waiting point, it has important impact for nucleosynthesis (Atanasov et al 2015).
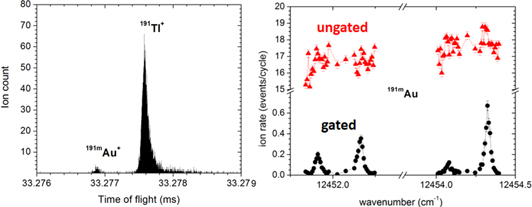
Even using the neutron converter and laser ionization, there is almost always an abundant surface-ionized isobar that will accompany the one of interest. Such was the case during experiments on 197,219At and 180,185,188,190Au, which were part of a study of shape coexistence south-west of 208Pb (Manea et al 2017) and 191mAu for which the MR-ToF was indispensable. Figure 7 (left) shows the A = 191 spectrum, illustrating the dominance of the surface-ionized 191Tl compare to 191Au. Another example was for 198At (where 198Tl was also present) which was also sent to the spectroscopy station in order to characterize an isomeric state (Stanja et al 2013). Coupling the MR-ToF with nuclear spectroscopy has even further highlighted its versatility by measuring beta-decay half-lives (Wolf et al 2016).
Figure 7. (Left) MR-ToF spectrum of the ISOLDE A = 191 beam showing the laser-ionized 191mAu and dominating surface-ionized 191Tl isobaric components. (Right) Hyperfine spectrum for laser-ionized 191mAu produced by recording an MR-ToF spectrum each laser frequency step. The crucial effect of mass separation by the MR-ToF can be seen by comparing the overwhelming Tl peak to gating on the low-abundance Au. Thus, in combination with laser ionization, the MR-ToF can address both masses and charge radii in one experiment.
Download figure:
Standard image High-resolution imageDuring these experiments we discovered that monitoring the MR-ToF spectra while scanning the ionization laser frequency produces reliable measurements of hyperfine structure from which nuclear charge radii can be determined. An example of such a spectrum for the 191mAu case mentioned above is shown in figure 7 (right). Laser scanning using alpha-decay rates had been used previously but in cases where the decay half-lives were too long or there was simply no alpha-decay branch, the MR-ToF was the tool of choice. This synergy is highlighted in (Marsh et al 2013) and results using the MR-ToF for the study of Au, Hg and Bi charge radii are soon to be published.
The successes mentioned above have not come without their share of difficulties. In 2010 the cooler-trap magnet quenched. ISOLTRAP ran several experiments that year before it quenched again due to the same vacuum leak. During the long shut-down (LS1, in 2012) ISOLTRAP underwent a total overhaul; parts of the apparatus had not seen the light of day for almost 20 years! Some of the most remarkable ISOLTRAP results followed that successful intervention.
More results are under analysis and will soon be published, including measurements of Cu isotopes out to the N = 50 shell closure (Welker 2017) and neutron-rich Cr isotopes near N = 40 (Mougeot 2017). The ISOLTRAP website (http://isoltrap.web.cern.ch) offers a database of all our measurements. As always, ISOLTRAP continues its technical development, for example implementing the phased-imaging ion-cyclotron resonance technique pioneered by TRIGATRAP (Eitel et al 2009) and SHIPTRAP (Eliseev 2013). Both of these Penning-trap installations are offspring of ISOLTRAP.
5. Summary
Perhaps the greatest tribute to the success of ISOLTRAP is the existence of the many on-line Penning-trap spectrometers that have followed. After CPT, built at Chalk River National Laboratory in Canada and then moved south of the border to Argonne National Laboratory, came SHIPTRAP at GSI and JYFLTRAP at the IGISOL facility, followed by LEBIT at NSCL, TITAN at TRIUMF's ISAC facility, MLLTRAP at LMU (moved to the ALTO facility in Orsay, while waiting for an eventual installation at the SPIRAL2-DESIR facility at GANIL), and TRIGATRAP at the TRIGA reactor in Mainz. Descriptions of these instruments can be found in (Schweikhard and Bollen 2006). While all of them have contributed to advancing mass spectrometry and beam handling, ISOLTRAP continues to play a major role, as demonstrated by the integration of the linear Paul trap and the MR-ToF ion trap, described in this article. Such devices have even found general-purpose applications, for example ISCOOL for bunching ISOLDE beams for all experiments and REX-TRAP as the first step of the HIE-ISOLDE post-accelerator. As radioactive-ion production techniques advance at ISOLDE and elsewhere, trapped-ion mass spectrometry is sure to keep pace, ensuring a bright future for mass measurements, nuclear structure and the associated fields.
Table 1. Doctoral student contributions to the ISOLTRAP program.
| Year | Name | Thesis title |
|---|---|---|
| 1986 | H Schnatz | Design and testing of equipment for direct mass measurement of short-lived nuclides |
| 1989 | F Kern | Development of experiment control, data analysis and mass determination of radioactive Cs isotopes |
| 1989 | G Bollen | First mass measurement on unstable isotopes using a Penning trap |
| 1992 | G Rouleau | A tandem Paul-Penning trap mass measurement system for radionuclides |
| H Stolzenberg | Precision mass determination of unstable isotopes cesium and barium using a Penning trap | |
| 1993 | T Otto | Penning trap mass spectrometry of neutron-deficient rubidium and strontium isotopes |
| 1994 | H Raimbault-Hartmann | Precision mass determination of neutron-rich rubidium and strontium isotopes and development and testing of a new concept for ion accumulation and cooling for ISOLTRAP |
| 1995 | M Koenig | Precision mass determination of unstable isotopes of cesium and barium in a Penning trap and investigation of ion motion in the azimuthal quadrupole excitation |
| 1997 | D Beck | Mass determination of unstable isotopes of the rare earths to 146Gd with the ISOLTRAP spectrometer |
| E Schark | First direct mass measurement of radioactive isotopes of rare earths on ISOLTRAP Experiment | |
| 1998 | S Schwarz | Manipulation of radioactive ion beams with a Paul trap and direct mass measurements of neutron-rich isotopes of mercury with the ISOLTRAP spectrometer |
| 1999 | A Kohl | Direct determination of mass in the lead area and investigation of a Stark effect in the Penning trap |
| 2001 | F Herfurth | A new ion beam cooler and buncher for ISOLTRAP and mass measurements of radioactive argon isotopes |
| 2001 | J Dilling | Direct mass measurements on exotic nuclei with SHIPTRAP and ISOLTRAP |
| S Henry | Trapping and cooling of exotic ions for mass measurements | |
| 2002 | A Kellerbauer | A study of the accuracy of the Penning trap mass spectrometer ISOLTRAP and standard-model tests with super-allowed beta decays |
| 2003 | G Sikler | Mass spectrometry of short-lived Sr and Sn isotopes and construction of the SHIPTRAP Penning trap |
| D Rodriquez | A radiofrequency quadrupole buncher for accumulation and cooling of heavy radionuclides at SHIPTRAP and high precision mass measurements of unstable krypton isotopes at ISOLTRAP | |
| 2004 | C Weber | Design of a cryogenic Penning trap setup for SHIPTRAP and mass determination of radionuclides to the Z = 82 shell closure at ISOLTRAP |
| M Mukherjee | The mass of 22Mg and a concept for a novel laser ion source trap | |
| 2005 | C Guénaut | Optimized ion trapping of exotic nuclides for mass measurements in the N = 40 (magic?) region |
| S Rahaman | First on-line mass measurements at SHIPTRAP and mass determinations of neutron-rich Fr and Ra isotopes at ISOLTRAP | |
| 2006 | C Yazidjian | A new detector setup for ISOLTRAP and test of the isobaric-multiplet-mass equation |
| 2008 | S Baruah | Precision mass measurements on neutron-rich Zn isotopes and their consequences on the astrophysical r-process |
| 2009 | M Breitenfeldt | Mass measurements on short-lived Cd and Ag nuclides at the online mass spectrometer ISOLTRAP |
| S George | First Ramsey-type mass measurements with ISOLTRAP and design studies of the new PENTATRAP project | |
| 2010 | S Naimi | Onsets of nuclear deformation from measurements with the ISOLTRAP mass spectrometer |
| D Neidherr | Nuclear structure studies in the xenon and radon region and the discovery of a new radon isotope by Penning trap mass spectrometry | |
| 2012 | C Borgmann | Mass measurements of exotic ions in the heavy mass region for nuclear structure studies at ISOLTRAP |
| 2013 | J Stanja | Synergy of decay spectroscopy and mass spectrometry for the study of exotic nuclides |
| R Wolf | First on-line applications of a multi-reflection time-of-flight mass separator at ISOLTRAP and the mass measurement of 82Zn | |
| 2014 | V Manea | Penning-trap mass measurements of exotic rubidium and gold isotopes for a mean-field study of pairing and quadrupole correlations |
| 2015 | C Boehm | High-precision mass measurements of neutron-deficient Tl isotopes at ISOLTRAP and the development of an ultra-stable voltage source for the PENTATRAP experiment |
| 2016 | M Rosenbusch | Development of new ion-separation techniques and the first mass measurement of 52,53K |
| D Atanasov | Precision mass measurements for studies of nucleosynthesis via the rapid neutron-capture process | |
| 2017 | A Welker | Phase-imaging ion cyclotron resonance for ISOLTRAP and mass measurements of exotic copper |
| F Wienholtz | Time-of-flight mass spectrometry for probing nuclear magicity in exotic calcium isotopes produced at CERN-ISOLDE | |
| N Althubiti | Decay spectroscopy and mass spectrometry of neutron-deficient polonium isomeric states | |
| 2018 | M Mougeot | Nuclear collectivity studied by high-performance mass spectrometry |
Acknowledgments
Many have contributed to make ISOLTRAP what it is today. The principal investigator Juergen Kluge has described best the history of these contributions in his review work (Kluge 2013). In presentations, Juergen shows a slide that reads: 'ISOLTRAP has many fathers—most of them students.' Table 1 lists the contributions (expressed as thesis titles) of the doctoral students that have held the responsibility for keeping ISOLTRAP ready for action. Several pos-doctoral (CERN) fellows have also dedicated crucial efforts to this task: Guy Savard (1988–1990), Jurek Szerypo (1992–1994), Harald Raimbault-Hartmann (1994–1997), Klaus Blaum (2000–2003), Alexander Herlert (2003–2006), Pierre Delahaye (2004–2006) Magdalena Kowalska (2007–2010) and Susanne Kreim (2010–2013). Since 2014, the local contact for ISOLTRAP is Vladimir Manea.