Abstract
In plasma-driven biocatalysis, enzymes are employed to carry out reactions using species generated by non-thermal plasmas as the precursors. We have previously demonstrated that this is feasible in principle, but that the approach suffers from the short lifetime of the biocatalyst under operating conditions. In this work, protection strategies were investigated to prevent the dielectric barrier discharge plasma-induced inactivation of biocatalysts, using recombinant unspecific peroxygenase from Agrocybe aegerita( rAaeUPO), one of the most promising enzymes for plasma-driven biocatalysis. Treatment in oxygen-free atmospheres did not provide any advantage over treatment in synthetic air, indicating that the detrimental reactive species did not originate from oxygen in the plasma phase. Chemical scavengers were employed to eliminate undesired reactive species, without any long-term effect on enzyme lifetime. Similarly, chaperones, including the known stress response proteins Hsp33, CnoX, and RidA did not increase the lifetime of rAaeUPO. Immobilization of the biocatalyst proved effective in preserving enzyme activity. The residual activity of rAaeUPO after plasma treatment strongly depended on the specific immobilization support. Essentially complete protection for at least 15 min of plasma exposure was achieved with an epoxy-butyl-functionalized carrier. This study presents new insights into plasma–protein interactions and plots a path forward for protecting biocatalytic proteins from plasma-mediated inactivation.
Export citation and abstract BibTeX RIS
1. Introduction
Plasmas are ionized gases that can be generated through the application of electric fields. The electric field selectively accelerates free electrons that collide with gas particles, resulting in the formation of ions, radicals, and metastables [1]. These reactive species are the main active component of plasmas and offer a variety of applications. Non-thermal plasmas in particular, which do not experience heating above the ambient temperature, are being increasingly explored for their use in biology and medicine [2]. These include, among others, disinfection, wound healing and cancer treatment [3]. The plasma-generated reactive species react with the biological target and cause inactivation in many cases, e.g. of bacteria and cancer cells [4, 5].
On a molecular level, the inactivation of enzymes by non-thermal plasmas is being studied in detail. When purified proteins were treated with non-thermal plasmas, several effects have been observed. Reactive oxygen and nitrogen species (RONS) react with amino acid side chains of the protein and cause their modification [6, 7]. This leads to a change in enzyme structure and potentially to complete loss of the structural integrity [8, 9]. When proteins contain cofactors, these can also be impaired by plasma treatment [10, 11]. Even fragmentation of proteins has been observed, which was reported to result from the interaction of ROS with the protein backbone [12]. It is also conceivable that the applied electric field leads to protein unfolding [13, 14]. In all of the aforementioned scenarios, proteins are inactivated.
Recently, we reported on the use of non-thermal plasmas in biocatalysis [15]. Therein, a solution containing an enzyme and starting material is treated with a non-thermal plasma, creating hydrogen peroxide (H2O2) in the liquid. The H2O2 is then used by the enzyme to convert the starting material. Compared to other H2O2 delivery methods for biocatalysis [16, 17], plasma-driven biocatalysis represents the only truly non-invasive approach to date that does not require the addition of other components to the reaction solution. Plasmas also provide the benefit of being easily tunable, allowing for specific adjustments depending on the reaction setup and biocatalysts employed. While the approach was proven to be feasible in principle, it was severely limited by the rapid inactivation of the biocatalysts due to cofactor inactivation and protein fragmentation upon plasma exposure [15].
Here, we used the in vitro-evolved, recombinant unspecific peroxygenase from Agrocybe aegerita( rAaeUPO) as a model for H2O2-utilizing enzymes to test different protection strategies including chaperone-based protection, chemical scavenging of species, different gases for plasma ignition, as well as immobilization to address the inactivation of enzymes triggered by plasma treatment with the goal of increasing the efficiency of plasma-driven biocatalysis.
2. Materials and methods
2.1. Enzymes
Purification of rAaeUPO (PaDa-I-variant) was performed as described in previous work [18]. Briefly, culture supernatant of the Pichia pastoris expression clone was used after a one-step ion exchange purification [19]. Hsp33, CnoX, and RidA were purified as His6-tag fusion proteins. E. coli cultures harboring pCA24N::hslO, pCA24N::cnoX, or pCA24N::ridA were grown in LB medium with 35 µg ml−1 chloramphenicol [20]. Cultures were induced with 100 µM IPTG at OD600 = 0.5 and grown for 4 h before harvesting. The cells were then disrupted by sonication, centrifuged for 30 min at 28 166 x g, and the supernatant subjected to Ni-NTA chromatography (Ni-NTA Agarose, Qiagen, Hilden, Germany). Proteins were eluted with 50 mM phosphate buffer containing 300 mM NaCl and 150 mM imidazole. Hsp33, CnoX, and RidA solutions were concentrated using centrifugal filters and stored in potassium phosphate buffer (100 mM, pH 7.5) at −20 °C until use.
Pre-activation of Hsp33 was carried out by incubation with a 50-fold molar excess of HOCl at 30 °C and 300 rpm for 10 min. Subsequently, Hsp33 was purified using spin columns (P30, Bio-Rad, Munich, Germany) according to manufacturer's instructions and eluted in potassium phosphate buffer (100 mM, pH 7.5).
2.2. Plasma treatment
Plasma treatment was performed using a Cinogy PlasmaDerm device [21]. Protein samples were placed onto a PTFE-coated glass slide on a grounded metal plate and treated with plasma at a distance to the electrode of 2 mm. For treatments including chaperones, plasma exposure was performed on metal plates to ensure complete sample recovery. Plasma was ignited at 13.5 kV applied voltage and 300 Hz trigger frequency in ambient air, using a 20 mm electrode. The concentration of free rAaeUPO was 100 nM during treatment. Protective enzymes were added at 100 or 1000 nM each. Uric acid, Trolox, or mannitol were added at 1, 0.5, and 100 mM, respectively.
For experiments using different gas atmospheres, a modified Cinogy DBD was used [22]. The electrode diameter was decreased to 10 mm and placed into a vacuum chamber with gas feed and vacuum pump inlets. The sample was transferred to a glass slide and placed into the chamber at 2 mm distance to the electrode. Lateral gas flow was set to correspond to 1 bar of pressure. Before igniting the plasma, the chamber was flushed for 3 min. Plasma parameters were 24 kVpp at 300 Hz to accommodate for the different gas atmospheres.
2.3. rAaeUPO activity assay
Treated samples were diluted 1:20 in buffer containing 5 mM 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as substrate in 100 mM sodium citrate buffer, pH 5. The reaction was started by adding the same volume of 2 mM H2O2 and monitored in a microplate reader at 405 nm (Biotek Epoch, Bad Friedrichshall, Germany). Final concentrations were 2.5 mM ABTS, 50 mM citrate, 1 mM H2O2, and 2.5 nM rAaeUPO. For immobilized rAaeUPO, samples were diluted 1:50 in 500 µl substrate buffer prior to addition of H2O2 and vigorously shaken during turnover to ensure sufficient substrate supply. Every two minutes for a total of ten minutes reaction time, aliquots of 100 µl were withdrawn and measured using a microplate reader. Calculations of activities were then corrected for the remaining volumes.
2.4. Immobilization
To immobilize rAaeUPO, Lifetech ECR resins were used (Purolite ECR1 kit, Llantrisant, Wales). For the amino-based resin, 100 mg were weighed into a suitable vessel, washed thrice with deionized water and incubated with glutaraldehyde (0.4% final concentration) in 100 mM potassium phosphate buffer (pH 7.5) for 1 h. Subsequently, the carrier was washed thrice with potassium phosphate buffer and rAaeUPO was added. Similarly, all other resins were washed thrice with deionized water and added to potassium phosphate buffer containing rAaeUPO. For 100 mg of resin, 1 nmol of enzyme was used. The resins were then incubated for 16 h at 8 °C with overhead shaking after which the supernatant was extracted. The beads were then washed thrice with buffer to remove unbound rAaeUPO and stored at 4 °C until further use. Binding efficiency was checked by comparing activity of the immobilization supernatant and virgin rAaeUPO.
3. Results and discussion
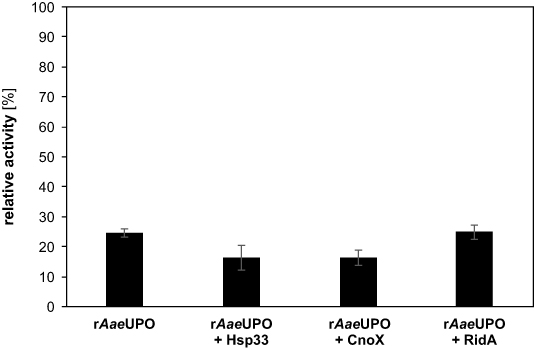
It has been observed that proteins aggregate under plasma treatment. A protective effect has been shown for Hsp33 in plasma-treated protein extracts as well as whole cells, leading to less aggregation and higher survival rates, respectively [23]. Thus, in order to prevent inactivation of rAaeUPO by plasma treatment, we first tested the chaperones Hsp33, CnoX, and RidA of E. coli[ 24–26] for their ability to prevent protein inactivation under plasma treatment. The chaperones were overexpressed in E. coli and purified using standard techniques. Then, the chaperones were mixed with rAaeUPO and the solution was placed onto a glass slide and treated for 1 min with a dielectric barrier discharge (DBD) plasma. The residual activity of rAaeUPO was then measured in a colorimetric assay. The residual activity was found to be essentially the same (approximately 20% of untreated controls) for all samples including the negative control without chaperone, indicating that none of the chaperones increased the lifetime of rAaeUPO (figure 1).
Figure 1. Residual activity of rAaeUPO after plasma treatment with different chaperones. A mixture of 100 nM rAaeUPO and 100 nM of the respective chaperone were treated with the DBD plasma for 1 min. Immediately after plasma exposure, the treated sample was diluted in reaction buffer containing the colorimetric substrate (100 mM citrate buffer, pH 5 with 5 mM ABTS). The reaction was started by addition of H2O2( 1 mM final concentration) and absorption monitored at 405 nm. Enzyme activity was calculated from the linear slope at the beginning of the reaction. The relative activity shown here is the activity after treatment divided by the activity prior to plasma exposure. Addition of the chaperones had no effect on the basal rAaeUPO activity before plasma treatment. Bars show means and standard deviations of three independent replicates. The significance of differences to rAaeUPO without protective enzymes was determined with Student's t-test (p > 0.05 in all cases).
Download figure:
Standard image High-resolution imageAnticipating the inactivation of the chaperones themselves, the concentration of the protective proteins was increased tenfold which, however, showed no significant change in the residual activity of rAaeUPO after treatment (supplementary figure 1, available online at https://stacks.iop.org/JPD/54/035204/mmedia). Hsp33 only acts as a chaperone in its oxidized form [24]. Therefore, Hsp33 was activated by bleach treatment prior to plasma exposure with rAaeUPO, but no benefit to rAaeUPO lifetime was observed (supplementary figure 2). Similarly, Hsp33 had been shown to be partially activated through plasma treatment [23], indicating that the activity of the chaperones themselves was presumably not critical in this case. Rather, aggregation of rAaeUPO was not the cause for its inactivation, therefore chaperones offered no benefit.
Next, several chemical scavengers were tested. Uric acid, trolox, and mannitol have previously been shown to scavenge peroxynitrite (ONOO−), hydroperoxyl radicals (•OOH) and hydroxyl radicals (•OH), respectively [27–29]. All three of these reactive species were shown to be produced in plasmas or plasma-treated liquids [30, 31]. Therefore, the scavengers were added to rAaeUPO prior to plasma treatment and the subsequent enzyme activity was measured (figure 2).
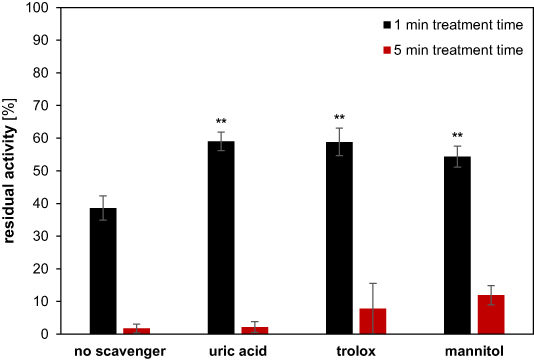
Figure 2. Influence of chemical scavengers on residual activity of rAaeUPO after plasma exposure. A mixture of 100 nM rAaeUPO and chemical scavenger (1, 0.5, and 100 mM of uric acid, Trolox, and mannitol, respectively) was treated with the DBD plasma for 1 min (black) or 5 min (red). Activity measurements were conducted as described above. Residual activity was calculated using untreated samples of rAaeUPO with the respective scavenger. Bars show means and standard deviations of three independent replicates. The significance of differences to the samples without scavenger was calculated using Student's t-test and is indicated by the asterisks (** = p < 0.01).
Download figure:
Standard image High-resolution imageAll scavengers offered a minor degree of protection against ROS during the 1 min treatment. The residual activity of rAaeUPO increased from 39% without any scavenger to 59%, 59% and 54% when using uric acid, trolox, and mannitol, respectively. For any application in biocatalysis, however, running times of hours are desirable [32]. Therefore, the treatment time with scavengers was increased to 5 min. With the increased treatment time, the protective effects diminished. Uric acid and trolox offered no significant protection against ROS after 5 min treatment. When rAaeUPO was treated in the presence of mannitol, the residual activity increased from 1.7% without the scavenger to 11.9% after 5 min of plasma exposure. Mannitol may therefore offer a moderate degree of long-term protection by scavenging •OH which is highly reactive. This is in congruence with previous studies where •OH was implied in protein fragmentation [12]. Since the concentration of mannitol used here was 100 mM, which certainly exceeds the concentration of •OH, better protection is not to be expected by adding more scavenger.
Since many protein-damaging reactive species are oxygen-derived, the use of pure nitrogen or argon for plasma ignition was investigated. To accommodate the change in gas atmosphere, the DBD source used in the experiments shown above was equipped with a vacuum housing with lateral gas flow. Before exposing rAaeUPO to the plasma, the housing was flushed with the respective gas to ensure that no impurities were present. Pure rAaeUPO was treated for up to 5 min and the activity was measured as described before (figure 3).
Figure 3. Activity of rAaeUPO after plasma treatment in different atmospheres. The DBD electrode was placed into a vacuum chamber with lateral gas flow that was set to amount to 1 bar of pressure for the gas used (synthetic air, nitrogen, or argon). The enzyme was placed onto a glass slide and treated for the indicated amount of time. The activity of rAaeUPO was determined and untreated activity set to 100%. Half-life values of the enzyme were obtained from non-linear regression curves. Means and standard deviations shown here were calculated from three independent experiments. Student's t-test revealed no significant difference between synthetic air and nitrogen (p > 0.05), but a significant difference between synthetic air and argon (p < 0.01) atmospheres.
Download figure:
Standard image High-resolution imageUsing synthetic air and nitrogen, the inactivation kinetics showed only minor differences. The half-life of the enzyme was estimated to be 75.3 and 82.3 s for synthetic air and nitrogen, respectively. Plasma ignited in argon turned out to inactivate rAaeUPO significantly faster than in synthetic air or nitrogen with an enzyme half-life of 28.1 s. This may be because argon is an atomic gas so that rotational or vibrational excitation does not occur and electron impact leads to ionization with a higher probability [33, 34]. With the same setup, the H2O2 production rates in the treated liquid were significantly higher for argon, indicating a higher influx of reactive species from the plasma [35]. The difference between Ar and the molecular gases may also indicate that crucial reactive species are generated at the plasma-liquid interface by reaction with the bulk water molecules rather than reactive species dissolving into the liquid [31, 36]. Nevertheless, both nitrogen and argon performed worse than air so that no further efforts were made to change the gas atmosphere.
We previously observed that when rAaeUPO was covalently bound to an inert carrier, enzyme activity after treatment was significantly higher than for the free enzyme [15]. We ascribe the protective effect of immobilization to the enzyme-free buffer zone above the macroscopic immobilization carrier. In this zone, reactive species can recombine without reaching the enzyme. We therefore tested different materials for rAaeUPO immobilization and evaluated their benefit in protecting the biocatalyst during plasma treatment. Immobilization of rAaeUPO was carried out according to the manufacturer's instructions and the binding efficiency was checked by determining the activity of residual free rAaeUPO in the supernatant (supplementary figure 3). After establishing that immobilization was successful for all carriers, the immobilized rAaeUPO was treated with plasma for 15 min and enzyme activity was measured (figure 4).
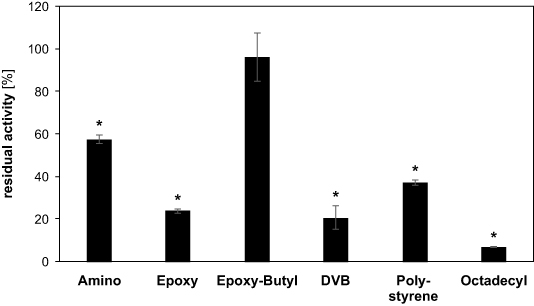
Figure 4. Residual activity of immobilized rAaeUPO after plasma exposure. Immobilization of rAaeUPO was carried out on Lifetech ECR carriers (Purolite) as described by the manufacturer's instructions. The immobilized rAaeUPO was then treated for 15 min and residual activity determined by setting the activity of the respective untreated, immobilized rAaeUPOs to 100%. Data shows means and standard deviations of three independent experiments. Significance was determined with Student's t-test. Data series with p < 0.05 compared to the respective untreated control are indicated with an asterisk.
Download figure:
Standard image High-resolution imageSurprisingly, the choice of the carrier greatly influenced the activity loss during plasma treatment, showing that increasing the distance between liquid surface and enzyme is not the only reason for increased lifetime. An overview of the different immobilization supports and their characteristics is given in table 1.
Table 1. Immobilization carriers used for rAaeUPO. Detailed information about immobilization carriers can be found in the methods section.
| Functional group | Immobilization type | Surface | Particle size (µm) | Pore diameter (nm) | Residual activity after plasma treatment (%) |
|---|---|---|---|---|---|
| Amino | covalent | hydrophilic | 150–300 | 60–120 | 57.47 |
| Epoxy | covalent | hydrophilic | 150–300 | 30–60 | 23.82 |
| Epoxy-Butyl | covalent | hydrophobic | 250–1000 | 45–65 | 96.23 |
| DVB | adsorption | hydrophobic | 300–710 | 22–34 | 37.03 |
| Styrene/DVB | adsorption | hydrophobic | 300–710 | 20–30 | 20.68 |
| Octadecyl | adsorption | hydrophobic | 300–710 | 40–65 | 6.93 |
The covalent immobilization on amino, epoxy, or epoxy-butyl carriers seemed to offer greater protection than the non-covalent immobilization on DVB, polystyrene, or octadecyl carriers. Also, the difference between epoxy and epoxy-butyl indicates that hydrophobic surfaces may offer an advantage to preserve rAaeUPO lifetime. The hydrophobic surface presumably repels the hydrophilic ROS and RNS, making them react with each other rather than with the enzyme itself. However, since all of the non-covalent methods also rely on hydrophobic interactions, this may only be the case in conjunction with covalent immobilization. When rAaeUPO was bound using epoxy-butyl carrier, activity after plasma treatment was 96.3% compared to the untreated sample, showing that no significant inactivation occurred.
Interestingly, carriers that showed the highest activity prior to plasma treatment also offered the best level of protection (supplementary figure 4). This may be attributed to a more general stabilization of the protein that helps during the turnover as well as during plasma treatment. This is in line with the observation that covalent immobilization offers greater protection since covalent techniques are reported to introduce greater rigidity in the protein structure [37]. However, the optimal method of immobilization may depend on the enzyme and operating conditions and can therefore not be generalized. Hence, the immobilization carriers used here may offer different degrees of plasma protection to different enzymes and need to be tested empirically for each enzyme.
In summary, this work shows that enzyme immobilization is the most effective technique to preserve enzymes during plasma treatment. Enzyme immobilization almost completely prevented inactivation of rAaeUPO. It also enables reuse of the enzyme-loaded carrier material, making immobilization an attractive strategy for plasma-driven biocatalysis. The mechanism of protection remains to be elucidated in future experiments. Nevertheless, the great variety of immobilization supports available will enable the improvement of enzyme lifetime under direct plasma exposure.
Acknowledgments
We acknowledge Cinogy for kindly providing the PlasmaDerm DBD sources. We thank Marco Krewing for fruitful discussions. The German Research Foundation is kindly acknowledged for providing funding (CRC1316-1 to JEB and PA and RTG2341 to JEB). Abdulkadir Yayci gratefully acknowledges travel support from the Japan Society for the Promotion of Science (JSPS) Core-to-Core Program JPJSCCA2019002.