Abstract
To investigate the potential role of the hydroxyl radical (•OH) in cold atmospheric plasma (CAP) jet treatment, two fluorescence-based methodologies are utilised to measure DNA strand breaks. The first comprises a model system of a double-stranded DNA oligomer, where the respective strand ends are tagged with fluorophore and quencher molecules; and the second, a cell culture system reporting DNA strand breaks using the γ-H2AX assay. During the various CAP jet treatments, optical emission spectroscopy is used to detect the •OH in the gas phase and electron spin resonance is used to detect the •OH in solution. The CAP jet production of the •OH is shown to correlate to CAP jet induced DNA damage both with the DNA model and in biological cells. Results indicate that the CAP jet induces a higher degree of DNA damage when the CAP plume is in contact with the target solution. The potential of a 'plasma screen' based upon a hydrogel film, as a method to remove the DNA-damaging •OH species from reaching skin cells, is shown to significantly reduce DNA damage whilst facilitating the delivery of hydrogen peroxide. These findings could aid in the development of CAP jet-based applications where DNA damage is the objective (e.g. in cancer treatment) and others where it is to be avoided, e.g. in open-wound treatment and dermatology.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
II Potential healthcare applications for cold atmospheric plasma (CAP) include dermatology [1, 2], wound decontamination and healing [3, 4], cancer therapy [5] and dentistry [6]. The possible (beneficial) effects of CAP in healthcare are often attributed to the broad range of reactive oxygen and nitrogen species (RONS) that are delivered from plasma to and/or created within the biological target [7, 8]. In addition to RONS, physical components of CAP (electric field, UV photons, charged particles, radiation, etc.) also play a major role in plasma-induced biological effects in tissues [9–12].
Within the human body, endogenous RONS act as key cell-signalling molecules with the ability to control/intervene in a variety of cellular and physiological processes. For instance, through very specific and highly regulated mechanisms, hydrogen peroxide (H2O2) has been shown to induce cell proliferation, cell differentiation and angiogenesis (generally at concentrations of <10 µM), whilst inducing apoptosis, necrosis and growth arrest in cells at concentrations higher than 0.1 mM [13–15].
Similarly, the superoxide radical (O2 •–) and the hydroxyl radical (•OH), along with H2O2 are produced by neutrophils and macrophages during the process of phagocytosis [16]. These act as potent microbiocidal agents that kill specific bacteria and viruses [17, 18]. Peroxynitrite (ONOO–) and nitric oxide (NO) have been reported as important intercellular messenger molecules for cell signalling and neural network activation at low concentrations (nM range) [19].
Amongst the wide range of endogenous RONS, the •OH is regarded as an important species due to its highly reactive nature and the ability to directly react with almost all biomolecules including DNA, unlike H2O2 [20–22]. The •OH reacts with DNA either by abstracting hydrogen from the amine group in the DNA bases or from the carbon groups of the sugar moiety [22, 23]. The former will result in oxidative base damage, while the latter causes cleavage of the phosphodiester backbone, i.e. strand breaks.
In biological systems, H2O2 can form the •OH upon reaction with metal ions bound to the DNA (for example: copper ions present in chromosomes) [24] or present within the cells in normal conditions (such as ferric ions) [25] via Fenton chemistry (reaction 1).
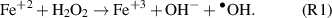
This intracellular production of the •OH is tightly regulated by anti-oxidant defense mechanisms which includes scavenging enzymes such as catalase, superoxide dismutase, glutathione peroxidase, peroxiredoxin and thioredoxin peroxidase [26, 27]. Another limiting/regulating factor for the production of the •OH in cells is the availability and location of metal ions, which are sequestered within the protein and DNA [28].
In certain healthcare applications, such as in the eradication of invading microbes or in cancer treatment, it is often desirable to induce detrimental changes in the target cells, such as irreversible DNA damage, in order to arrest the progression of a disease. However, in other applications such as the treatment of an open wound, the imperative should be to minimise unnecessary DNA damage.
If the objective is to produce the •OH within a biological target, the anti-oxidant defense mechanisms can be overcome through the exogenous production and delivery of RONS, e.g. through CAP treatment. During CAP treatment, the •OH is produced in the gas-phase (reaction 2) [29] and/or via interaction between the UV photons and liquid surface through the process of UV photolysis (reaction 3) [30]. The •OH can be subsequently converted to stable H2O2 (reaction 4) through recombination with neighbouring molecules [30], which play an essential role in cellular signalling processes [31].
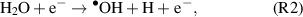
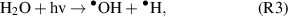
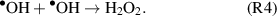
However generated, the plasma produced •OH has been linked to a number of biological effects including phagocytosis, apoptosis and DNA damage [32, 33]. This indicates that the plasma delivery of the highly reactive •OH can potentially be harnessed for the treatment of various refractory indications such as cancers. Given the potential beneficial effects of the •OH in plasma healthcare, yet at the same time considering its potential mutagenic risk, it is necessary to understand how to safely and effectively deliver the •OH to achieve specific biological effects.
To a certain degree, different biological effects can be achieved in plasma treatments by 'tuning' the plasma composition, including RONS, which can be accomplished by tailoring the design of the CAP device, or the CAP operational parameters (e.g. input power, frequency, flow rate) or in the manner of the CAP treatment (e.g. treatment distance, treatment time). Herein, we have investigated how the proximity of a target solution to a helium plasma jet influences the intrinsic plasma properties, how this correlates with the generation of the •OH in solution and the effects in terms of subsequent DNA modification, in the form of DNA strand breaks.
In an attempt to establish a 'safer' more real-world, implementable plasma treatment, where for example it is difficult to precisely control the plasma-target distance, the concept of a plasma screen (a hydrogel dressing) has been developed and is tested. By placing the hydrogel dressing in-between the biological target and CAP jet, we show that the delivery of the highly reactive •OH to the cells/tissue fluid is limited, without compromising the delivery of H2O2. Such an approach may be beneficial for chronic wound treatments by preventing undesirable damage from the CAP to the surrounding healthy cells and tissue. The results and conclusions drawn from this study strengthen our knowledge about the interactions of CAP with biological targets (cells and DNA) and this knowledge may help in the future development of CAP technology in various biomedical applications.
2. Materials and methods
2.1. Helium CAP jet
The CAP jet assembly consisted of a glass tube with a 6 mm outer and 4 mm inner diameter, respectively. The glass tube was tapered to 800 µm at the nozzle. A single, cylindrical copper electrode of 15 mm in length and a distance of 40 mm from the nozzle, was fitted over the glass tube so that the was no visible air-gap between the glass and copper. The electrode was powered by a PVM500 (Information Unlimited, USA) power supply. The flow rate of high purity helium gas (BOC) through the glass tube was controlled by a digital mass flow controller (APEX, USA) and fixed at 1 standard litres per minute (slpm). The input voltage supplied to the electrode was fixed at 10 kVp-p (peak-to-peak) at a frequency of 30 kHz. A schematic of the plasma jet assembly is shown in figure 1(a).
Figure 1. (a) A schematic of the CAP jet assembly. (b) A photograph of the CAP jet showing the position of the optical emission spectrometer optical fibre used to capture the optical emission in situ in a custom-built dark enclosure.
Download figure:
Standard image High-resolution image2.2. Electrical and optical plasma diagnostics
The voltage and current during plasma operation were recorded with a commercial voltage probe (Tektronix P6015) and current monitor (Pearson Electronics, model 2877), respectively, connected to a digital oscilloscope (Tektronix TDS2014B). Optical emission between 300 and 750 nm, was measured with a commercial fibre-optic spectrometer (Ocean Optics, Model—Flame-TX-R1-ES, Grating—#31-500/250) through a collimating lens. Measurements were taken in a custom-built 'dark box' to prevent interference from ambient light, as shown in figure 1(b). The optical spectrometer was placed perpendicular with the direction of CAP jet. The emission from the plasma plume was measured laterally at a distance of 20 mm from the end of the nozzle of the glass tube of the CAP jet assembly. The integration time of the spectrometer was set to 10 ms with a wavelength range of 280–780 nm.
2.3. Plasma screen
A 4 mm thick commercially available hydrogel dressing, IntraSite Conformable (Smith & Nephew, Catalogue no. 66000324), was used in this study. IntraSite Conformable is a hydrogel cream on a non-woven dressing material. The dressing was cut into approximately 20 × 20 mm2 pieces. For the plasma treatments through hydrogel dressing, 400 µl of the cell culture media or a model DNA (see section 2.4 below) in a 96-well plate was treated with the CAP jet. The hydrogel dressing was slightly pressed down into the well to ensure it contacted the solution.
2.4. Preparation of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer
HEPES buffer was prepared by dissolving 238.3 mg of HEPES (Sigma-Aldrich, Catalogue no. 3375), 624 mg of sodium chloride (Optigen Scientific, Catalogue no. OS-22760), 22.4 mg of sodium hydroxide (Sigma-Aldrich, Catalogue no. 367176) and 29.22 mg of Ethylenedinitrilo tetraacetic acid disodium salt (Optigen Scientific, Catalogue no. OS-53170) in 100 ml of water (Sigma-Aldrich, Catalogue no. 95283). The pH of the HEPES solution was adjusted to 7.4 using 10 mM of NaOH solution. The solution was filtered using a 0.2 μm syringe filter.
2.5. Assessment of DNA strand breaks in HEPES buffer
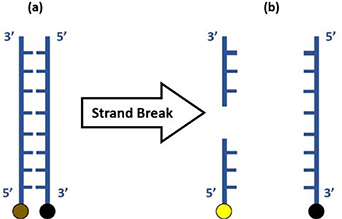
Double-stranded DNA with a length of 12 nucleotides was purchased from Sigma-Aldrich. The forward oligomer was labelled with a fluorescein amidite (6-FAM) fluorophore at the 5' end with sequence: 5'-[6-FAM]GCACTGAAGCGC-3'. The reverse oligomer was labelled with Black Hole Quencher (BHQ-1) at the 3' end with complimentary sequence: 5'-GCGCTTCAGTGC[BHQ-1]-3'. Intact, the BHQ-1 inhibits the fluorescence of 6-FAM in the double-stranded DNA. DNA strand break(s) leads to a separation of the BHQ-1 and 6-FAM and a switch-on of fluorescence, as shown in figure 2.
Figure 2. Schematic of the double-strand DNA used as a fluorescent probe to assess DNA strand breaks. (a) The DNA molecule consists a fluorophore (6-FAM, brown dot) at the 5' end of one strand and a quencher (BHQ-1, black dot) at the 3' end of the second strand. (b) One (as shown) or multiple DNA strand breaks anywhere on the molecule result in a separation of the fluorophore and quencher and subsequent switch-on of fluorophore fluorescence (yellow dot).
Download figure:
Standard image High-resolution imageA volume of 405 μl and 753 μl of 10 mM Tris-base buffer was added to 40.5 nmol of forward and 75.3 nmol of reverse oligomer, respectively, to obtain oligomer solutions of 100 μM each. Synthetic DNA solution with a working concentration of 1 μM was prepared by diluting both combined oligomer solutions in HEPES buffer. The double-stranded DNA probe was prepared by heating this solution at 95 °C for 3 min to first reversibly denature the double-stranded DNA, followed by annealing at ambient temperature for 3 h in the dark to allow re-hybridisation. This procedure ensured the correct conformation of the double-stranded DNA in solution.
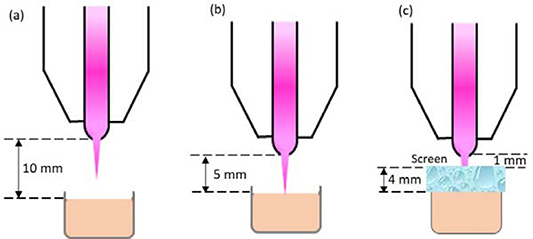
A volume of 200 µl of the DNA-HEPES solution was then dispensed into the well of a 96-well plate. The direct CAP jet treatment of the DNA solution (without a hydrogel dressing on top) was performed at two different distances of 5 mm and 10 mm such that the plasma plume is in contact (at d = 5 mm) and not in contact (d = 10 mm) with the DNA solution (figure 3(a) and (b)). For the indirect CAP jet treatment, 400 µl of DNA solution in a 96-well plate was treated through the screen (hydrogel dressing) at d = 1 mm (figure 3(c)). Fluorescence measurements were recorded using a microplate reader (BMG Labtech Fluostar Optima) at an excitation wavelength of 498 nm and emission wavelength of 522 nm.
Figure 3. Experimental setup for the three types of CAP jet treatment conditions: (a) non-contact (plasma plume is not in contact with the target solution in a well of a 96-well plate); (b) contact (plasma plume is in contact with the target solution); and (c) through a ca. 4 mm screen (a hydrogel dressing is placed on top of the well such that the plasma plume is not in direct contact with the target solution).
Download figure:
Standard image High-resolution image2.6. Measurement of H2O2
The concentration of H2O2 in HEPES after the CAP jet treatment was measured using an electrochemical probe (ISO-HPO-2, World Precision Instruments) interfaced to the Free Radical analyser (TBR4100-416, World Precision Instruments) [34].
2.7. Measurement of the •OH
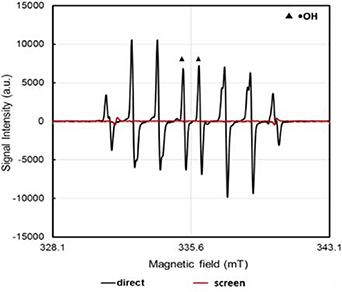
Electron spin resonance (ESR) spectroscopy was used to measure the •OH delivered by the CAP jet into solution. 5-(2,2-Dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) was used as the spin-trapping agent [35]. A 100 µl volume of 10 mM CYPMPO (Shidai system, Catalogue no. RR-121-A) was prepared in HEPES and treated with the plasma jet in a 96-well plate. Following CAP jet treatment, the ESR reaction sample was transferred to a flat cell (JEOL resonance, Catalogue no. DP-1055). The ESR signal was measured using an ESR spectrometer (JES-X310, JEOL Resonance, Tokyo, Japan), operating at 9.42 GHz and at 100 kHz field modulation. The settings of the spectrometer were: centre magnetic field = 335.6 mT; field width = ±15.0 mT; microwave power = 4 mW; modulation amplitude = 0.1 mT; and time constant = 0.1 s. The relative intensities of the ESR spectra were calculated by referencing against a manganese (Mn2+) internal standard. Relative magnetic intensities were plotted by subtracting peak signal intensities of untreated (control) from the intensities of CAP jet treated solution.
2.8. HaCaT cell culture
HaCaT keratinocyte-like cells were cultured in DMEM (Gibco®, Catalogue no. 11965118) supplemented with 10% (v/v) fetal bovine serum (Gibco®, Catalogue no. 1099141), 100 IU ml−1 of penicillin and 100 µg ml−1 of streptomycin (Gibco®, Catalogue no. 15140122) in T75 cell culture flasks (Corning®, Catalogue no. 353110). The cells were cultured at 37 °C in a humidified incubator with 5% CO2. The cells were passaged following the trypsinisation process such that a total of 7000 cells in 200 µl of DMEM was added to wells of a 96-well tissue cell culture plate for the direct CAP jet treatment (without screen on top). For the CAP jet treatments through the screen, 400 µl cell suspension (with 7000 cells in total) was treated for 240 s at d = 1 mm. The hydrogel dressing was not removed from the plate until the γ-H2AX assay was performed. After the CAP jet treatments, the cells (with and without the hydrogel dressing) were incubated overnight at 37 °C, 5% CO2, prior to the γ-H2AX assay.
2.9. γ-H2AX assay for the assessment of DNA strand breaks in HaCaTs
The cells were fixed with 4% (v/v) paraformaldehyde solution (ProSciTech, Catalogue no. EMS15710-S), permeabalised with 0.5% (v/v) Triton X-100 (Sigma-Aldrich, Catalogue no. T8787) and blocked with 3% (w/v) bovine serum albumin (BSA) solution (Sigma-Aldrich, Catalogue no. A7906). The cells were incubated with the primary antibody mouse monoclonal [9F3] to γ-H2AX [phospho S139] (Abcam, Catalogue no. ab26350) diluted at 1:1000 and the secondary antibody goat anti-mouse IgG H&L [Alexa Fluor® 488] (Abcam, Catalogue no. ab150113) diluted at 1:1000. The nucleus was stained using 300 nM of 4',6-diamidino-2-phenylindole (DAPI, Thermo Fischer, Catalogue no. D1306).
Fluorescence images were recorded on an Olympus microscope using a DP80 camera. The images were taken at an excitation/emission of 358/461 nm for DAPI and 495/519 nm for Alexa Fluor®. The exposure time for all of the images was kept constant at 137.93 ms for the DAPI channel and 500 ms for the Alexa Fluor® channel. The γ-H2AX positive nuclei from a total of up to 100 cells (DAPI stained nuclei) were counted for each condition for three biologically independent replicates. The images were processed using the Olympus cellSens Dimension software.
3. Results
The voltage waveform and input power (Pin) of the CAP jet for each treatment parameter (shown in figures 4(a)–(c)) was measured by electrical diagnosis. A discharge current (Id) was calculated by subtracting the displacement current (Idis) from the total current (It) as per the equation below [36]:

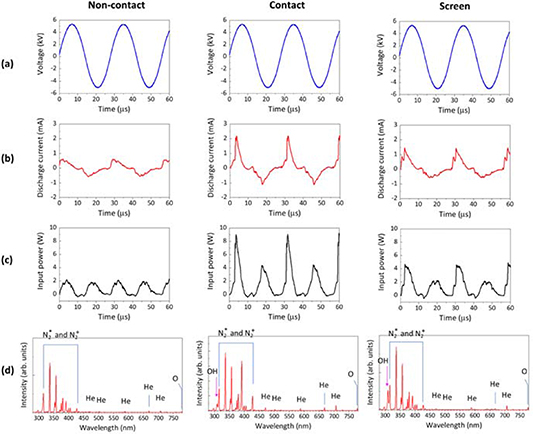
Figure 4. The electrical and optical emission properties of the CAP jet during treatment of the HEPES buffer solution. The four rows show (a) Vp-p, (b) Id, (c) Pin and (d) optical emission spectra between 280 and 780 nm. The three columns show results taken for each of the three CAP jet treatment conditions—i.e. plasma plume non-contact with HEPES buffer solution, plasma plume contact with HEPES buffer solution and through screen treatment (with the plasma plume in contact with the hydrogel dressing).
Download figure:
Standard image High-resolution imagewhere Idis is the current measured in absence of the plasma discharge (i.e. without applying Vp-p) and It is current measured for the plasma discharge with the applied Vp-p. Values of V and Id were used to calculate averaged Pin at a fixed frequency ( ) using the formula:
) using the formula:
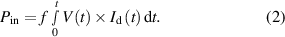
Figure 4(a) shows that the sinusoidal waveforms of Vp-p for the He plasma discharges are identical for the direct (plasma contact and non-contact with a HEPES buffer solution) CAP jet treatments and for treatment through the screen (plasma contacts the hydrogel dressing). However, as shown in figure 4(b), the shape and peak value of Id varies according to the target. The highest Id (2.21 mA), with a narrow peak width, is seen when the CAP jet is in contact with the liquid, the lowest is seen for the non-contact mode (0.63 mA), and the peak value for Id is somewhere between these two when the CAP jet is in contact with the screen (1.46 mA). A similar trend is observed for Pin as shown in figure 4(c). The closer treatment distance produced the highest value of Pin at 9.26 W. However, CAP jet treatment through the screen resulted in a Pin value of 4.89 W, double than that of the non-contact treatment condition, but half for the solution contact condition. Considering the current pulse width, the averaged-power for the contact treatment condition and the through-screen treatment is not very different (1.64 ± 0.03 for contact and 1.25 ± 0.04 for screen, respectively).
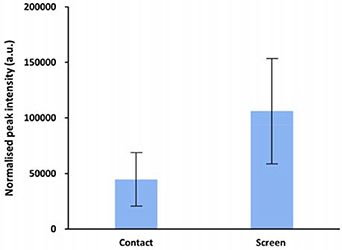
Following the electrical characterisation, an optical diagnosis of the CAP jet was performed by optical emission spectroscopy (OES). The typical optical emission spectra from 280 to 780 nm are shown in figure 4(d). For all treatment conditions (contact, non-contact and through the screen), the spectra in figure 4(d) confirm the presence of N2 species (N2 + second positive and N2* first negative systems) by spectral lines in the UV-A region (310–430 nm), excited He at 471.3, 501.6, 587.6, 667.8, and 706.5 nm and excited O at 777.5 nm (assignments made from the NIST Atomic Spectra Database). A slight increase in the signal intensities for He* is observed at the closer distance between the CAP jet and target of 5 mm (compared to the 10 mm treatment distance). No •OH emission is observed in the plasma plume for the non-contact treatment condition. However, when plasma is touching the liquid surface (contact condition) or the screen, •OH emission is observed at 308 nm. The peak intensity for •OH is higher for plasma treatment through the screen than the direct contact treatment. The •OH emission spectra normalised to the He peak at 706.5 nm is shown in figure 5 to directly compare the differences in •OH emission, which corroborates with our discussion directly above.
Figure 5. Normalised •OH peak intensities for the direct (contact) and indirect (through screen) plasma treatment conditions. The •OH emission intensities at 308 nm are normalised against the He emissions at 706.52 nm.
Download figure:
Standard image High-resolution imageThe extent of DNA damage induced by the CAP jet by the direct CAP jet and screen treatments was assessed using the double-strand DNA fluorescent probe synthetic DNA probe. Direct CAP jet treatments for the non-contact and contact condition were conducted at 1 slpm for 15, 30 and 60 s. A third condition comprises treatment through the screen for 240 s (to produce a solution H2O2 concentration in the same range as contact treatments; this will be discussed later) with the plasma plume in contact with the screen.
To measure the relative amount of DNA breaks, the normalised fluorescence intensity (FI) of the synthetic DNA probe was measured by:
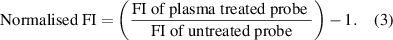
The data are presented in figure 6 and represent the mean value of three replicates (n = 3) and ±standard deviation (STDEV) error of the mean. For both contact and non-contact conditions, the results in figure 6 show that the extent of DNA damage increases with the plasma exposure time. At any equivalent exposure time, a much higher degree of DNA damage is observed for the contact versus non-contact scenario. These data allow the assessment that in tissue fluid, 60 s of 'non-contact' CAP jet treatment induces an equivalent level of DNA damage to 15 s of 'contact' CAP jet treatment. Since DNA damage was not eliminated with any of the direct CAP jet treatments, these data provide the basis to assess the role of the screen to limit DNA damage. As shown in figure 6, exposing synthetic DNA to plasma for 240 s through the screen reduces DNA damage to a lower level than the 15 s of direct CAP jet treatments in both contact and non-contact conditions. This implies that by employing the screen between the target and the CAP jet, the extent of DNA damage can be significantly restricted.
Figure 6. DNA damage measured in terms of DNA strand breaks shown by normalised F.I. Direct CAP jet treatments were carried out at 1 slpm for 15, 30 and 60 s or through the screen for 240 s. The data above represents the mean value of three replicates (n = 3) and ±standard deviation (STDEV) error of the mean.
Download figure:
Standard image High-resolution imageAs previously stated, it is speculated that the •OH delivered by the CAP jet is a major contributing factor in DNA damage, and that a reduction in DNA damage can be achieved by inhibiting the delivery of the •OH to the target (for example to cells). Therefore, we assessed by ESR the relative amount of the •OH delivered by the non-contact and contact direct CAP jet treatments and the screen treatment into the HEPES buffer. A typical ESR spectrum is shown in figure 7 for a direct CAP jet treatment and screen treatment. As shown in figure 7, when CYPMPO spin-trap in HEPES was directly treated with the CAP jet, the signal for the CYPMPO-OH adduct (i.e. •OH) is clearly observed. However, in the presence of the screen repeating the experiment, there are now no visible peaks corresponding to the trapped •OH. This suggests that the plasma screen can inhibit the CAP jet generation of the •OH in the HEPES, presumably by quenching the •OH within the gel matrix.
Figure 7. The ESR spectrum of the CYPMPO-OH adduct in HEPES after it was directly treated with the CAP jet (d = 10 mm, treatment time = 60 s) and indirectly through the hydrogel dressing/screen (d = 1 mm, treatment time = 240 s).
Download figure:
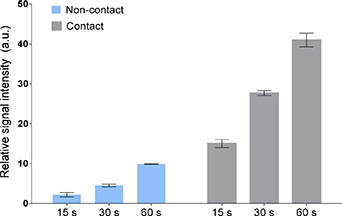
Standard image High-resolution imageThe relative signal intensities for the •OH in the ESR spectrum (calculated using the Mn2+ internal standard) for each CAP jet treatment parameter was calculated, and the results are shown in figure 8. As seen in figure 8, the relative signal intensities of the •OH peak increases as function of the CAP jet treatment time, for both non-contact and contact conditions. However, the production of the •OH is higher in the contact condition as compared to non-contact.
Figure 8. The relative signal intensities of the •OH after direct CAP jet treatments of the CYPMPO spin-trap in HEPES buffer. The peak intensities were calculated by referencing to a Mn2+ internal standard. Data are expressed as the mean ± STDEV (n = 3).
Download figure:
Standard image High-resolution imageIn the next set of experiments the concentration of H2O2 delivered by the CAP jet into HEPES in non-contact and contact conditions and in the presence of the screen were measured (figure 9). H2O2 was used to give an indication of the total RONS concentration on the basis that H2O2 is the major longer-lived RONS generated in aqueous solution for the He CAP jet configuration utilised in this study [37–39]. Moreover, H2O2 plays a significant role in cell proliferation and other important signalling mechanisms [31]. As shown in figure 9, the concentrations of H2O2 increases in a dose-dependent manner. Higher concentrations of H2O2 are produced for the contact condition (up to 300 µM) than the non-contact (up to 100 µM). However, a shorter treatment time of 15 s in contact condition generates an equivalent concentration of H2O2 compared to the longer treatment time of 60 s in the non-contact condition. It is also evident that the CAP jet can deliver a similar concentration of H2O2 through the screen as compared to the direct CAP jet treatments.
Figure 9. Concentration of H2O2 in HEPES generated by the direct CAP jet treatments and by the screen treatment.
Download figure:
Standard image High-resolution imageThe results so far show that the screen method of treatment can inhibit CAP jet induced DNA strand breaks for naked DNA freely suspended in HEPES. A further step in this study was to evaluate if this result could be translated to preventing DNA damage in skin cells. Therefore, in the following experiment, the γ-H2AX assay was performed using HaCaT keratinocytes that were subjected to the direct CAP jet treatment and to the screen treatment. The γ-H2AX assay is a marker for the DNA damage response (DDR), e.g. after a DNA strand break event in cells. To confirm that the biological effects are induced by CAP treatment, neutral He gas treatments (i.e. no plasma) were performed as a negative control (figure SI.03 (available online at stacks.iop.org/JPhysD/54/035203/mmedia) of the supplementary information). A neutral He gas treatment did not induce any DDR. Figure 10 shows a series of immunofluorescence images for all cell nuclei stained with DAPI-stained (blue) and γ-H2AX positive (green) nuclei captured 24 h after performing each of the treatments. The percentage of nuclei displaying phosphorylated H2AX (γ-H2AX) are calculated to provide a measure of the percentage of DNA strand breaks (figure 11). The percentage of DNA-strand breaks for each treatment condition is calculated using the following formula:
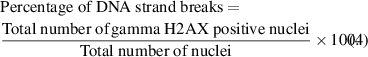
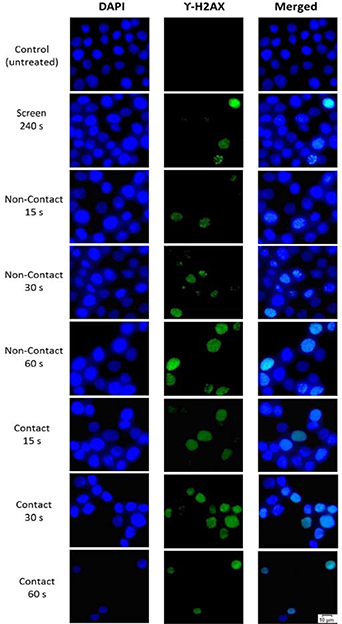
Figure 10. Micrographs from the γ-H2AX assay for direct CAP jet and screen treatments. The first, second and third column show the immunofluorescence images of DAPI stained nuclei, γ-H2AX positive nuclei and the overlayed images of columns 1 and 2, respectively.
Download figure:
Standard image High-resolution imageFigure 11. Percentage of DNA-strand breaks observed in HaCaT cells the γ-H2AX assay following direct CAP jet and screen treatments. Untreated control cells are shown for comparison. The γ-H2AX positive nuclei from a total of up to 100 cells (DAPI stained nuclei) were counted. Multi-way ANOVA was used to determine the statistical significance of the values. The values with * marks represent statistically significantly different values compared to the untreated (p < 0.05), whereas ns implies 'not-significant' (p > 0.05) i.e. the values are statistically insignificant compared to untreated.
Download figure:
Standard image High-resolution imageThe results of the γ-H2AX assay in figures 10 and 11 show that all direct plasma jet treatments activate the DDR in HaCaT cells, as compared to the untreated (control) cells (p < 0.05). The DNA strand breaks manifest in γ-H2AX positive nuclei, as shown in figure 10. The number of γ-H2AX nuclei increase with the treatment time and are higher with the plasma plume in contact with the cell media during treatment. A marginal increase γ-H2AX nuclei is observed for the screen treatment (p > 0.05). Figure 10 also highlights different types of γ-H2AX staining—pan-nuclear pattern, wherein the entire nucleus shows green γ-H2AX stain (e.g. in case of 15 s direct contact CAP jet treatment) and focal pattern, wherein distinct γ-H2AX foci are formed in the nuclei (e.g. in case of screen treatment). The frequency of pan-nuclear stained cells increased with the treatment time for non-contact and contact direct CAP jet treatments.
CAP jet treatments can potentially change the pH and temperature of the cell media that (in addition to RONS such as the •OH) might influence cellular activity. However, although we were unable to measure pH or temperature of the CAP jet treated media (because our pH and temperature probes were incompatible with the experimental set-up), based on our previous studies [40–42] and studies by other groups [43–45], we expect that neither pH nor temperature would have significantly impacted the cell results presented in this study.
4. Discussion
Previously, we have shown that H2O2 does not directly induce DNA strand breaks in a number modelled environments including surrogates of tissue fluid, cells and tissue [41]. This is in agreement with Verlackt et al, who also found that H2O2 does not directly break the phosphodiester backbone of DNA through a simulation study [46]. With the premise that the longer-lived RONS delivered by the plasma into the target are not involved in direct DNA damage, we focused on the highly reactive •OH, also recognised for its ability to directly induce DNA strand breaks in cells (e.g. in x-ray radiation therapy).
The emission spectra obtained from OES provides information of the presence of oxygen and nitrogen radicals (and metastables) in the plasma-phase (figure 4(d)). Although He*, O* and N2 species were produced in all the CAP jet treatment conditions (contact, non-contact and screen), the emission peak for the •OH only became prominent when the plasma plume was in contact with the target (HEPES, cell media or screen). Furthermore, the signal intensity for the •OH increased as the treatment distance reduced from 5 mm (direct contact treatment) to 1 mm (through-screen treatment). At small distances, the plasma induces higher evaporation of water, thus creating a source of more water molecules that can be dissociated by UV photons, emanating from the CAP jet, into the •OH. Secondary reactions with the highly reactive RONS (e.g. the •OH) in the solution generate longer-lived but less reactive molecules such as H2O2 and NO2 – [47, 48].
In the CAP jet treatment through the screen, the hydrogel dressing quenches the •OH within the hydrogel matrix (which was confirmed by the ESR results in figure 7). This prevented the delivery of the •OH into the target solutions. However, the screen facilitated the delivery of longer-lived H2O2 (figure 9). It should be noted that these findings are not limited to the 'commercial' dressing used in this study, but are also applicable to other types of hydrogels. We showed that a simple hydrogel film fabricated from gelatin (figure SI.02 of supplementary information) can significantly perturb the delivery of the •OH cf to the direct plasma jet treatments. The short-lived oxygen species, e.g. the •OH and O* produced near the screen, can undergo reactions to form secondary RONS including H2O2 near and within the screen surface. These longer-lived species, along with other plasma species, may accumulate within the screen and undergo subsequent hydration or solvation before penetrating through the screen via a yet-to-be defined slow molecular transport process [38]. These reactions may lead to production of secondary RONS such as the O2 •–and ONOO–. In our study we also detected the production of O2 •– in treatment through the screen (figure SI.01 in supplementary information). The low levels of DNA strand breaks observed in solution (figures 6 and 11), despite the presence of screen inhibiting the generation of the •OH in solution (figure 7), can be linked to the formation of the O2 •– and potentially other species. Further indication that other CAP jet generated molecules might also be involved in DNA strand breaks is seen by a similar level of DNA strand breaks produced between the 60 s non-contact treatment and 15 s contact treatment (figure 6) despite the latter producing more of the •OH in solution (figure 8). Studying the potential role of other CAP jet generated molecules involved in DNA strand breaks could be the subject of an interesting follow-up study.
To assess the biological significance of this result, DNA damage was assessed in HaCaT keratinocyte cells. If cells experience DNA damage such as DNA strand breaks, cells will initiate the DDR to repair DNA [49]. The earliest DDR process, which occurs within seconds after DNA damage, is phosphorylation of the histone variant H2AX to form γ-H2AX, which is mediated by phosphatidylinositol 3-kinase related kinases (PIKK) [50]. γ-H2AX formation is important for recruiting the DNA repair factors, the proteins important for cell cycle progression and the repair and remodelling of chromatin and DNA [51]. Therefore, γ-H2AX is considered as a good marker for DNA damage [52, 53].
In this study, we utilised a commercially available hydrogel dressing as screen to shield the target solution from the CAP jet to prevent the delivery of the •OH (figure 7) that mitigated DNA damage in the HaCaT cells (figures 10 and 11). The differential staining showing the pan-nuclear pattern and focal pattern for γ-H2AX after the direct CAP jet and screen treatments (figure 10), indicates different types of cellular response to the DNA damage. Studies conducted with UV rays and ionising irradiation have observed similar staining patterns [53, 54]. The focal staining pattern can be linked to localised DDR, while the pan-nuclear staining pattern indicates apoptotic (or pre-apoptotic) cells. Therefore, it is fair to say that CAP jet-induced effects in cells are a complex phenomenon, and that the γ-H2AX assay can serve as a good biomarker to distinguish DDR cells from apoptotic cells. The results from figure 10 imply that at shorter CAP jet treatment distances and/or longer exposure durations where there is an enhanced production/delivery of H2O2 and •OH, cells undergo apoptosis (pan-nuclear cells). However, during CAP jet treatment through the screen, where the delivery of the •OH to the cells is limited, localised DDR (focal staining) rather than apoptosis is observed. Overall, these results indicate that the •OH generated by the CAP jet treatments has a major role in causing DNA damage.
5. Conclusion
In this study, we show that a hydrogel dressing can screen out the •OH from being generated in solution during CAP jet treatment. We show that preventing the delivery of the •OH during CAP jet treatment, completely abrogates the occurrence of DNA strand breaks and DNA damage in HaCaT cells, which is not possible with the direct CAP jet treatments in this study. However, the screen approach still enables the delivery of the CAP jet generated H2O2 (a well-known antimicrobial agent and cell stimulatory molecule) at a concentration in the same range as the direct CAP jet treatments investigated in this study. These data indicate that the screen is a promising development for minimising unwanted potential genotoxic events in cells such as for applications in CAP jet treatments of open wounds.
Acknowledgments
NG, EJS, AJC and RDS express gratitude to the Australian Government's Cooperative Research Centre's Program and the Wound Management Innovation CRC for partially supporting this research through project SP09-02. EJS acknowledges the support from the Australian Research Council through the Future Fellowship (No. FT190100263) award. HK acknowledges JSPS KAKENHI Grant Number (26390096, 17K05095) and the Naito Research Grant. AM acknowledges a MEXT KAKENHI Grant Number 24108005. JSO and MI acknowledge MEXT Supported Program for the Strategic Research Foundation at Private Universities (S1511021).