Abstract
All-inorganic perovskite nanocrystals (NCs), such as CsPbX3 (X = Cl, Br, I) NCs, have become particularly interesting for lighting applications due to their efficient synthesis, higher stability over hybrid organic–inorganic perovskites (HOIP) and narrow emission bands associated with high photoluminescence quantum yields (PLQYs). However, a critical aspect is how to prepare pin-hole free films using solvent-based deposition techniques and additives to avoid electrochemically-induced degradation of the perovskites under device operation conditions. Here, we have synthesized all-inorganic CsPbX3 NCs using a hot injection synthesis route and characterized their structural, morphological and photophysical properties in detail. Furthermore, we have integrated these NCs into light-emitting electrochemical cells (LECs), achieving a brightness of 8 cd m−2 for the NCs comprising a mixture of bromide and iodide at low driving currents. Additionally, we have demonstrated that the use of the salt KCF3SO3 in the active layer not only decreases the injection voltage but also prevents halide segregation in perovskite NCs due to a further stabilization of the lattice with potassium ions. Overall, we believe that all-inorganic perovskite NCs are promising emitters for lighting devices, however, further efforts towards elucidating the interplay of the components of the active layer and driving schemes are necessary.
Export citation and abstract BibTeX RIS
Introduction
Hybrid organic–inorganic (HOIP, CH3NH3PbX3, X = Cl, Br, I) and all-inorganic lead halide perovskite (CsPbX3) materials have the potential to revolutionize both photovoltaic and lighting technologies [1–4]. However, the decomposition of the HOIP into PbX2 and volatile products, such as CH3NH2, HI and I2 due to the low lattice energy, has shifted the attention to the all-inorganic perovskite nanocrystals (NCs). These are more stable to degradation induced by moisture, oxygen, heat, light or combined effects thereof than their hybrid counterparts [5, 6]. Therefore, the interest in these NCs has been at high levels after the first demonstration of their synthesis by Protesescu et al [6–9].
The remarkable photoluminescence (PL) properties and simple synthetic routes of all-inorganic perovskite NCs have already established their place in lighting applications [10, 11]. They exhibit narrow size distributions as well as narrow PL emission bands with a full width at half maximum (FWHM) of 12–50 nm [5] along with high PL quantum yields (PLQYs) up to 95%–100% [6, 7, 12]. Furthermore, this class of NCs exhibits high defect tolerance, meaning that they are highly luminescent without any further electronic surface passivation, whereas such passivation is obligatory to obtain a high PLQY from conventional quantum dots (QDs) based on metal chalcogenides or metal pnictides [5, 13]. Additionally, tunable band gaps via quantum confinement or simple changes in composition as well as the solution processability have revealed their potential in light-emitting electrochemical cells (LECs), consisting of an active layer sandwiched between two electrodes [5, 6, 14–16].
Recently, the incorporation of conventional QDs in LECs was successfully demonstrated [17–19]. Inspired by these findings, we fabricated the first LECs based on HOIP NCs using an up-scalable spray-coating technique [20]. These devices showed luminance of 1–2 cd m−2 at injection voltages of 11–12 V. Additionally, we demonstrated the important role of the electrolyte matrix in the device operating mechanism, as devices without this component displayed no detectable luminance. Moreover, the devices were analyzed by applying both constant voltage and pulsed current driving schemes, showing that LECs based on HOIP and all-inorganic NCs follow a common LEC behavior [20, 21].
Here, we show the successful synthesis of all-inorganic CsPbX3 perovskite NCs via the hot injection method as well as their application in lighting schemes following the LEC concept. LECs were fabricated by blending the CsPbX3 NCs with an ionic polyelectrolyte comprised of trimethylolpropane ethoxylate (TMPE) and KCF3SO3. These devices featured a luminance of 8 cd m−2 for mixed halide CsPb(Br/I)3 NCs at low injection voltages of 4 V unlike their hybrid counterparts, which require injection voltages of around 11–12 V. Additionally, the spectral electroluminescence (EL) width of the devices (~30 nm) is narrower than that of the HOIP-based NCs (~150 nm), owing to the homogeneous size distribution and superior structure stability of the all-inorganic NCs. We also show that the addition of the salt KCF3SO3 enhances the device performance due to improved carrier injection and suppresses halide segregation in mixed halide systems with further stabilization of the lattice by potassium cations. As a result, we believe that all-inorganic perovskite NC-based LECs provide an alternative route to circumvent complicated fabrication processes of other perovskite-based lighting devices.
Methods
Synthesis of CsPbX3 NCs and characterization details
The NCs were synthesized according to a published report by Protesescu et al [6]. All chemicals except olelylamine (OLA) were purchased from Sigma Aldrich and used as received. OLA (approximate C18-content 80%–90%) was purchased from Acros Organics.
Preparation of Cs-oleate.
Cs2CO3 (0.814 g), octadecene (ODE, 40 ml) and oleic acid (OA, 2.5 ml) were loaded into a 250 ml 3-neck flask and dried under vacuum for 1 h at 120 °C. Then, the solution was heated under N2 to 150 °C until Cs2CO3 reacted with OA.
Synthesis of CsPbX3 NCs.
OLA and OA were dried under vacuum for 2 h at 120 °C before use. ODE (10 ml) and PbX2 (0.376 mmol, X = Cl, Br, I) or mixtures of the lead halide salts were loaded into a 100 ml 3-neck flask and dried under vacuum for 1 h at 120 °C. Dried OLA (1 ml) and OA (1 ml) were injected into the flask at 120 °C under a N2 atmosphere. After all PbX2 was solubilized, the temperature was increased to 140 °C–200 °C, the Cs-oleate solution (0.8 ml, prepared as described above) was quickly injected and the reaction mixture was then rapidly cooled by an ice-water bath. In the synthesis of CsPbCl3 NCs, the higher temperature of 150 °C and 1 ml of trioctylphosphine (TOP, Fluka) were necessary to solubilize the lead salt. The resulting solution was centrifuged at 10 000 rpm for 10 min and the solids were redispersed in 1 ml toluene. The NCs were centrifuged at 12 000 rpm for 10 min and the precipitate was used for the fabrication of LECs.
Characterization details.
The powder XRD measurements were performed using a Cu-Kα1-source on a G670 Imaging Plate Guinier diffractometer (Huber Diffraktionstechnik GmbH & Co. KG). Steady-state absorption spectra were measured with a Lambda 1050 UV–VIS spectrophotometer (PerkinElmer, Inc.) using an integrating sphere. Steady-state photoluminescence measurements were performed with a Fluotime 300 spectrofluorometer (Picoquant GmbH). The excitation wavelength was fixed at 405 nm. TEM measurements were performed with a JEM-2011 (JEOL GmbH) equipped with LaB6 operated at 200 kV and an EDS (EDAX) detector. AFM characterization was performed under ambient conditions using a NANOINK atomic force microscope with Si n-type tip with a radius of <10 nm in tapping mode with a scan rate of 0.2 Hz, a proportional gain of 50 and an integral gain of 30. The PLQYs were measured using an integrating sphere in a Fluorolog-3 FL3-22 (Horiba Jobin Yvon GmbH) spectrometer. For the photostability measurements, a colloidal solution of CsPbBr3 NCs was continuously illuminated at an excitation of 405 nm, and the intensity of the PL maxima (515 nm) was recorded as a function of excitation time.
Fabrication of LECs and characterization details
Indium-doped tin oxide (ITO) coated glass substrates were patterned by etching with zinc powder and an HCl solution (3 M). The substrates were washed with deionized water and a 2% Hellmanex detergent solution. They were consecutively cleaned with deionized water, acetone, and ethanol and dried by pressurized air. The substrates were further cleaned with 2-propanol before the deposition of poly(3,4-ethylenedioxyth-iophene):poly(styrenesulfonate) (PEDOT:PSS). PEDOT:PSS (Heraeus) was diluted with 2-propanol at a ratio of 1:2 and this solution was spin-coated (dynamic dispense) on ITO substrates at 2000 rpm for 45 s. The layers (50–60 nm) were annealed at 120 °C for 10 min. The precipitate from the purification of NCs was used for creating the active layer. 0.25 ml of trimethylolpropane ethoxylate (TMPE, 5 mg ml−1 in hexane) was added to the NCs. Thereafter, 5 µl of KCF3SO3 (1 mg ml−1 in THF) was added to the mixture of polymer and NCs. This mixture was deposited on PEDOT:PSS layers via spin-coating (static dispense) at 1000 rpm for 30 s, resulting in a thickness of 60–70 nm. The aluminum cathode (90 nm) was thermally evaporated using a shadow mask under high vacuum (<1 × 10−6 mbar) using a Covap evaporator (Angstrom Engineering) integrated into the inert atmosphere glovebox. Current density and luminance versus voltage were measured using an Avaspec-ULS2048LTEC spectrophotometer (Avantes) calibrated with a white LED in conjunction with 1916-c optical power meter (Newport Corporation) equipped with a calibrated silicon diode and an OLT OLED Lifetime-Test system (Botest Systems GmbH). The EL spectrum was recorded using the above-mentioned Avantes spectrophotometer.
Results
Synthesis and characterization of CsPbX3 NCs
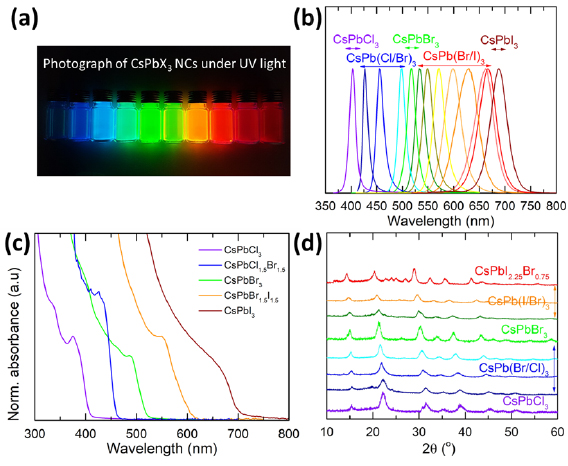
We synthesized CsPbX3 NCs (X = I, Br, Cl) following the hot injection method, where the previously prepared cesium-oleate solution was reacted with a warm solution of the PbX2 in a high boiling solvent (octadecene) at 170 °C (for details, see methods section) [6]. OLA and OA were added to the reaction flask to dissolve PbX2 and to stabilize the NCs. Mixed-halide perovskite NCs were prepared by mixing the appropriate ratios of PbX2 salts. Photos of the NC dispersions under UV light are displayed in figure 1(a).
Figure 1. (a) Photographs of NC dispersions with different halide composition under UV-illumination (366 nm) and their corresponding (b) PL spectra, (c) UV–VIS spectra and (d) XRD patterns.
Download figure:
Standard image High-resolution imageWe investigated the optical features of the NCs in detail by obtaining steady-state PL and absorption spectra of their dispersions (figures 1(b) and (c)). Importantly, both can be tuned over the entire visible spectral region by changing the halide ratio during the synthesis. Notably, all samples exhibit sharp band edges in the absorption spectra. Additionally, the dispersions all showed narrow emission line widths of 11–48 nm (see table S1) and high PLQYs of 40%–80%, whereby the low PLQYs (<50%) belong to chloride-based NCs. Furthermore, the high PLQYs suggest that surface dangling bonds do not play a significant role in the emission. We emphasize that the photostability of CsPbBr3 NCs in solution is exceedingly high. Under continuous excitation at 405 nm, only a small drop in PL intensity at the beginning of the measurement was observed, as seen in figure S1 (stacks.iop.org/JPhysD/51/334001/mmedia). These results highlight the suitability of the NCs for lighting applications.
Cesium-based lead halide perovskites are known to crystallize in orthorhombic, tetragonal and cubic polymorphs of the perovskite lattice [6, 22, 23]. However, here x-ray diffraction (XRD) data show that all CsPbX3 NCs are in the cubic phase (figure 1(d)). The reflections in the XRD patterns are shifted due to the different unit cell sizes, resulting from variation of the halide component. As expected, upon incorporation of Cl− the lattice shrinks, and all the peaks shift to higher angles while incorporation of I− expands the cell and the peaks shift to lower angles due to the larger unit cell. Furthermore, transmission electron microscopy (TEM) images not only confirm the high crystallinity of the NCs but also show the fairly homogeneous size distribution of the perovskite NCs (figure 2).
Figure 2. TEM images of (a) CsPbBr3 and (b) CsPbI3 NCs at different magnifications.
Download figure:
Standard image High-resolution imageFabrication of light-emitting electrochemical cells (LECs)
Taking advantage of the remarkable features of these perovskite NCs, we have successfully fabricated LECs. We initially purified the NCs by washing them with toluene once after the synthesis and found this process to be crucial for the formation of homogeneous films, as shown in AFM images in figure S2. Whereas the films prepared from the non-purified NCs showed the formation of micron-sized rods due to unreacted excess ligands in the solution, the films prepared with purified NCs possessed a homogeneous morphology without any visible rods. For device fabrication, we mixed the purified NCs with TMPE polymer and KCF3SO3 and spin-coated the solution on ITO-coated glass substrates modified by the deposition of PEDOT:PSS. The devices were completed by thermal evaporation of aluminum (Al) serving as the top electrode. A schematic structure of these devices is shown in the inset of figure 3(a) (see methods section for further experimental details).
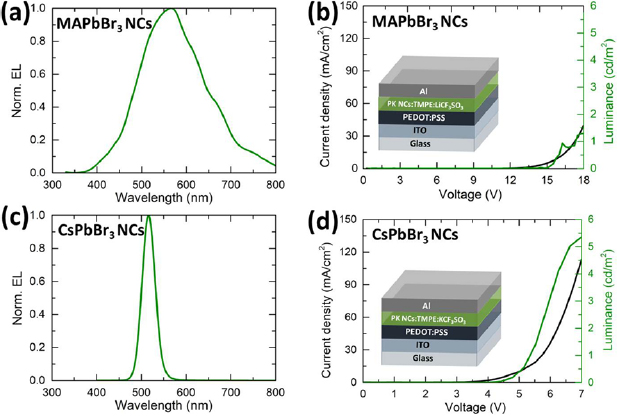
Figure 3. EL spectra (left) and luminance-current density versus applied voltage characterization (right) of LECs with (a) and (b) MAPbBr3 NCs and (c) and (d) CsPbBr3. The insets in (b) and (d) show the architecture of LECs used in this work. (a), (b) Reprinted with permission from [20]. Copyright 2015 American Chemical Society.
Download figure:
Standard image High-resolution imageTo assess the quality of the all-inorganic NC-based LECs we compare their performance with that of our previously demonstrated HOIP perovskite NC-based LECs using methylammonium lead bromide (MAPbBr3) NCs [20]. Corresponding luminance-current density versus applied voltage (LIV) plots are depicted in figures 3(b) and (d) for the HOIP NCs and all-inorganic CsPbBr3 without any purification, respectively. The LIV assays show an injection voltage of around 4 V with a maximum luminance of 5.4 cd m−2 for the CsPbBr3 NC based LECs. In comparison, the HOIP-LECs display an injection voltage around 12 V with a maximum luminance of 1.4 cd m−2. Additionally, the inorganic NCs show very sharp and narrow EL spectra in stark contrast to the very broad EL emission of the HOIP-LECs (figures 3(a) and (c)). This highlights some of the advantages of all-inorganic perovskites for LEC devices.
In general, the main reason for the rather low performance of single-layer devices is still unknown, but it has been tentatively attributed to the electrochemical stability with respect to (i) halide segregation and (ii) formation of hot spots located at surface defects where non-radiative electron–hole recombination occurs [24]. Therefore, some passivation is still needed to achieve stable devices [24–26].
Here, we found that devices with mixed halide perovskite compositions exhibit different behavior in the case that the salt KCF3SO3 is added to the purified NC and TMPE polymer mixture (figure 4). To study this effect, we focused on LECs with CsPbBr1.25I1.75 NCs. Here, devices without the salt exhibited an EL emission with a maximum at 640 nm and a secondary peak at 520 nm (figure 4(a)) evolved upon applying a high voltage (7 V) and low luminance of <1 cd m−2 (figure 4(b)). The low brightness of the devices is due to the reduced ionic mobility in the active layer and the lack of efficient electron–hole recombination. This is a result of the working principle of LECs, as ionic polyelectrolytes without salt do not control the growing doped regions near the electrodes. On the other hand, devices with mobile ions show an injection voltage of around 4 V as well as a luminance of 8 cd m−2 along with an EL spectrum centered at 640 nm up to an applied bias of 10 V (figures 4(c) and (d)).
Figure 4. EL of the LECs comprised of CsPbBr1.25I1.75 NCs (a) without salt and (c) with salt (KCF3SO3). LIV characterization of the same devices (b) without salt and (d) with salt at 10 V.
Download figure:
Standard image High-resolution imageDiscussion
The working principle of LECs is based on a p-i-n junction, where the ions accumulate at the electrode interfaces once a voltage is applied. The doped regions grow with time, leading to the formation of a p-i-n junction between them and the drop in the applied potential triggers the charge recombination and light emission [16, 27]. Therefore, an optimized salt concentration balances the space charge effects at the interfaces, improving the conductivity of the layer by electrochemical doping and increasing the current density as well as the recombination rate in devices [28–31]. For perovskite NC-based LECs, the addition of salt, KCF3SO3, analogously provides small, mobile ions that can accumulate in high concentrations near the electrodes. Therefore, the improvements in injection voltage and luminance values are rationalized in terms of balanced charge carrier injection after increased redistribution of ionic space charges, confirming the results of Li et al who demonstrated a single layer light-emitting diode using a perovskite/poly(ethylene oxide) composite film with a low turn-on voltage thanks to the ionic conductivity of the film and thus the formation of a p-i-n homojunction [32].
Our results show that all-inorganic perovskite-based LECs outperform their HOIP NC-based counterparts, both in terms of injection voltages and total luminance [20]. The improvement in the luminance values can be attributed to the significantly higher PLQYs of the all-inorganic NCs (40%–80%), in comparison to only 5%–15% obtained from HOIP NCs in our previous work. Additionally, time-resolved PL measurements indicate that the inorganic perovskite NCs exhibit significantly higher radiative recombination rates, as the PL lifetimes in the HOIP NCs are longer despite a significantly lower quantum yield, as shown in figure S3.
Interestingly, we observe lower injection voltages in all-inorganic LECs, which we attribute to the higher conductivity of inorganic perovskite CsPbBr3 NCs than HOIP ones [21]. Additionally, the striking difference in the FWHM of the EL spectra between LECs based on the HOIP NCs and all-inorganic NCs is likely due to their large distribution in size of the former that causes degradation of the active layer upon application of the electric field and changes of the spatial light distribution. Thus, this finding suggests that inorganic perovskite NCs are more stable and maintain the same spectral shape in the devices.
Mixed halide perovskites are known to exhibit halide segregation upon illumination [33, 34]. We have also observed the same phenomenon with the application of voltage in our devices [35]. However, it is noteworthy that this can be overcome with the incorporation of the salt KCF3SO3. This may be due to the fact that the small K+ cation can find a place in the cystal structure and cause a contraction of the PbX6 octahedral volume, thus improving the phase stability as Nam et al reported for CsPbI2Br1 perovskite with the incorporation of KI [36]. Calculations have demonstrated that the K+ ions preferentially locate on interstitial sites rather than on substitutional sites due to energetic reasons [37, 38]. Thus, in light of these observations, the inclusion of K+ cations in the active layer seems to prevent halide segregation by occupying intersititial sites.
Conclusion
In this work, we have successfully synthesized all-inorganic perovskite NCs with different halide compositions via a hot injection method. These NCs show high PLQYs and narrow PL emission as well as a rather narrow size distribution. Taking advantage of the excellent optoelectronic properties of these NCs, we have fabricated working LECs. Furthermore, we demonstrate an enhancement of luminance values of LECs based on inorganic perovskite NCs as well as a decrease in the injection voltages from 7 V to 4 V with the addition of a KCF3SO3 salt, which we attribute to improved control of space charge effects and balanced carrier injection. Our results also show that the salt helps to stabilize the perovskite structure in mixed halide systems by suppressing halide segregation. Moreover, our results suggest that all-inorganic perovskite NCs are more efficient than hybrid organic–inorganic perovskite NCs due to their higher PLQYs and narrower size distribution. Therefore, we believe that the use of all-inorganic perovskite NCs in LECs shows promising potential for the development of efficient lighting technologies with single layer solution-processable devices.
Acknowledgment
The authors specially thank Dr Steffen Schmidt from LMU München for TEM images. The authors acknowledge funding from the Bavarian Collaborative Research Program 'Solar Technologies Go Hybrid' (SolTech), the Center for NanoScience (CeNS), and the DFG Excellence Cluster 'Nanosystems Initiative Munich' (NIM). M F A acknowledges the Scientific and Technological Research Council of Turkey. B M D P gratefully acknowledges the support of the Cluster of Excellence 'Engineering of Advanced Materials (EAM)' at the University of Erlangen-Nuremberg, which is funded by the German Research Foundation within the framework of the 'Excellence Initiative'. Y T acknowledges the China Scholarship Council (CSC). R D C acknowledges the program 'Ayudas para la atracción de talento investigador—Modalidad 1' of the Consejería de Educación, Juventud y Deporte—Comunidad de Madrid, with the reference number 2016-T1/IND-1463 and Spanish MINECO for the Ramón y Cajal program (RYC-2016-20891). R D C and B M D P thank Professor Dirk M Guldi for his kind support and advice. M F A and P D acknowledge support from the Federal Ministry of Education and Research (BMBF) under the project ID 03SF0516B. M F A, Y T, A S U, and P D, acknowledge the CeNS Seed Funding.