Abstract
The biological approach to synthesis of AuNPs is eco-friendly and an ideal method to develop environmentally sustainable nanoparticles alternative to existing methods. We have developed a simple, fast, clean, efficient, low-cost and eco-friendly single-step green chemistry approach for the synthesis of biocompatible gold nanoparticles (AuNPs) from chloroauric acid (HAuCl4) using a water extract of Eclipta Alba leaves at room temperature. The AuNPs using Eclipta extract have been formed in very short time, even in less than 10 min. The as-synthesized AuNPs were thoroughly characterized by several physico-chemical techniques. The in vitro stability of as-synthesized AuNPs was studied in different buffer solutions. A plausible mechanism for the synthesis of AuNPs by Eclipta extract has been discussed. The biocompatibility of AuNPs was observed by in vitro cell culture assays. Finally, we have designed and developed a AuNPs-based drug delivery system (DDS) (Au-DOX) containing doxorubicin (DOX), a FDA approved anticancer drug. Administration of this DDS to breast cancer cells (MCF-7 and MDA-MB-231) shows significant inhibition of breast cancer cell proliferation compared to pristine doxorubicin. Therefore we strongly believe that the use of Eclipta Alba offers large-scale production of biocompatible AuNPs that can be used as a delivery vehicle for the treatment of cancer diseases.
Export citation and abstract BibTeX RIS
1. Introduction
Nanotechnology is one of the fastest growing areas of science and technology, with an exponential progress in biomedical applications including imaging, diagnostics, drug delivery and therapeutics using metal nanoparticles [1–8]. Among several nanoparticles, AuNPs have potential applications in the field of electronics, catalysis, biological sensors, cancer diagnostics, therapeutics, and nanomedicine, due to advantages such as (i) easy synthesis by simple, economically cheap, safe wet chemical and other reliable physical methods, (ii) size control (5–100 nm), (iii) easy characterization due to its red coloration, (iv) monodispersity, (v) easy functionalization, and (vi) biocompatibility and non-toxicity [2, 7, 9, 10]. Many groups, including our group, have already established the diagnostic and therapeutic applications of AuNPs, where the gold nanoparticles were synthesized by chemical methods [7, 9–16]. Although these AuNPs synthesized by chemical methods are biocompatible, however the synthesis of eco-friendly nanoparticles is a continuing interest of nanoscience for biomedical application [2, 3]. Recently, a biosynthetic approach for the synthesis of non-toxic AuNPs has attracted considerable attention because synthesis of AuNPs by green chemistry methods is simple, economically cheap, convenient and environmentally safe. On the other hand, synthesis of nanoparticles by chemical methods is not economically cheap and eco-friendly, as the chemicals are expensive and some toxic chemicals may be adsorbed on the surface of as-synthesized AuNPs. Therefore, recently, several groups have been engaged in green synthesis of biocompatible AuNPs with different size and shape, taking the advantage of efficient bioresources such as plants, fungi, algae, microorganism etc as the sources of reducing agent [17–22].
In this report we have utilized a single-step green chemistry approach for the synthesis of biocompatible AuNPs in aqueous solution using a medicinally important herbal plant, 'Eclipta Alba' (known as Bhringraj locally in India). The in vitro stability and biocompatibility of as-synthesized AuNPs was observed by several assays. Finally, we have designed and developed a AuNPs-based drug delivery system (DDS) containing doxorubicin (Au-DOX) and found that administration of this DDS to breast cancer cells shows significant inhibition of breast cancer cell proliferation. Therefore we strongly believe that the use of Eclipta Alba leaves allows the large-scale production of biocompatible AuNPs that can be used as delivery vehicles for the treatment of several cancers. To the best of our knowledge there are no reports involving Eclipta Alba-assisted synthesis of biocompatible AuNPs and their potential application as delivery vehicles for cancer therapy.
2. Experimental procedures
2.1. Materials
Tetrachloroauric acid (HAuCl4) was purchased from Sigma, Aldrich Chemicals, St Louis, MO, USA and was used without further purification. Initially, we have prepared 10−2 M of HAuCl4 solution in sterile Millipore water and used this stock for the synthesis of AuNPs and its nanobioconjugates. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) reagent was purchased from Calbiochem. Eclipta Alba was collected from the village of Hyderabad, Andhra Pradesh, India. Breast cancer cells (MCF-7; MDA-MB-231) were purchased from the American Type Culture Collection (ATCC), USA.
2.2. Preparation of aqueous extract from leaf of Eclipta Alba
Initially, 37.5 g of Eclipta Alba was thoroughly washed with distilled water followed by sterile Millipore water in a 500 ml beaker, boiled in 400 ml sterile Millipore water for 3 min at a setting of 800 W in a domestic microwave oven (Samsung 800 W, DE68-03844L), and then stirred for 3 h continuously on a magnetic stirrer. The color of the extract was dark green and was centrifuged at 10 000 rpm for 10 min at 4 °C. We discarded the precipitate that contains chlorophyll-like elements and collected the supernatant (light yellow). This light yellow color aqueous leaf extract of Eclipta Alba (denoted as EA) was used for the synthesis of AuNPs using different concentrations of extract.
2.3. Biosynthesis of gold nanoparticles (AuNPs)
We have synthesized AuNPs using a biosynthetic route, where we have used chloroauric acid as the AuNPs precursor, sterile Millipore water as the solvent and an extract of Eclipta Alba plant leaf as both a reducing agent and stabilizer of AuNPs. Briefly, we have carried out a set of reactions containing a final volume of 5 ml where we have used a constant amount of chloroauric acid (200 μl of 10−2 M) with different volumes of the Eclipta extract (EA) from 100 to 1000 μl (please see table 1). The reaction mixture was allowed to stir continuously on a magnetic stirrer from minimum of 10 min to 96 h, depending on the formation of AuNPs. Experiment number #5 in table 1 takes the minimum time for the appearance of the red color indicating the formation of AuNPs. Experiment number #1 takes more time (4 h) for the appearance of first red color. These results indicate that with increasing concentration of extract, the reaction occurs faster. The as-synthesized AuNPs were characterized by several physico-chemical techniques and used for cell culture experiments after 15 min of UV irradiation inside the cell culture hood.
Table 1. Reaction conditions for the green synthesis of gold nanoparticles (AuNPs): the biocompatible AuNPs were synthesized from HAuCl4 using different volumes of Eclipta Alba extract under ambient conditions. The total volume of HAuCl4 and final reaction mixture remained unchanged.
| Exp. No | EA-W (Extract) (μl) | HAuCl4 (μl) | Water (ml) | Total volume (ml) | Time (min or h)a |
|---|---|---|---|---|---|
| (1) | 100 | 200 | 4.7 | 5 | ∼4 h |
| (2) | 250 | 200 | 4.55 | 5 | ∼90 min |
| (3) | 500 | 200 | 4.3 | 5 | ∼60 min |
| (4) | 750 | 200 | 4.05 | 5 | ∼20 min |
| (5) | 1000 | 200 | 3.8 | 5 | ∼10 min |
aTime required for the appearance of the red coloration (formation of AuNPs).
2.4. Synthesis of AuNPs by a chemical method and its conjugation with mercapto undecanoic acid (MUA)
AuNPs were synthesized by sodium borohydride for the reduction of chloroauric acid according to our published articles [9, 11]. Initially, a stock solution of tetrachloroauric acid with 10−2 M was prepared by dissolving 1.0 g of HAuCl4 in autoclaved mili-Q water. 10−4 M of HAuCl4 was prepared by diluting the original stock, and an aqueous solution of sodium borohydride was added under vigorous stirring continued for 12–16 h to obtain the AuNPs [9–11]. We make a gold nanoconjugate (Au-MUA) by incubating 10 μg ml−1 of MUA in 1 ml of AuNPs solutions at RT for 45 min. Finally we collected the loose pellet of AuNP-MUA after centrifugation at 14 000 rpm at 10 °C for 1 h. These chemically synthesized AuNPs were used for in vitro toxicity studies in comparisons with biosynthesized AuNPs.
2.5. Preparation of the standard curve of doxorubicin (DOX)
We took different concentrations of DOX, starting from 1 to 20 μg ml−1 in water, measured the UV absorbance and plotted this versus concentration to get the standard curve of DOX.
2.6. Conjugation of doxorubicin with AuNP-EA-1000
We added 5 μg ml−1 of DOX in the as-synthesized AuNP-EA drop by drop and stirred vigorously for 45–60 min. Then we centrifuged the AuNP-DOX at 16 000 rpm at 10 °C for 1 h and collected the loose pellets of AuNP-DOX for treatments in the MCF-7 cell line to check the anticancer property of this DDS.
2.7. Cell culture studies
Breast cancer cells (MCF-7, MDA-MB-231) were incubated with AuNPs in a dose-dependent manner to check the biocompatibility. All cell lines were maintained in DMEM (Dulbecco's Modified Eagle Medium) supplemented with 10% fetal bovine serum (FBS), 5% l-glutamine, and 1% antibiotics (penicillin, streptomycin purchased from Sigma-Aldrich) in a humidified 5% CO2 incubator at 37 °C.
2.8. Cell viability test using MTT reagent
The MTT assay has been used to determine the cell viability in the presence of any cytotoxic potential medicinal agents/nanomaterials. Briefly, MCF-7 and MDA-MB-231 cells were incubated with Eclipta extract, and as-synthesized AuNPs, in particular AuNP-EA-1000 (Experiment number #5 from table 1), Au-MUA (chemically synthesized) and DOX in water and AuNP-DOX in a dose-dependent condition for 48 h and checked for biocompatibility of the AuNP-EA-1000. The detailed procedure is discussed in supporting information (available at stacks.iop.org/Nano/23/455103/mmedia).
3. Characterization techniques
The as-synthesized AuNPs were thoroughly characterized by several physico-chemical techniques, described in detail in supporting information (available at stacks.iop.org/Nano/23/455103/mmedia). Initially, the red intense loose pellets of as-synthesized AuNPs samples were collected by centrifugation at 14 000 rpm at 10 °C for 1 h in a Thermo Scientific, Sorvall-WX Ultra 100. These loose red AuNPs pellets were used for further characterizations. The absorption and crystallinity of as-synthesized AuNPs were measured by UV–vis spectroscopy and x-ray diffraction (XRD) analysis. The morphology and shape of nanoparticles were examined by transmission electron microscopy (TEM). An inductively coupled plasma optical emission spectrometer (ICP-OES) was used to determine the concentrations of gold ions in the aqueous solutions. Fourier transform infrared spectroscopy (FTIR) was used to identify the possible functional groups in the biomolecules (from plant extract) responsible for the synthesis of AuNPs. The hydrodynamic radius and surface charge of as-synthesized nanoparticles were measured in a zeta potential analyzer. In order to identify the specific Eclipta proteins responsible for the formation and stabilization of gold nanoparticles, [22, 23] we have carried out gel electrophoresis using 12% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). XPS measurements were obtained on a KRATOS-AXIS 165 instrument equipped with dual aluminum–magnesium anodes using Mg K radiation (hV 1253.6 eV), operating at 5 kV and 15 mA with a pass energy of 80 eV and an increment of 0.1 eV. The samples were degassed for several hours in the XPS chamber to minimize air contamination on the sample surface. To overcome the charging problem, a charge neutralizer of 2 eV was applied and the binding energy of C 1s core level (BE 1/4 284.6 eV) of adventitious hydrocarbon was used as a standard. The XPS spectra were fitted using a nonlinear squares method with the convolution of Lorentzian and Gaussian functions after a polynomial background was subtracted from the raw spectra. The characterization procedures are described in detail in supporting information (available at stacks.iop.org/Nano/23/455103/mmedia).
4. Results and discussion
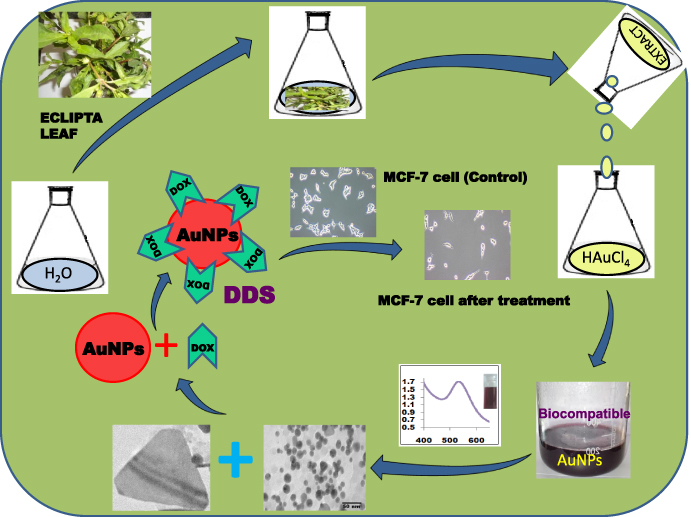
The biological approach to synthesis of AuNPs is an eco-friendly and ideal method to develop environmentally sustainable nanoparticles for use as an alternative to existing methods. The selection of an environmentally sustainable solvent system and eco-friendly reducing and stabilizing agent is the most important criteria for the green chemistry synthesis of nanoparticles. Here we have used water as the solvent and the plant extract as both an eco-friendly reducing and stabilizing agent for the green synthesis of biocompatible AuNPs. The overall procedures, schematically presented in scheme 1, include (i) biosynthesis of AuNPs from HAuCl4 using Eclipta plant leaf extract, (ii) characterization of AuNPs by physico-chemical techniques, (iii) biocompatibility tests, (iv) development of an AuNPs-based drug delivery system (DDS) and (v) administration of this DDS to breast cancer cells and assessment of its therapeutic efficacy.
Scheme 1. Synthesis, characterizations of biocompatible AuNPs by Eclipta Alba extract from HAuCl4 and its application in drug delivery for breast cancer therapy.
Download figure:
Standard image4.1. UV–visible spectroscopy
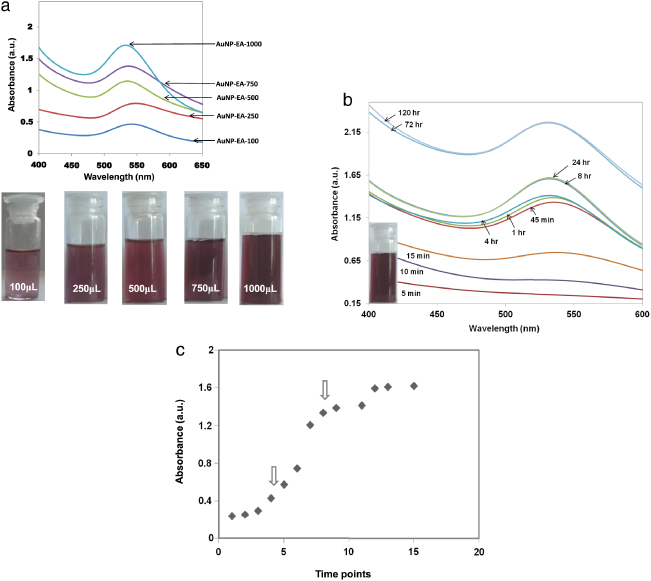
In order to find the optimized biocompatible AuNPs, initially we carried out a set of experiments for the formation of AuNPs by changing the reaction conditions (shown in table 1). Here table 1 demonstrates that the total volume of each reaction is 5 ml for the synthesis of AuNPs, where we have changed the volume of Eclipta extract (from 100–1000 μl) keeping the concentration of HAuCl4 constant (200 μl) (table 1). It is also evident from table 1 that with an increasing volume of extract, less time is required for the formation of AuNPs. We have obtained red color stable gold nanoparticles in less than 10 min when the volume of Eclipta extract is 1 ml, whereas it takes around 4 h when the volume of the Eclipta extract is 100 μl.
In all cases, we have observed the ruby red coloration that indicates the formation of AuNPs with different volume of extract (optical images are shown bottom of figure 1(a)). We continued the reactions for 24 h and the formation of AuNPs was also confirmed by UV visible spectroscopy after taking the absorbance of each red color solution; the results are shown in figure 1(a). The absorbance of all solutions (AuNP-EA-100 μl to AuNP-EA-1000 μl) was observed at λmax ∼ 530–555 nm, clearly indicating the formation of AuNPs [21, 22, 24–26]. The absorption at 530–555 nm is due to excitation of the surface plasmon vibrations in the AuNPs, as the plasmon band of AuNPs ranges from 510 to 560 nm [27]. AuNP-EA-1000 (Exp. No. 5 in table 1) in figure 1(a) clearly shows the absorbance value ∼1.71 at λmax = 532 nm, which gives the maximum concentration of AuNPs compared to the other set of reactions. Apart from that we have found that Exp. No #5 takes less time (only 10 min) to form AuNPs and there is no aggregation of these AuNPs over a long period (discussed in detail later). Therefore, from table 1 we have selected AuNP-EA-1000 as the optimized AuNPs from the experiment number # 5 in table 1 for further characterizations and in vitro cell culture studies. We continued the experiment number # 5 (AuNP-EA-1000) up to 5 days and found that the intensity of the absorbance increases with time (figure 1(b)). A similar pattern (variation of absorbance with time) was also observed for the experiments numbered #1, #2, #3 and #4, and the corresponding results are shown in SI-figure 1, SI-figure 2, SI-figure 3, and SI-figure 4, respectively (available at stacks.iop.org/Nano/23/455103/mmedia). The change in absorbance with time and change of wavelength with time for AuNP-EA-1000 are shown in SI-figure 5(a) and SI-figure 5(b) respectively (available at stacks.iop.org/Nano/23/455103/mmedia), and we have observed that after a certain time, the intensity of absorbance remain almost unchanged, indicating the completion of the reaction between HAuCl4 and the water extract of Eclipta leaves. It is also evident from SI-figures 5(a) and (b) (available at stacks.iop.org/Nano/23/455103/mmedia) that both the absorbance and wavelength for AuNP-EA-1000 (Exp.No # 5) at different times are more consistent than synthesized AuNPs from Exp. No. 1–4, suggesting more stability of AuNP-EA-1000 compared to the others. This is also another reason to select AuNP-EA-1000 (Exp. No. #5) for further characterization, stability, biocompatibility and in vitro cell culture studies.
Figure 1. UV visible spectra of green synthesized gold nanoparticles (AuNPs) at different reaction conditions along with the formation kinetics study. (a) Change of absorbance of as-synthesized gold nanoparticles (at λmax = 532 nm) obtained from the reduction of HAuCl4 using different volumes of Eclipta extract (100–1000 μl). Numerical values in AuNP-EA-100, AuNP-EA-250, AuNP-EA-500, AuNP-EA-750 and AuNP-EA-1000 indicate the volume of Eclipta extract in μl. The bottom pictures show the ruby red color of corresponding AuNPs. (b) Change of absorbance of as-synthesized AuNP-EA-1000 (Expt. No. #5 in table 1) with time (1 min to 120 h). The pictures show that absorption intensity of AuNPs increases with time, suggesting the formation of more AuNPs. The inset picture shows the as-synthesized AuNP-EA-1000. (c) The formation kinetics study of AuNP-EA-1000 was determined by taking the absorbance of AuNP-EA-1000 with time (1 min to 2 days). Arrows show initiation and growth processes in the sigmoid curve.
Download figure:
Standard imageFor AuNP-EA-1000 (Exp.No. #5 in table 1), we monitored the time-dependent reduction process of HAuCl4 by the phytochemicals from EA and the growth kinetics of AuNPs by UV–vis spectroscopy. As previously reported, the synthesis process has been categorized into two steps, namely nucleation process and growth process [28]. In the initial 15 min of the synthesis, there is a steep rise of the peak in the time versus absorbance curve (figure 1(c)), indicating the initiation of the AuNPs synthesis (shown by arrow). After 15 min the synthesis rate decreases and the growth process has started (shown by arrow). The process continues up to 3–4 days, resulting the formation of larger particles due to growth process. The generation of large particles can be explained by earlier literature reporting that a weak reducing agent normally generates large nanoparticles [29]. However, fewer larger nanoparticles were formed, as was clearly observed from TEM. Also some hexagonal and triangular shapes of AuNP were formed, as supported by TEM (as will be discussed later). These results indicate that although our EAW is a weak reducing agent with respect to chemical reducing agents such as NaBH4, its reducing ability is still more than other so-called weak reducing agents.
4.2. X-ray diffraction (XRD) spectroscopy
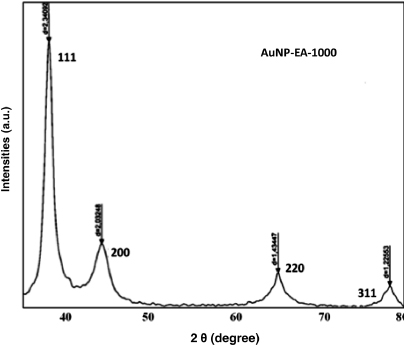
The crystal structures of the as-synthesized products obtained after the reduction of HAuCl4 using Eclipta extract were identified using x-ray diffraction (XRD) analysis and the results are presented in figure 2. The XRD pattern indicates the crystalline nature of the product and all reflections are distinctly indexed to a face-centered cubic (fcc) phase of AuNPs. No peaks from other phases were detected, indicating the purity of the as-synthesized samples. The diffraction peaks were consistent with the standard data files (the JCPDS card No.04-0784) for all reflections. These results corroborate with earlier published literature [30].
Figure 2. X-ray diffraction pattern of as-synthesized AuNP-EA-1000. XRD pattern of AuNP-EA-1000 (Exp No. #5 in table 1) shows the crystalline (FCC) structure.
Download figure:
Standard image4.3. Transmission electron microscopy (TEM)
In order to reveal the morphology of as-synthesized AuNPs, we have carried out TEM. The TEM images of as-synthesized AuNP-EA-1000 (figures 3(a)–(d)), obtained at different time intervals (1 h to 7 days) indicate the formation of mainly spherical gold nanoparticles (5–25 nm) along with some hexagonal nanoparticles (15–50 nm) (figure 3(e)) and the triangular AuNPs (20–200 nm) (figure 3(f)). TEM images of AuNP-EA-1000 obtained after 1 and 5 h show mixtures of spherical AuNPs with sizes range from 10 to 25 nm (figures 3(a) and (b)). However, after 24 h to 7 days of reaction it shows very few triangular and hexagonal size nanoparticles along with spherical nanoparticles (figures 3(c) and (d)). The TEM picture clearly shows that the AuNPs are almost monodispersed, although a little aggregation is visible for some cases. The inset of figure 3(b) indicates the SAED pattern of as-synthesized Au nanoparticles (AuNP-EA-1000), showing Debye–Scherrer rings assigned to the (111), (200), (220) and (311) lattice planes of the face-centered cubic (fcc) structure. It also indirectly proves the crystalline nature of AuNPs, further supported by the XRD pattern (figure 2). Triangular and hexagonal sizes of larger nanoparticles are shown in figures 3(e) and (f) at high magnification. These results suggest that the biosynthesis of AuNPs occurs in two steps, namely initiation and growth processes. After the initiation process at the early stages, the growth process starts and with increasing time, the concentration of AuNPs increases, leading to some of them being deposited on the surface of other AuNPs and making the particle size bigger. These results also correlate with the fact that weak reducing agents can generally form larger nanoparticles. SI-figures 6(a)–(d) (available at stacks.iop.org/Nano/23/455103/mmedia) show the TEM images of a mixture of spherical AuNPs along with hexagonal and triangular shapes obtained from experiment number #1–4, where we have used different volumes of extract in the range (100–750 μl) without changing the volume of HAuCl4.
Figure 3. TEM images of different as-synthesized AuNP-EA-1000. TEM images are obtained after (a) 1 h, (b) 5 h of AuNP-EA-1000; inset picture indicates the SAED of the corresponding sample, (c) 24 h and (d) 7 days of AuNP-EA-1000; (e) Triangular shapes and (f) hexagonal shapes of AuNP-EA-1000 were obtained at high magnifications.
Download figure:
Standard imageWe can see from figure 3(d) a mixture of spherical, triangular and hexagonal gold nanoparticles that supports the high degree of polydispersion of nanoparticles. However, at a early times, especially at 1 h (figure 3(a)) and 5 h (figure 3(b)), we obtain mostly spherical gold nanoparticles, supporting the monodispersion of the nanoparticles. Hence, at optimum reaction conditions we can control the monodispersity of the spherical nanoparticles by reducing the time. Moreover, we have found monodispersion of the spherical nanoparticles from HAuCl4 with different volumes of Eclipta extract (e.g. 100, 250, 500 and 750 μl; see table 1 for detailed information, Exp.No. 1–4, and also SI-figures 6(a)–(d) in supporting information available at stacks.iop.org/Nano/23/455103/mmedia). In most cases we get spherical gold nanoparticles with high monodispersity with lower reaction times.
4.4. Dynamic light scattering (DLS) technique
The dynamic light scattering (DLS) method was employed to calculate the hydrodynamic radii or size of AuNPs coated with phytochemicals or proteins [31]. The range of hydrodynamic diameter of AuNP-EA-1000 is 23–166 nm, as obtained by DLS, which matches with the diameter of nanoparticles measured by TEM (5–200 nm) (see table 2). The insets of SI-figures 6(a)–(d) (available at stacks.iop.org/Nano/23/455103/mmedia) show the DLS size distribution, indicating a high degree of monodispersion of spherical nanoparticles. Difference in size of AuNPs obtained from TEM and DLS, suggesting that AuNPs are coated with phytochemicals including low molecular weight as well as high molecular weight proteins, flavonoids (phenolic compounds) such as wedelolactone, desmethylwedelolactone, desmethyl-wedelolactone-7glucoside and stigmasterol etc [32, 33]. The charge or zeta potential (ξ) is an essential parameter for the dispersion of synthesized nanoparticles. It also indicates the repulsive forces present between the nanoparticles, which can be helpful to give an idea regarding the long-term stability of these biosynthesized AuNPs [22]. The mutual balancing between the attractive and repulsive forces will lead to particle stability. The ξ value of AuNP-EA-1000 is negative (−19.4 ± 0.8 i.e.), which helps to make AuNPs stable for a long time without any aggregation between AuNPs. AuNPs obtained from other experiments also show the surface negative charge (table 2).
Table 2. Size determination of AuNPs by TEM and DLS technique. The size of green synthesized AuNPs was obtained at different reaction conditions by both TEM and DLS.
| Sample name (Eclipta based) | Diameter (nm) from DLS | Size (TEM) (nm) | Zeta potential (ξ) Charge (mV) | ||
|---|---|---|---|---|---|
| Peak-1 | Peak-2 | Peak-3 | |||
| AuNPs-100 μl | — | 83.315 | — | 5–50 | −13.9 ± 0.6 |
| AuNPs-250 μl | 4.810 | 62.896 | — | 5–45 | −23.0 ± 1.6 |
| AuNPs-500 μl | 11.702 | — | 112.508 | 5–75 | −17.2 ± 1.3 |
| AuNPs-750 μl | 1.904 | 55.848 | — | 5–50 | −19.5 ± 1.0 |
| AuNPs-1000 μl | 1.279 | 23.279 | 166.721 | 5–200 | −19.4 ± 0.8 |
4.5. Fourier transformed infrared (FTIR) spectroscopy
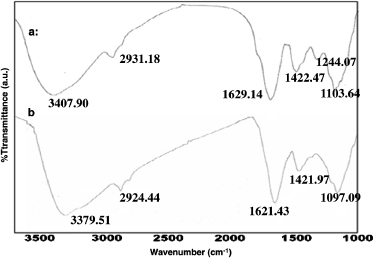
In order to determine the possible functional groups of phytochemicals or proteins present in Eclipta leaves that help in the reduction of HAuCl4 to gold nanoparticles and its stabilization, we have carried out FTIR spectroscopy. Figures 4(a) and (b) indicate the FTIR spectra of Eclipta extract and AuNP-EA-1000, respectively. The major stretching frequencies of Eclipta extract are observed at 3407.90, 2931.18, 1629.14, 1422.47, 1244.07, 1103.64 cm−1 (curve (a) in figure 4) whereas the stretching frequencies of AuNP-EA-1000 are observed at 3379.51, 2924.44, 1621.43, 1421.97 and 1097.09 cm−1 (curve (b) of figure 4). Here the major IR spectrum, arises at ν = 3407.90 cm−1 in curve (a), due to the O–H stretching modes which are significantly reduced and become sharper upon coordination with gold nanoparticles, and shifts to 3379 cm−1 in curve (b) [13], suggesting the role of phenolic groups in the reduction of HAuCl4 to AuNPs. On the other hand, the IR spectrum at ν = 1244 cm−1 (curve (a)) due to the presence of amide III in proteins of Eclipta Alba leaves [21] disappeareds in curve (b) (AuNP-EA-1000) due to the involvement of that protein in the reduction of HAuCl4 during the synthesis of AuNPs. This is further confirmed by SDS gel electrophoresis (discussed later).
Figure 4. FTIR spectra. FTIR spectra of (a) Eclipta Alba extract and (b) as-synthesized AuNP-EA-1000 (Expt. No. #5 in table 1). Relative transmittance abscissa: wavenumber (cm−1). FTIR spectra indicate the presence of polyphenols and proteins in Eclipta extract which help in the synthesis and stabilization of AuNPs.
Download figure:
Standard imageThe remaining peaks at ν = 2931 cm−1,ν = 1629 cm−1, ν = 1422 cm−1 and ν = 1103 cm−1 of curve (a), which may be assigned due to –CH2 stretching of aliphatic groups [34], -NH stretching of secondary amines [35], C=O groups from aromatic rings having conjugation [36], bending vibrations of the C–OH alcoholic group [37] and C–O single bond vibrations of ether linkages [24, 35] respectively are almost unchanged in curve (b) of figure 4. Therefore we believe that the presence of phenolic and amine groups present in the Eclipta leaf might be a plausible reason for the formation and stabilization of gold nanoparticles.
4.6. X-ray photoelectron spectroscopy (XPS)
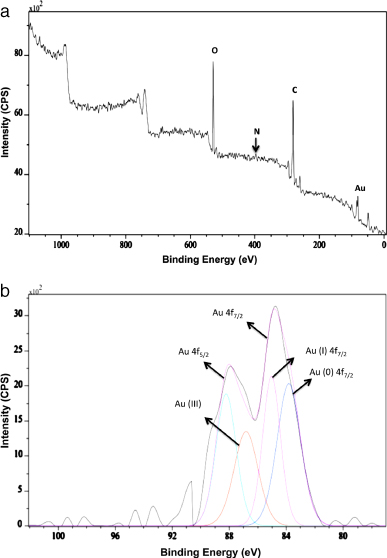
Figure 5(a) shows the XPS survey data of AuNP-EA-1000, indicating the binding energy (BE) of different atoms (Au, C, O, N). The BEs at around 84, 285, 533 and 400 eV can be assigned due to the presence of Au(4f), C(1s), O(1s) and N(1s) elements, respectively. Figure 5(b) shows that the two spin orbit components Au 4f7/2 and Au 4f5/2 arise due to resolving the Au 4f spectrum at a binding energy (BE) 83.8 and 88.2 eV respectively. The Au 4f7/2 core level decomposes into two components centered at 83.8 and 85.5 eV BEs, which can attributed to Au(0) or metallic gold and Au(I) respectively (figure 5(b)). The presence of Au(I) on the surface of the gold nanoparticles is responsible for the high BE peak and helps to stabilize the gold nanoparticles from aggregations [25, 38]. The small peak at BE at 86.7 eV suggests the presence of a very small amount of Au (III). Similarly, the C(1s), O(1s) and N(1s) BE versus intensity spectra were also recorded by XPS and shown in supporting information (SI-figures 7(a)–(c) available at stacks.iop.org/Nano/23/455103/mmedia) suggesting the presence of C, O, and N elements along with gold nanoparticles. The BE peaks for C(1s) at 284.6, 286 and 287.5 eV can be assigned as peaks due to carbon atoms present in the carbonyl group (carbonyl or amide) and carbon atoms present in the α carbonyl carbon atom, which also may arise due to the carbon complexed with some electron-withdrawing group such as carbonyl or carbon coordinated with some hydroxyl groups respectively [39–42]. The O(1s) spectrum can be resolved into two distinct components at BE values 532.2 and 533.7 eV that may arise due to oxygen from the phenolic hydroxyl group present in the wedelolactone and other phenolic compounds present in Eclipta Alba leaves. We have observed a BE peak for N(1s) at 399.7 eV that can be assigned to the presence of a weak N 1s signal (figure 5(a)). This N 1s signal may arise due to the presence of N atoms in the amino group in the protein molecules [42]. Although the signal for N 1s is very weak from figure 5(a), we can observe the presence of the N 1s signal from SI-figure 5(c) (available at stacks.iop.org/Nano/23/455103/mmedia) indicating the low abundance of N present on the surface of the AuNP-EA-1000.
Figure 5. XPS spectra obtained from AuNP-EA-1000. (a) The BEs at around 84, 285, 533 and 400 eV can be assigned due to the presence of Au (4f), C(1s), O(1s) and N(1s) elements, respectively. (b) High-resolution XPS spectrum of Au atom present in AuNP-EA-1000.
Download figure:
Standard image4.7. In vitro stability studies of AuNPs
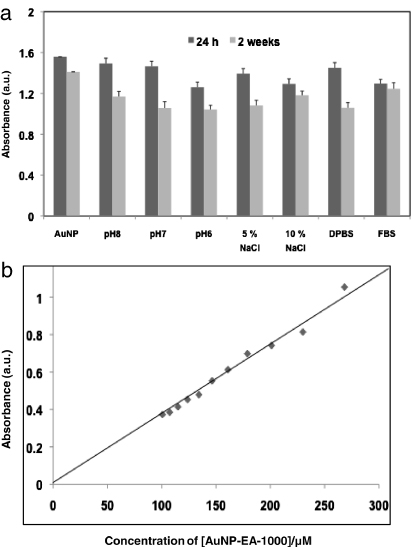
The stability of the AuNPs is very important for biomedical applications [11, 12, 22, 24]. Therefore, we have investigated the in vitro stability of AuNP-EA-1000 in different types of buffer solution and biological fluid, including DPBS, 5%–10% NaCl solution, buffer saline with pH = 6, 7 and 8, fetal bovine serum (FBS) using AuNP-EA-1000 (Expt. No. #5) obtained after 24 h. Briefly, 800 μl of AuNP-EA-1000 was mixed with 200 μl of corresponding buffer/biological fluids and the resultant mixture was incubated for 24 h–14 days. After 24 h of incubation we took the UV spectra of each resulting solution and found almost no change of absorbance with respect to only AuNP-EA-1000 (figure 6(a) and SI-figure 8(a) (available at stacks.iop.org/Nano/23/455103/mmedia)). The incubation was continued for 2 weeks and we found a slightly lower absorbance value compared to 24 h results (SI-figure 8(b) available at stacks.iop.org/Nano/23/455103/mmedia and figure 6(a)). These results clearly support the stability of AuNP-EA-1000 in different buffer solutions and biological fluids. According to the reported literature we have measured the plasmon wavelength (λmax) and plasmon bandwidth (Δλ) for 2-week samples and found a plasmon wavelength and plasmon bandwidth shift of approximately 5 nm suggesting the high in vitro stability of our as-synthesized AuNPs [22, 24].
Figure 6. In vitro stability studies of AuNP-EA-1000 and the effect of plasmon absorption of AuNP-EA-1000 with dilution. In vitro stability studies were performed by (a) bar-diagram of AuNP-EA-1000 in different buffer solutions (24 h to 14 days). (b) Change in the plasmon absorption of AuNP-EA-1000 under various dilution conditions with water. The linear fit supports the greater stability of AuNP-EA-1000.
Download figure:
Standard imageIn general, biomedical applications require a lower concentration of AuNPs. Therefore, it is vital to check that the AuNPs solutions do not change their physico-chemical properties on dilution, which indirectly supports in vitro stability of AuNPs [22]. In order to find the in vitro stability, we have carried out an experiment where we have taken 1 ml AuNPs and diluted it with 0.2 ml of Mili-Q water in successive steps. After each addition of 0.2 ml water we monitored the plasmon wavelength (λmax) and plasmon bandwidth (Δλ). The absorption intensity at λmax532 nm shows a linear dependence with changing concentration of AuNPs according to the Lambert–Beer law (figure 6(b)). Also λmax and Δλ did not change significantly on dilution from 10−5 to 10−6 M, suggesting the greater stability of AuNPs. Biosynthetically prepared AuNPs using Eclipta extract are exceptionally stable as proteins, flavonoids, alkaloids etc present in the Eclipta extract provide extra stability of AuNPs by coating the surface of the AuNPs and protecting them from aggregation. Because of long-term stability of green synthesized AuNPs, they can be very useful for cancer drug delivery.
4.8. Silver nitrate staining
Numerous publications demonstrated that proteins or phenolic compounds present in the plant extract are responsible for the formation and stabilization of gold nanoparticles [22, 23, 43]. Some groups have reported that amino acids help to synthesize gold nanoparticles and maintain their stabilization [44, 45]. In our study we have observed that Eclipta Alba extract helps in the formation of AuNPs by the reduction of HAuCl4 solution and that it shows extra stability over a long time. Therefore Eclipta leaf extract not only acts as a reducing agent but also as a stabilizing agent. In order to find out the specific proteins of Eclipta Alba that are responsible for the formation of AuNPs, we have run SDS-PAGE where we have used the concentrated water extract of Eclipta leaves and the supernatant of AuNP-EA-1000 obtained after centrifugation. According to figure 7, lanes-M, −1, and −2 correspond to standard protein marker, water extract of Eclipta Alba leaves, and supernatant of Eclipta- AuNPs obtained after centrifugation respectively. Proteins ∼10–15 kDa and ∼150 kDa present in the Eclipta extract (Lane-1) fully or partially disappear in the supernatant of AuNP-EA-1000 (Lane-2), suggesting that both low (∼15–50 kDa) as well as high (∼150 kDa) molecular weight proteins present in Eclipta leaves are responsible for formation and stabilization of AuNPs (figure 7). Our results support the previously reported literature [22, 23, 43].
Figure 7. SDS-PAGE profile of Eclipta extract with silver nitrate staining. M indicates the standard protein marker. Lane-1 and lane-2 indicate the Eclipta extract and supernatant of AuNP-EA-1000 obtained after centrifugation respectively. The silver nitrate lane staining after gel electrophoresis shows the presence of low (10–15 kDa) and high (∼150 kDa) molecular weight proteins only in the Eclipta extract in lane-1 and the disappearance of 10–15 kDa proteins or reduction of ∼150 kDa protein molecules in the Eclipta extract associated with AuNPs in lane-2. The results confirm the role of these proteins in the reduction and stabilization of AuNPs.
Download figure:
Standard image4.9. Cell viability assay (MTT assay) with AuNP-EA-1000 and ICP-OES data
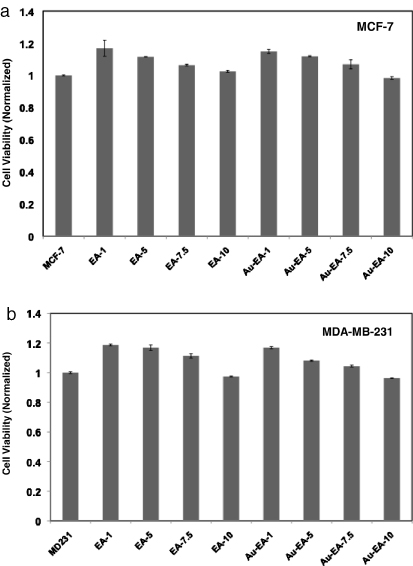
In order to evaluate the biocompatibility of the as-synthesized AuNP-EA-1000, we carried out cell viability assay on breast cancer cells (MCF-7 and MDA-MB-231) using MTT reagents. Before incubating the cells with AuNP-EA-1000, we determined the concentration of AuNP-EA-1000 using ICP-OES analysis. From ICP-OES data we have found that the concentration of Au in AuNP-EA-1000 is 6.75 μg ml−1. Both types of breast cancer cells were incubated with different volumes (1–10 μl) of AuNP-EA-1000 for 48 h and were found viable up to 10 μl of AuNP-EA-1000, suggesting the biocompatibility of green synthesized AuNP-EA-1000 (figures 8(a) and (b)). Here 1, 5, 7.5 and 10 μl of AuNP-EA-1000 correspond to 11.4, 57.1, 85.7 and 114.2 μM of Au concentration, according to ICP-OES data.
Figure 8. Cell viability test using MTT reagents. Biocompatibility of AuNP-EA-1000 was tested in breast cancer cells (a) MCF-7 and (b) MDA-MB-231 in a dose-dependent manner. The as-synthesized AuNP-EA-1000 shows the biocompatibility to breast cancer cells. Numerical values in EA-1, EA-5, EA-7.5, EA-10 indicate the volume of Eclipta Alba extract in μl whereas the numerical values in Au-EA-1, Au-EA-5, Au-EA-7.5, Au-EA-10 indicate the volume of AuNP-EA-1000 in μl. Here 1, 5, 7.5 and 10 μl of AuNP-EA-1000 correspond to 11.4, 57.1, 85.7 and 114.2 μM of Au concentration, according to ICP-OES data analysis.
Download figure:
Standard image4.10. Quantification of doxorubicin in AuNP-EA-1000 and its delivery to breast cancer
Drug delivery systems (DDS) have become important tools for cancer therapy. We have fabricated a DDS containing green synthesized AuNPs and doxorubicin, where AuNPs have been used as a delivery vehicle and doxorubicin as an FDA approved anticancer drug. In order to check the functional activity of doxorubicin in gold nanoparticles based DDS, we have performed cell viability tests using MTT reagent on breast cancer cells (MCF-7). Before performing the cell viability tests, we have quantified the doxorubicin (DOX) present in the AuNP conjugates (DDS) using UV–visible spectroscopy. SI-figure 9 (available at stacks.iop.org/Nano/23/455103/mmedia) indicates the UV–vis spectra of DOX in aqueous solution with different concentrations of DOX (from 1 to 20 μg ml−1) and the change of absorption spectra with an increasing concentration of DOX. To quantify the amount of DOX in the nanoconjugate, first a standard curve of DOX (figure 9(a)) in aqueous solution using the absorbance of DOX (at λmax = 490 nm) at varying concentrations of DOX (from 1 to 20 μg ml−1) was prepared according to the literature [46]. Using this standard curve we can calculate the amount of DOX in an unknown solution. In our study, we have prepared two types of gold nanoconjugates, namely AuNP-EA-DOX and AuNP-EA, and taken the UV absorbance of both nanoconjugates and their corresponding supernatant (figure 9(b)). Finally, from the standard curve and the UV spectrum of the supernatant of AuNP-EA-DOX, we have found that 15% DOX is attached to the AuNP-EA-1000, maybe due to the weak electrostatic attractive force [47, 48].
Figure 9. Standard curve of doxorubicin in water and UV visible spectra measurements. (a) Standard curve of DOX in aqueous solution was determined by taking the absorbance of DOX at different concentrations. This standard curve of DOX can be used to determine an unknown concentration of pristine DOX or DOX conjugated to AuNPs. (b) UV visible spectra of AuNP-EA-1000 and its conjugation with DOX.
Download figure:
Standard imageIn this context, we have carried out a DLS study (especially zeta potential measurement) in order to find the nature of interaction leading to the linkage of DOX with AuNP-EA-1000. We have found the zeta potentials of AuNP-EA and AuNP-EA-DOX are −19.4 ± 0.8 and −14.7 ± 0.6 respectively. In both cases, the zeta potential values are negative. However, the negative zeta potential value of AuNP-EA, which is −19.4 ± 0.8, decreases to −14.7 ± 0.6 in the case of AuNP-EA-DOX, suggesting the presence of positively charged doxorubicin. The positively charged DOX in aqueous solution is further confirmed by the positive zeta potential value of DOX, which is +2.6 ± 0.6 (measured by DLS study). These results indicate that the major interaction between the AuNP-EA-1000 and DOX might be due to the presence of weak electrostatic attractive forces. Finally, our observation is supported by published reports [47, 48].
We have already shown that green synthesized AuNPs are biocompatible. Therefore, in order to check the functional activity of DOX in AuNP-EA-DOX, we have incubated MCF-7 cells with Au-MUA (chemically synthesized gold nanoparticles conjugated with MUA; concentration Au is 6.6 μg ml−1), EA (Eclipta extract itself), AuNP-EA-1000, DOX alone and AuNP-EA-DOX. Here MCF-7 cells were treated with DOX at different concentrations (2.5–10 μM) for 48 h of incubation. We have found almost no inhibition of proliferation of both breast cancer cells when the cells were treated with Au-MUA, EA and AuNP-EA-1000 at different concentrations (figure 10). However, we have observed ∼16%, 30% and 50% of inhibition of MCF-7 cells when the cells were treated with 2.5 μM,5 μM and 10 μM of DOX respectively. The inhibition of MCF-7 cell proliferation by DOX increases from 16% to 34% (at 2.5 μM), 30% to 42% (at 5 μM) and 50% to 60% (at 10 μM) when DOX is attached to AuNPs (AuNP-EA-DOX) compared to free DOX. Thus, these results show that AuNP-DOX shows a greater anticancer effect than pristine doxorubicin. This study can be used as an alternative drug delivery system for cancer therapeutics where green synthesized biocompatible gold nanoparticles are used as a delivery vehicle.
Figure 10. In vitro efficacy of AuNP-EA-1000 on breast cancer cells (MCF-7). Treatment of MCF-7 cells with Au-MUA (chemically synthesized AuNP conjugated with MUA), Eclipta extract (EA), AuNP-EA-1000, free DOX and DOX conjugated with AuNP (AuNP-EA-1000-DOX). The gold nanoconjugated drug delivery system containing DOX shows a greater killing efficiency on MCF-7 cells than AuNPs or free DOX alone in water.
Download figure:
Standard image4.11. Plausible mechanism
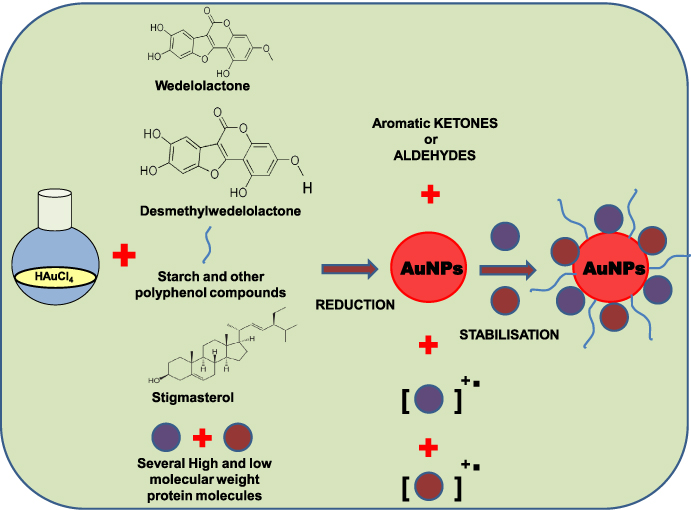
Numerous publications demonstrated that the plant extract is responsible for the formation and stabilization of gold nanoparticles [22, 23, 43–45]. In our present study, we have developed and designed a biosynthetic method for the synthesis of gold nanoparticles using Eclipta extract. However, the exact mechanisms for the formation of gold nanoparticles using plant extract have still not been thoroughly explored. Earlier literature provides a few clues, such as the involvement of low (10–15 kDa) and high molecular weight protein molecules (100–200 kDa), amino acids, phenolic compounds (wedelolactone, desmethylwedelolactone, stigmasterol etc), starches, polysaccharides, alkaloids, alcoholic compounds, vitamins, enzymes etc present in Eclipta Alba leaf help in the formation and stabilization of gold nanoparticles (AuNPs) [22, 23, 32, 33, 43–45, 49–51]. The overall mechanism for the formation and stabilization of AuNPs using Eclipta extract is schematically presented in scheme 2.
Scheme 2. The plausible mechanism for the formation and stabilization of AuNPs using Eclipta extract.
Download figure:
Standard imageNewman et al demonstrated that the involvement of proteins supports the formation and stabilization of AuNPs. They demonstrated that reduction of HAuCl4 occurs due to transfer of electrons from the amine to the metal ion, resulting in the formation of Au0 (gold nanoparticles) as follows [50].
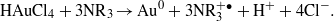
The standard reduction potential of gold (E0Au3+/Au0) is 1.50 V whereas the standard oxidation potential of alcohol to the ketone/aldehyde system ( ) is 1.80 V [52]. Therefore the phenolic/alcoholic compounds present in Eclipta extract can help in the reduction of Au3+ (in HAuCl4) to Au0 (gold nanoparticle). The direct participation of phenolic compounds and protein molecules for the synthesis and stabilization of AuNPs has been further supported by FTIR data and SDS gel electrophoresis (discussed before).
) is 1.80 V [52]. Therefore the phenolic/alcoholic compounds present in Eclipta extract can help in the reduction of Au3+ (in HAuCl4) to Au0 (gold nanoparticle). The direct participation of phenolic compounds and protein molecules for the synthesis and stabilization of AuNPs has been further supported by FTIR data and SDS gel electrophoresis (discussed before).
5. Conclusions
We have developed a simple, fast, clean, efficient, low-cost and eco-friendly single-step green chemistry approach for the synthesis of biocompatible gold nanoparticles (AuNPs) using Eclipta Alba, which acts as both a reducing and a stabilizing agent. The biosynthesis of gold nanoparticles was carried out at room temperature, at atmospheric pressure and in water (universal solvent), indicating a green process that presents a reliable and economic method, taking advantage of an efficient bioresource (Eclipta extract). The as-synthesized AuNPs were thoroughly characterized by several physico-chemical techniques. These AuNPs show very high stability in different buffer solutions. The extra stability of AuNPs is very useful for biomedical applications. The presence of phenolic and protein molecules present in eclipta extract is the plausible mechanism for the synthesis and stabilization of AuNPs. Biocompatibility of AuNPs was observed by in vitro cell culture assays. Administration of the drug delivery system based on green synthesized AuNPs containing doxorubicin to breast cancer cells (MCF-7 and MDA-MB-231) shows significant inhibition of breast cancer cell proliferation compared to pristine doxorubicin. Finally, this environmentally friendly method will be a competitive alternative to the existing methods of production of large-scale biocompatible gold nanoparticles that can be used in catalysis, sensors, electronics and biomedical applications, especially for cancer therapy.
Acknowledgments
This research was supported by Ramanujan Fellowship grant (SR/S2/RJN-04/2010; GAP0305), DST-New Delhi and CSIR Institute fund to CRP. SM and AKB are grateful to CSIR, New Delhi and UGC, New Delhi, respectively for Junior Research fellowships. The authors are grateful to Dr J S Yadav, Director, IICT-Hyderabad for his continued help, support and motivation for nanomedicine work at IICT.