Abstract
Radiosensitization using gold nanoparticles (AuNPs) has been shown to vary widely with cell line, irradiation energy, AuNP size, concentration and intracellular localization. We developed a Monte Carlo-based AuNP radiosensitization predictive model (ARP), which takes into account the detailed energy deposition at the nano-scale. This model was compared to experimental cell survival and macroscopic dose enhancement predictions. PC-3 prostate cancer cell survival was characterized after irradiation using a 300 kVp photon source with and without AuNPs present in the cell culture media. Detailed Monte Carlo simulations were conducted, producing individual tracks of photoelectric products escaping AuNPs and energy deposition was scored in nano-scale voxels in a model cell nucleus. Cell survival in our predictive model was calculated by integrating the radiation induced lethal event density over the nucleus volume. Experimental AuNP radiosensitization was observed with a sensitizer enhancement ratio (SER) of 1.21 ± 0.13. SERs estimated using the ARP model and the macroscopic enhancement model were 1.20 ± 0.12 and 1.07 ± 0.10 respectively. In the hypothetical case of AuNPs localized within the nucleus, the ARP model predicted a SER of 1.29 ± 0.13, demonstrating the influence of AuNP intracellular localization on radiosensitization.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
General scientific summary Nanoparticles of gold can enhance the curative effect of radiotherapy, a major anti-cancer treatment. This enhancing effect has been difficult to predict as it varies with cell line, irradiation machine, nanoparticle size, concentration, and where they localize inside the cell. We have developed a computer simulation model to predict this enhancing effect. The model incorporates cell geometry, sensitivity to radiation, and the nanoparticle localization to realize a very detailed simulation of radiation energy deposition and the subsequent location of cellular damages. We successfully compared predictions from our model to in vitro experimental results. Furthermore our model shows that accumulation of nanoparticles inside the cell nucleus dramatically increases the effect. The model provides a framework to explore and optimize nanoparticle radiation enhancement for clinical application.
1. Introduction
Gold nanoparticle (AuNP) radiosensitization represents a novel approach to enhance the local effects of radiation. The proof of principle of AuNP radiosensitization was first demonstrated by Hainfeld et al who effectively treated mammary carcinoma xenografts in mice with radiation after in-vivo administration of 1.9 nm AuNPs (Hainfeld et al 2004). The mechanism of AuNP radiosensitization relies on the enhanced attenuation of low-energy photons by high-atomic number (high-Z) materials through the photoelectric effect. Photoelectric absorptions within Au atoms result in the release of a localized spray of photoelectric products comprising characteristic photons, photoelectrons and Auger electron cascades. The conversion of photons into short-ranged Auger electrons exhibiting a potentially higher linear energy transfer (LET) can alter the local radiation quality (Cho et al 2009, Pradhan et al 2009). Previous designs of high-Z radiosensitizers including halogenated pyrimidines such as iododeoxyuridine and bromodeoxyuridine, relied on the incorporation of individual high-Z atoms into cellular DNA (Pignol et al 2003). These strategies failed to produce clinically positive results due to the limited accumulation of high-Z atoms into cancer cells (Pignol et al 2003, Nath et al 1990). With the advent of nanotechnologies and the nanoparticle as a vehicle to accumulate a large number of high-Z atoms within a specific target, novel research opportunities have emerged in the field of radiation dose enhancement. However, whereas halogenated pyrimidines are incorporated directly into the DNA, the sub-cellular location of nanoparticles within various cell compartment may place the DNA out of reach from the low energy products of the photoelectric cascade. In recent years gold nanoparticles (AuNPs) have been extensively studied for radiosensitization due to gold's high atomic number, general biocompatibility (Connor et al 2005), and a surface well suited for conjugation to tumor targeting moieties (Qian et al 2008, Chattopadhyay et al 2010).
Experiments have revealed that AuNP radiosensitization is highly sensitive to cancer cell type (Jain et al 2011), photon source energy (Chithrani et al 2010), and AuNP size (Butterworth et al 2012), concentration (Rahman et al 2009), and localization relative to cellular DNA (Brun et al 2009). Various metrics of AuNP radiosensitization have been evaluated including the macroscopic dose enhancement to the tumor (Cho 2005, Roeske et al 2007, Lechtman et al 2011), and the radial dose enhancement around AuNPs at the nanoscale (Carter et al 2007, Jones et al 2010, Leung et al 2011). While informative, translating these metrics directly into a radiobiological effect is not straightforward and generally underestimates experimental findings (Rahman et al 2009, Chithrani et al 2010, Jain et al 2011).
Recent radiobiological predictive models of AuNP radiosensitization (Pignol 2010, McMahon et al 2011) have been adapted from the Local Effect Model (LEM) used for heavy-ion therapy treatment planning (Scholz and Kraft 1996). The basic theory of the LEM proposes that cell survival can be better predicted by accurately accounting for the microscopic spatial energy deposition of a given radiation treatment. This paper describes a Monte Carlo-based AuNP-tailored adaptation of the LEM, called hereafter the AuNP Radiosensitization Predictive (ARP) model, to estimate cell survival within the parameter space of a given cancer cell type and radiation sensitivity, photon source energy, as well as AuNP size, concentration, and intracellular location. The current study provides an alternative adaptation of the LEM for AuNP radiosensitization presented by McMahon (McMahon et al 2011), by building upon a previously described Monte Carlo approach to investigate photoelectric absorptions within AuNPs and the subsequent spatial distribution of escaping energy presented by Lechtman et al (2011).
2. Materials and methods
2.1. Cell experimentation
2.1.1. Cell culture and characterization
In-vitro experiments were carried out on PC-3 human prostate adenocarcinoma (American Type Culture Collection, Manassas, VA). Cells were cultured in RPMI 1640, with L-glutamine and sodium bicarbonate supplemented with 10% fetal bovine serum (Cellgro laboratories, Manassas. VA), and 5% penicillin and streptomycin (Invitrogen, Carlsbad, CA). Exponentially growing cells were seeded in 35 mm treated culture dishes with 2 mL cell culture media and grown to 80% confluence. To characterize cellular volumes, live cells were imaged using confocal microscopy, and cell nucleus and cytoplasm volumes were measured using ImageJ as described by Cai et al (2010).
2.1.2. AuNP preparation
30 nm diameter AuNP colloids (Ted Pella Inc., Redding, CA) were PEGylated by reaction with 0.235 g MeO-PEG-SH (IRIS Biotech GmbH, Marktredwitz, Germany) at a molecular weight of 2000 daltons per 500 mL of AuNPs, and then concentrated through centrifugation. AuNP concentration was verified by UV-Vis absorption using the Nanodrop 2000 (Thermo Scientific, Wilmington, DE). Highly concentrated AuNPs were re-suspended in cell culture media at a concentration of 2 mg mL−1. Cell cultures were incubated with the final AuNP/cell media mixture 24 h prior to irradiation and analysis.
2.1.3. AuNP concentration and localization
After incubation with AuNPs, dishes were thoroughly washed four times with PBS to remove any AuNPs not taken up in cells. Cells were detached using 0.25% trypsin/EDTA, buffered in cell culture media, counted, and pelleted for analysis. AuNP cellular uptake was quantified using inductively coupled plasma mass spectroscopy (ICPMS) after pellets were digested in 10 mL of concentrated trace-metal analysis grade HCl (SeaStar Chemicals, Sidney, BC), and then further diluted (∼450×) with 3% HCl and 1% thiourea. Results were compared to an Au standard (Inorganic Ventures, VA), and measurements were converted to AuNPs per cell, and mg of gold per mL of cells. Nine ICPMS trials were conducted per point. AuNP intracellular localization was visualized at various magnifications using transmission electron microscopy (TEM) after cell pellets were fixed with Karnovsky's fixative, thin sectioned (60–70 nm) and stained. TEM imaging was conducted using a H7000 transmission electron microscope (Hitachi Corp, Tokyo, Japan) at various magnifications.
2.1.4. Radiobiological experiments
Irradiation was carried out on a clinical Gulmay D3300 (Chertsey, UK) 300 kVp x-ray therapy unit (average energy ≈100 keV) using a 10 cm diameter cone collimator. Radiation was delivered from above the culture dishes penetrating 4 mm of cell culture media. Cell cultures with no gold in the media were irradiated at 0, 1, 2, 4, and 8 Gy. Cell cultures incubated with gold for 24 h were irradiated at 0, 2, 4, and 8 Gy with AuNPs still present in the media. Experiments were repeated three times to obtain the average cell survival with standard deviations.
Immediately after irradiation, culture dishes were washed four times with PBS, trypsinized, counted, and plated into 60 mm cultured dishes producing 100 ± 50 colonies (three dishes per point). Colonies were fixed and stained with methylene blue after 11–13 days. Colonies of 50 cells or more were counted, and cell survival was calculated relative to the plating efficiency of controls receiving no radiation.
Clonogenic survival as a function of dose with and without AuNP present was fit to the linear-quadratic cell survival model  . Alpha and beta parameters were extracted from the data using nonlinear least-squares regression analysis in Matlab and expressed with 95% confidence intervals.
. Alpha and beta parameters were extracted from the data using nonlinear least-squares regression analysis in Matlab and expressed with 95% confidence intervals.
2.2. ARP model simulations
2.2.1. Monte Carlo simulation of AuNP radiosensitization
A detailed account of the sub-cellular spatial energy deposition around AuNPs was required as input for the ARP model. Monte Carlo simulation of this energy deposition was performed in steps to reduce computation time.
First, the 300 kVp source photon phase-space was simulated in air using the MCNP-5 code (X-5 Monte Carlo Team 2003) as described by Keller et al (2008). The rate of AuNP photoelectric absorption was calculated from a simulation of photons penetrating 4 mm of cell culture media, as described by Lechtman et al (2011). Second, the PENELOPE code version 2008.1 (Salvat et al 2008), was used to calculate the macroscopic dose enhancement due to AuNPs, and to perform detailed event-by-event simulations of the energy deposition around AuNPs from escaping electron and photon tracks (Lechtman et al 2011). For each simulated photoelectric absorption within a AuNP, the escaping electron and photon tracks were followed in 3D and the local nanoscale energy deposition was recorded using a customized tally. Although PENELOPE has limitations with regards to very low-energy electron inelastic scattering simulation and track structure calculations (Bernal and Liendo 2009, Nikjoo et al 2006), we chose this code due to its comprehensive coupled electron/photon transport simulation through various mediums including tissue and gold, and flexibility of its geometry package (Fernandez-Varea et al 2012). Furthermore, PENELOPE offers a highly customizable code allowing for user defined tallies that were required for this study (Salvat et al 2008).
2.2.2. The ARP model
The ARP model incorporated a three-compartment spherical cell model comprising a cytoplasm, a radiosensitive nucleus region, and an extracellular region. For each simulation AuNPs were randomly distributed within the three regions of the ARP cell model based on input concentrations. For computational efficiency, nanoparticles beyond a cutoff distance from the cell, corresponding to the maximum range of escaping photoelectrons, were not considered in the simulations (Lechtman et al 2011). Simulated energy deposition tracks were randomly selected, and their spatial coordinates were transposed in Matlab to represent individual photoelectric interactions with AuNPs. The number of photoelectric events was determined based on the calculated rate of photoelectric absorption. The energy released from AuNPs and deposited within nanometric nucleus voxels was then scored. Background dose delivered by photons was assumed homogeneously distributed throughout the nucleus. Each simulation was repeated eleven times with random AuNP locations, producing an average survival and maintaining a standard error of less than 1%. Cell survival was determined similarly to the local effect model (Kraft et al 1999):
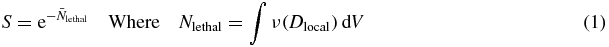
S is the surviving fraction,  is the average number of lethal events per cell, and dV is the differential voxel element. In our implementation of this model, we assumed voxel sizes of finite volume and therefore the integral was carried out as a Riemann sum. ν(Dlocal) is the lethal event density, which is a function of Dlocal, the local dose absorbed within voxels of the nucleus. In theory, the lethal event density can be formulated as any function of the local energy distribution. We followed Elsässer's two component formulation based on the well-known linear-quadratic cell survival model for local doses below a threshold value, Dt, and a purely exponential survival model for local doses above the threshold to account for the limitation of the linear quadratic model at very high energy densities (Elsässer et al 2008):
is the average number of lethal events per cell, and dV is the differential voxel element. In our implementation of this model, we assumed voxel sizes of finite volume and therefore the integral was carried out as a Riemann sum. ν(Dlocal) is the lethal event density, which is a function of Dlocal, the local dose absorbed within voxels of the nucleus. In theory, the lethal event density can be formulated as any function of the local energy distribution. We followed Elsässer's two component formulation based on the well-known linear-quadratic cell survival model for local doses below a threshold value, Dt, and a purely exponential survival model for local doses above the threshold to account for the limitation of the linear quadratic model at very high energy densities (Elsässer et al 2008):
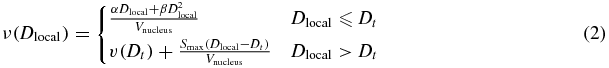
With α and β being the cell radiosensitivity parameters of the linear-quadratic model for low-LET photon radiation, and Smax = α + 2βDt. The threshold dose—an empirical parameter—has been shown to produce more accurate predictions of cell survival at increasingly high doses (Astrahan 2008).
ARP model survival calculated at delivered doses from 0 to 8 Gy was then fit to the linear quadratic model in order to compare against experimental results.
2.2.3. Macroscopic dose enhancement model
Another prediction of cell survival was modeled from the estimated macroscopic dose enhancement given by:

Where D is the dose delivered by the photon source, and Denhance is the macroscopic dose enhancement predicted from simulations (Lechtman et al 2011). The macroscopic dose enhancement survival at delivered doses from 0 to 8 Gy was fit to the linear quadratic model to compare against experimental results. Table 1 highlights the similarities and main differences between the macroscopic dose enhancement model and the ARP model.
Table 1. A comparison of two models used to predict AuNP radiosensitization.
| Macroscopic enhancement model | ARP model |
|---|---|
| MCNP Monte Carlo simulation to determine the rate of photoelectric absorption within AuNPs | |
| PENELOPE Monte Carlo simulation of secondary energy escaping AuNPs | |
| Macroscopic dose enhancement | Randomly selected tracks of escaping radiation |
| calculated in a volume around the AuNPs | simulated to interact with a model cell. Dose |
| corresponding to the cell culture media. | enhancement scored in nanoscale voxels of the nucleus. |
| Cell survival estimated using | Cell survival estimated by integrating |
| the linear quadratic model as | the local lethal event density over the cell |
| described by equation (3). | nucleus as described by equations (1) and (2). |
2.2.4. ARP validation and predictions
In order to quantify AuNP radiosensitization and to compare experimental and predicted survival, the mean inactivation dose (MID), represented by the area under the survival curves was calculated. The MID is a useful metric, as it represents radiosensitivity over the whole survival curve with a single parameter (Fertil et al 1984). Sensitizer enhancement ratios (SER) were calculated by dividing the MID without AuNPs by the MID with AuNPs (Jain et al 2011). MID and SER values were presented along with standard deviations. Predicted SER values were compared to experimental results and the effect of AuNP intracellular localization was explored using ARP.
3. Results
3.1. PC-3 cell characteristics and AuNP accumulation
The average cell volume and nucleus volume were measured from confocal images to be 2290 ± 1480 µm3 (n = 26) and 9480 ± 6040 µm3 (n = 27) respectively (figure 1). Therefore, the average cytoplasmic volume ignoring cytoplasmic organelles was estimated to be ∼7190 µm3.
Figure 1. Confocal images of live PC-3 cells. Cell surfaces were stained by wheat germ agglutinin-Alexa Fluor 594 conjugate, whereas cell nuclei were stained by Hoechst 33342 dye. Average cell and nucleus radius were measured to be 13.1 ± 2.5 µm and 8.2 ± 2.1 µm respectively.
Download figure:
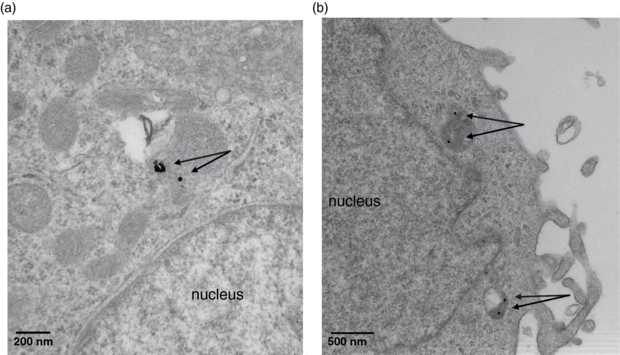
Standard imageThe amount of gold per PC-3 cell, as measured using ICPMS was 2.27 × 104 ± 1.47 × 104 AuNPs per cell. TEM imaging (figure 2) revealed 30 nm AuNPs to be randomly distributed within cytoplasmic vesicles, but were not observed to enter the cell nucleus. Given this observation, the concentration of gold within the cytoplasm was estimated to be 0.84 mg of gold per mL, resulting in a concentration uptake ratio (AuNP concentration inside the cells divided by the concentration in the surrounding media) of 0.42.
Figure 2. TEM images reveal 30 nm AuNPs accumulate in cytoplasmic vesicles both individually and in groups within vesicles. Nanoparticles of this sizes were not observed to enter the nucleus.
Download figure:
Standard image3.2. Clonogenic survival of PC-3 cells
PC-3 cells incubated with AuNPs at 2 mg mL−1 without irradiation did not show loss of clonogenicity. Figure 3 shows the clonogenic survival of cells irradiated without and without AuNPs. Radiosensitivity of PC-3 cells was observed to have a shouldered response, but this shoulder was less pronounced when irradiation was carried out with AuNPs in the media. Table 2 shows the linear quadratic parameters fitted to the experimental data.
Figure 3. Experimental survival for PC-3 cells irradiated using a 300 kVp source. When cells were incubated with AuNPs prior to irradiation, survival was significantly reduced. The linear quadratic model was fit to each set of data using nonlinear least-squares regression.
Download figure:
Standard image3.3. ARP model parameters and comparison to clonogenic assays
For the 300 kVp source, the rate of photoelectric absorption within a 30 nm AuNP was calculated from simulations to be 1.00 × 10−3 per AuNP per Gy absorbed dose. The maximum range of photoelectrons escaping AuNPs was determined from simulations to be 147 µm, and this value was used as the cutoff distance for simulating AuNPs in the media surrounding the ARP model cell. Outside the cell AuNPs were assumed randomly distributed at a concentration of 2 mg mL−1. The ARP tally voxel size was set to 20 × 20 × 20 nm3. The threshold dose was set to 23.9 Gy—a value that fit the ARP model well to experimental cell survival at 2 Gy with 2 mg mL−1 AuNPs. At AuNP concentrations of 2 mg mL−1, macroscopic dose enhancement was calculated to be 6.45 × 10−2 Gy per Gy delivered.
Figure 4 shows a good agreement between experimental survival and the ARP model survival curves, while the macroscopic dose enhancement survival model underestimates AuNP radiosensitization. Table 2 shows the extracted linear quadratic parameters of both models compared to experimental results along with the MID and SER values. The SER obtained by the ARP model is in close agreement with experimental results, while the macroscopic model underestimates sensitization by 12%. It should also be noted that the macroscopic model assumed a uniform concentration of 2 mg mL−1 AuNP concentration both inside and outside the cell, but we observed a lower concentration within the cells experimentally. While the macroscopic dose enhancement model underestimates the radiobiological effect of short ranged Auger electrons, in this case it overestimated the intracellular AuNP concentration, and therefore appears to show better agreement than the model warrants.
Figure 4. Comparing the ARP model and the macroscopic model with experimental results. The ARP model fits experimental data more closely by taking into account energy deposition at the nanoscale, as well as experimental details such as cell dimension, and intracellular AuNP concentration.
Download figure:
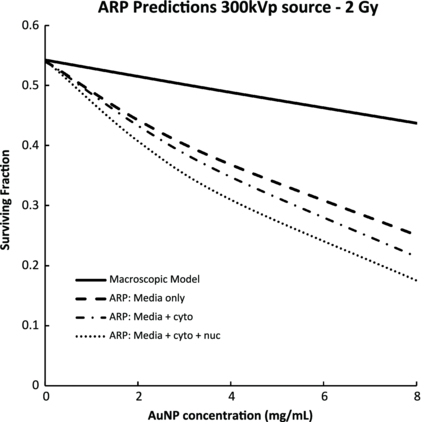
Standard imageFigure 5 shows ARP survival for a 2 Gy delivered dose with varying AuNP concentrations and distributions within the cell. Three hypothetical cases were considered: (i) 2 mg mL−1 AuNPs distributed in the media only, (ii) 2 mg mL−1 AuNPs distributed in the media and the cytoplasm, (iii) 2 mg mL−1 AuNPs distributed in the media, cytoplasm and nucleus. The ARP model was compared to the macroscopic dose enhancement prediction, which did not incorporate AuNP localization and assumed a homogeneously distributed AuNP concentration of 2 mg mL−1.
Figure 5. The predicted effects of AuNP cellular and nuclear accumulation. The ARP model shows a strong influence of AuNP intracellular distribution on radiosensitization. These effects are not taken into account in the macroscopic doe enhancement model.
Download figure:
Standard image4. Discussion
Based on the experimental data presented, the ARP model provides a more accurate prediction of AuNP radiosensitization—with a predicted SER in close agreement to the experimental value—compared to macroscopic dose enhancement model predictions. Furthermore, as opposed to macroscopic dose enhancement predictions (Roeske et al 2007, Lechtman et al 2011, Cho 2005), the ARP model incorporates detailed information regarding the radiation source, cell line, and characteristics of AuNPs including size, concentration, and cellular distribution to predict the extent of radiosensitization. This model builds on the local effect model adapted for AuNPs proposed by McMahon, by incorporating structural compartments of the cell and AuNP localization as input parameters (McMahon et al 2011). This gives the ARP model flexibility to examine radiosensitization through a vast parameter space of pharmacological and cell modelization. With increasing efforts to conjugate AuNPs to targeted moieties, toward the aim improving cellular and possibly nuclear incorporation (Chattopadhyay et al 2010, 2012, Nativo et al 2008, Ryan et al 2007), the ARP model can help provide a framework for predicting the benefits of such targeting strategies.
Table 2. Parameters of cell survival.
| α (confidence bounds) (Gy−1) | β (confidence bounds) (Gy−2) | Mean inactivation dose ± SD (Gy) | SER ± SD | |
|---|---|---|---|---|
| No AuNPs | 0.217 (0.141, 0.293) | 0.044 (0.016, 0.072) | 2.56 ± 0.25 | – |
| (experimental) | ||||
| 2 mg ml−1 AuNPs | 0.345 (0.288, 0.402) | 0.037 (0.017, 0.057) | 2.11 ± 0.10 | 1.21 ± 0.13 |
| (experimental) | ||||
| Macroscopic | 0.231 (0.231, 0.231) | 0.050 (0.050, 0.050) | 2.40 ± 0.00 | 1.07 ± 0.10 |
| dose model | ||||
| ARP model | 0.315 (0.314, 0.316) | 0.045 (0.045 0.046) | 2.13 ± 0.02 | 1.20 ± 0.12 |
Figure 5 reveals the radiobiological benefit of high-LET Auger and delta electrons as predicted by the ARP model. These escaping electrons travel an average distance of 1.06 µm from the AuNP surface, as calculated from PENELOPE simulations, and therefore only contribute to cell killing when AuNPs are sufficiently close to the radiosensitive DNA. Significant radiosensitization is observed when AuNPs are localized in the cytoplasm, resulting in a predicted SER of 1.23 ± 0.11 at an AuNP concentration of 2 mg mL−1. The largest effect is predicted when AuNPs are located in the nucleus, resulting in a SER of 1.29 ± 0.13 at an AuNP concentration of 2 mg mL−1. When AuNPs are exclusively located in the surrounding cell culture media, the ARP model still predicts significant, though less substantial radiosensitization. This effect is likely due to longer ranged photoelectrons traveling 40 microns on average that can reach the nucleus from outside the cell.
The influence of AuNP localization is expected to be most pronounced for photon sources with average energies below the k-edge, at which escaping photoelectrons have shorter average ranges and Auger and delta electrons comprise a significant percentage of the total escaping energy. For higher energy photon sources, longer ranged photoelectrons are the main contributor to radiosensitization (Lechtman et al 2011).
Comparing the extracted linear quadratic parameters from experimental survival with and without AuNPs, we observed an increase in the α component but only a small change in the β component. This suggests that AuNP radiosensitization involves radiobiological mechanisms similar to that of high-LET radiation, which exhibits a predominantly linear dose response (Kellerer and Rossi 1971). This finding agrees with other experimental findings of AuNP radiosensitization (Chithrani et al 2010), and demonstrates that the ARP model is able to effectively represent this feature of AuNP radiosensitization.
In our development of the ARP model, we have incorporated two fundamental distinctions from the LEM model. The first involves a more detailed simulation of the energy deposition at the sub-cellular scale, rather than representing the energy deposition as a radial dose function (Kraft et al 1999), or scoring dose in radial bins (McMahon et al 2011). This provides additional information of the Auger cascade, and avoids variations in the calculation of dose associated with the increasingly large concentric volumes further away from the AuNP (Lechtman et al 2011). Another distinction of the ARP model is the calculation of lethal event density within finite sized voxel elements. This method, rather than a point response approach, may be a better representation of radiobiological mechanisms that occur over finite distances, such as diffusion of free radicals, double strand breaks, multiply damaged sites, and clustered effects (Ward 1994, Nikjoo et al 2001). At the smallest relevant scale, the DNA helix is 2.2–2.6 nm wide and each base 0.33–0.34 nm long (Mandelkern et al 1981). The maximum distance of double strand breaks has been reported to lie between 20–43 base pairs, corresponding to a distance of 6.6–13.2 nm, with some reported distances beyond 20 nm (Shao et al 1999). Radical diffusion, which further extends the distance of radiobiological action, is known to occur within 4 nm of DNA molecules (Elsässer and Scholz 2007). We therefore chose a voxel dimension of 20 nm in order to encompass these features. Although PENELOPE's low-energy simulations differ from more accurate models below a few hundred eV (Fernandez-Varea et al 2012, Dingfelder et al 1999, Emfietzoglou et al 2005), and furthermore the transport of electrons below a few hundred eV is still poorly understood (Emfietzoglou and Nikjoo 2005, Dingfelder 2006, Liljequist et al 2012, Thomson and Kawrakow 2011), the ARP model voxel dimension of 20 nm, corresponding to the average path length of ≈500 eV electrons (Cole 1969), minimizes the inherent uncertainty in transporting electrons below this energy. It should also be noted that the ARP survival predictions do not change significantly as a function of voxel size below ≈30 nm because of the purely exponential survival function above the threshold dose.
The ARP model also includes several simplifications. First it assumes that cancer cells have a spherical shape, with a nucleus that is centered in the middle of the cytoplasm. While this assumption is somewhat acceptable for undifferentiated cancer cells, the shape of the cell may have an impact on the biological efficiency of AuNP radiosensitization. Second, the ARP model assumes a random distribution of AuNPs inside the cytoplasm instead of an accumulation into the phagolysosomes—an observation noted in several studies (Rahman et al 2009, Coulter et al 2012, Jain et al 2011, Chithrani et al 2010). While this assumption may result in AuNPs closer or further away from the nucleus compared to reality, it is not yet understood where the nanoparticle would be localized at the sub-cellular scale in vivo during irradiation, as AuNPs have been shown to exhibit complex trajectories once inside cells (Chithrani 2010). Finally, similar to the LEM model developed by Kraft, the ARP model includes the concept of a threshold dose, beyond which the cell survival response is expected to be purely exponential (Elsässer et al 2008). While there is evidence that survival takes on a purely exponential shape after a threshold dose (Park et al 2008, Astrahan 2008), extracting this value from experimental results may be difficult. All these assumptions may limit the use of the ARP model as an 'absolute' radiobiological predictive tool. However, this limitation should not impact its capacity to provide relative comparisons between different clinically relevant uses of AuNPs; for example comparing different AuNP sizes, concentrations, intracellular localizations, and various beam energies, radiation doses, and cancer cell radiation sensitivities. The ARP model therefore remains a useful tool to define the best scenario for a clinical application of AuNP radiosensitization.
Acknowledgments
This research was supported by a grant from the Canadian Breast Cancer Research Alliance (grant 019374) and the Canadian Institute for Health Research Terry Fox New Frontiers Program Project in Ultrasound for Cancer Therapy.