Abstract
The removal of a high mercury contamination on a Pt reference mass by thermal desorption was studied directly by x-ray photoemission spectroscopy (XPS). Subsequently the contamination mechanism was investigated. Samples of PtIr and AuPt exposed to vapour of mercury in air were studied using XPS and gravimetric mass determination. We find an extremely rapid mercury contamination which takes place within minutes and reaches an initial equilibrium state after 2 h to 4 h. Roughly 1 to 2 monolayers of mercury adsorbs directly on the metal surface. A natural contamination of carbon and oxygen compounds is at the top. Due to the accumulation of mercury, we find a gain in mass which corresponds to 20 µg to 26 µg for a PtIr standard. XPS data from a historical Pt standard give strong evidence for further average mercury accumulation of (1.3 ± 0.1) µg/year during a period of more than a century. This can be explained by a two-step mechanism presented in this study. The speed of contamination depends on the initial surface conditions. Polishing activates the surface and results in an enhanced accumulation of mercury. Natural contamination by C and O can delay but not prevent contamination. We further demonstrate that the mercury contamination can be removed by both hydrogen plasma and thermal desorption. The removal of mercury by hydrogen plasma can directly be attributed to the synthesis of gaseous mercury dihydrides at low pressures.
Export citation and abstract BibTeX RIS
1. Introduction
Primary mass standards are of central and essential interest for the current definition of the mass unit but also for a future new definition based on natural constants. The unit of mass is currently represented by a PtIr artefact, the international prototype of the kilogram (IPK). The dissemination of the unit is realized by direct mass comparison of this prototype with platinum–iridium working standards and copies owned by national metrological institutes. While the definition itself potentially can be based on natural constants via the watt balance [1–7] and Avogadro [8–11] route, the dissemination still relies on standards which are subject to airborne contamination resulting in a limited stability in mass.
The use of PtIr for primary standards of highest quality exhibits many advantages. The material has excellent mechanical properties. It is hard but ductile and consequently abrasion and wear are strongly reduced. Because of its high density the volume and surface area are small, air-buoyancy and sorption effects on the surface are minimized. Among the potential materials it is one of the chemically most resistant and forms no oxides under normal conditions. Physisorbed contaminations can largely be removed by various cleaning methods such as nettoyage-lavage [12], UV/ozone [13–15] or hydrogen plasma [16, 17]. These characteristics make PtIr quite favourable as a material for mass standards of highest stability.
The surprising discovery of mercury contamination on PtIr samples [18] did awake the mass community. In order to monitor the environmental influences on the recontamination, the authors placed a set of samples at different positions in a regular mass laboratory. After a very short period of exposure of only 19 days, all samples showed a significant contamination of mercury. In a subsequent study using a quartz crystal microbalance (QCM) [19] they demonstrated that the growth of mercury is initially very rapid, followed by a further but slow increase in mass which never stops. In their first study [18] they found that mercury adsorbs directly on the metal surface. Additional layers of natural contamination containing carbon and oxygen are at the top. All attempts to remove the mercury by the established BIPM cleaning procedure [12] failed.
In this paper we first analyse a historical Pt primary standard which is found to be highly contaminated with mercury. The contamination was successfully removed by a thermal desorption. The contamination mechanism was further investigated using surface chemical analysis by x-ray photoemission spectroscopy (XPS) on small PtIr and Au alloy samples exposed to Hg vapour. The contamination reaches saturation of roughly 1 to 2 monolayers after 2 h extremely rapidly. The contamination mechanism can be explained by a two-step model which was recently developed for the natural contamination of reference standards [20]. The speed of contamination depends strongly on the surface preparation. It can be forced and increased by initial polishing. Unexpectedly, we find hydrogen plasma can remove Hg very efficiently. We attribute this to the synthesis of gaseous mercury dihydrides at low pressures, a process which was discovered recently [21, 22].
2. Experiment
For our study we started with the historical kilogram prototype C3, which was ordered in 1863 by the Swiss Federal Council from the Conservatoire des Arts et des Métiers. It is the third copy of the platinum kilogram of the Conservatoire, manufactured from pure platinum metal by the Collot brothers in Paris. Since then it has been kept in the national mass laboratory of Switzerland. Over this period of time, the mass laboratory was moved five times to different buildings. Subsequent measurements were performed with several sets of metal samples made of an alloy containing mass fractions of 90% platinum and 10% iridium, machined from the same material that is used for the production of kilogram prototypes, and a gold alloy (mass fractions: Au 75%, Ag 9%, Cu 12%, Pt 4%) which is a potential material for future mass standards [23]. All samples were carefully polished and cleaned with a chamois soaked in pure ethanol/ether in order to achieve equal starting conditions. They were mounted on a multiple sample holder which fits up to six samples; in this way an entire set of samples could be exposed simultaneously to the same processes, and transported and stored under identical conditions for better comparison.
All XPS measurements were performed in a multifunctional UHV chamber at a pressure below 5 × 10−7 Pa. The instrument and the experimental layout have been described in more detail recently [16, 20]. For surface chemical analysis by XPS we used an Alpha 110 hemispherical photoelectron analyser normally operated in the constant energy mode (CAE) with pass energies of 50 eV for survey and 20 eV for higher resolution scans. The samples were irradiated by Mg Kα(hν = 1253.6 eV) at 300 W/15 kV generated with a twin anode x-ray source (Thermo Electron Corp.). Raw XPS data were analysed using the manufacturer's 'Avantage' data processing software. For curve fitting we used published values of binding energies [24] and a common Shirley background. The intensities of the mercury photoelectron lines were extracted using both the raw and the differentiated spectra for better background subtraction.
For the low-pressure plasma cleaning, a system integrated in the load lock of the XPS chamber was used. The load lock was equipped with a precision leak valve for the supply of high purity H2 process gas (Alphagas 99.999%). An anode and a cathode were attached on opposite viewports laterally to the load lock and supplied by a 40 kHz generator to produce a high-frequency field to ionize the gas. During the plasma process, the pressure in the load lock was kept at 70 Pa.
The thermal desorption was carried out at 200 °C to 220 °C in a Vacutherm 6000M vacuum furnace (Heraeus Instruments) under an argon atmosphere at 5 × 104 Pa.
3. Results
3.1. Cleaning of the Pt reference mass C3 by thermal desorption
Without any cleaning, the historical Pt reference mass C3 was first analysed by XPS. Figure 1 shows a widescan spectrum. It proves that the standard is pure platinum metal. Surprisingly, we found, in addition to the natural contamination with carbon and oxygen compounds, a huge amount of mercury. Angle-resolved XPS (ARXPS) measurements revealed the following layer structure: mercury is directly adsorbed on the Pt metal surface with a sharp interface between mercury and platinum. The natural contamination by carbon and oxygen compounds is at the top. As assumed earlier for all reference kilograms [18], this old Pt standard, conserved at the mass laboratory for over 150 years, is completely contaminated by mercury. The observed layer structure is in good agreement with earlier findings [18]. From our XPS measurements we calculated a layer thickness of (2.0 ± 0.5) nm. Using the conversion formula from [18] we estimate a total mass of mercury of (200 ± 30) µg for the standard, which is much more than 22 µg to 32 µg found previously [18]. As we will show later, this unexpectedly high amount of mercury can be explained by the extremely long exposure time of more than a century.
Figure 1. The overview XPS spectrum of the initial state of the reference mass C3 reveals a serious contamination of mercury.
Download figure:
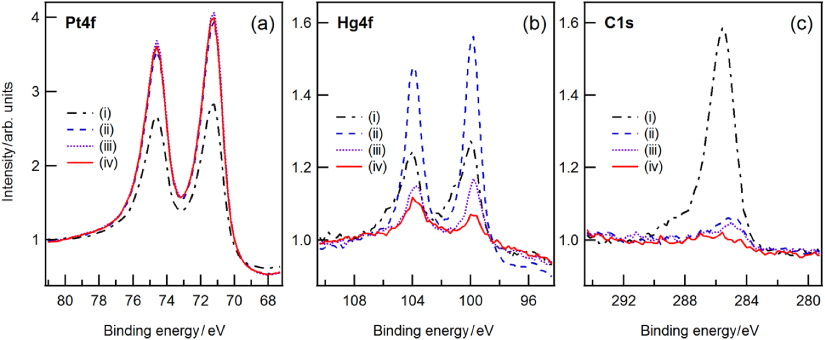
Standard imageIn order to remove the mercury by thermal desorption, the standard was heated twice for 2 h at 200 °C to 220 °C in a vacuum furnace under an argon atmosphere at 5 × 104 Pa. Figure 2 shows the evolution of the platinum, mercury and carbon photoelectron lines upon individual steps of treatment. The standard was first analysed by XPS (figure 2, (i)) and then cleaned by hydrogen plasma to reduce the natural carbon and oxygen contamination, followed by an additional XPS analysis (figure 2, (ii)). There was a significant decrease in carbon after hydrogen cleaning resulting in an increase in the mercury and platinum intensities, due to the reduction of carbon and oxygen. After each heating, the standard was cleaned by hydrogen plasma to reduce again the natural contamination of carbon and oxygen arisen during the heating process, and to reach a similar level of contamination for a better comparison of mercury removal (figure 2, (iii+iv)). The measurements show a remarkable reduction of mercury after heating. We estimate a layer thickness of 0.6 nm after the second heating, which corresponds to a reduction of 1.4 nm.
Figure 2. The initial state of the mass standard C3 shows a huge amount of carbon and low intensities of Hg and Pt (i). After hydrogen plasma cleaning the carbon was reduced resulting in higher intensities of Hg and Pt (ii). First (iii) and second (iv) heating caused a decrease in Hg and carbon intensities, resulting in a slight increase in Pt intensity.
Download figure:
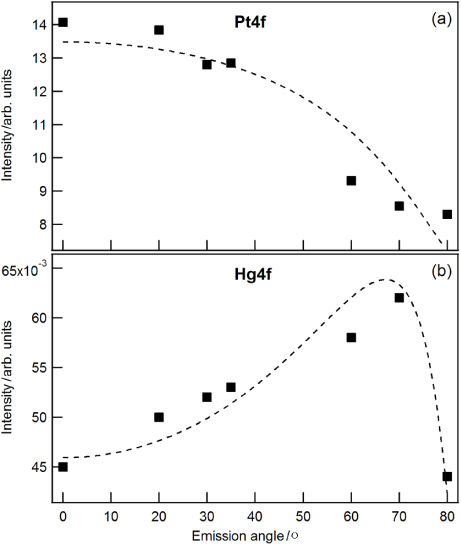
Standard imageWe attribute this reduction to thermal desorption of mercury at elevated temperatures. The remaining contamination is still located at the surface. With ARXPS measurements we found a sharp interface between Hg and Pt, but no indication of Hg diffusing into Pt. This is in good agreement with earlier findings [18]. The authors outlined that the diffusion of Hg into bulk Pt at room temperature was extremely small; the time needed for a mercury atom to move one atomic spacing exceeds 100 years. Thus, interdiffusion of Hg into bulk Pt can be ruled out also in our experiment. Figure 3 shows the intensity of bulk Pt and Hg obtained from XPS measurements as a function of the emission angle. The dashed lines are best fits to the data based on a physical model for bulk (Pt) and layer (Hg, C) materials following strictly an approach by Cumpson and Seah [18]:



The layer thicknesses of mercury, dHg, and carbon, dC, are fitting parameters using attenuation lengths of λHg = 3.05 nm and λC = 2.8 nm which are in good agreement with published values [25, 26].
Figure 3. ARXPS of the mass standard C3 after 2 h of heating in a furnace. As the emission angle is increased the intensity of Pt decreases (a) and the intensity of the overlying Hg increases and reaches a maximum at around 70°(b). The decrease beyond this angle is an effect due to the carbon layer which is located at the top of the surface.
Download figure:
Standard imageOur findings are in good agreement with results obtained by thermogravimetry (TG) and scanning electron microscopy (SEM) on a Pt–20% Rh alloy [27]. The authors found the desorption of mercury occurs in three well-defined temperature ranges. From ambient to 175 °C mainly bulk mercury is desorbed very rapidly, resulting in a loss of 80.8% of the total mercury. The remaining mercury was found to form an intermetallic PtHg4 surface film. In a second step from 175 °C to 224 °C the intermetallic PtHg4 is decomposed into PtHg2 and Hg which desorbs and leads to a further reduction of 11% of the total mercury deposited on the surface. The remaining intermetallic film does not completely cover the surface. The final removal of mercury in the range from 224 °C to 305 °C is attributed to the thermal decomposition of intermetallic species PtHg2 and RhHg2. For the maximum temperature of 200 °C to 220 °C used in our experiment we assume a thermal desorption of bulk Hg and an incomplete intermetallic layer of PtHg2 and RhHg2.
In parallel, we tried to measure gravimetrically the loss in mass under vacuum due to the evaporation of mercury. Unexpectedly, we found huge changes in mass of 2 mg to 3 mg which cannot be explained by the removal of mercury. Similar effects have been reported for standards manufactured by forging before the metre convention. Such standards, including our standard C3, exhibit a density which is up to 5% smaller than for cast Pt, indicating the presence of porosities and inclusions which are held responsible for loss in mass when measured under vacuum [28]. Historically, as a consequence vacuum measurements were stopped around the year 1870.
3.2. Contamination mechanism
In order to verify the results with high-quality materials used in today's mass laboratories, we continued this study with materials that are currently used for mass prototypes standards such as PtIr as well as a material for potential future standards made of an AuPt alloy [23]. First, we focused on the contamination mechanism to find out the speed of action. Therefore, we exposed polished samples of both materials to saturated mercury vapour in air. The samples were placed in a glass exsiccator which contained 1 cm3 of spilled mercury and monitored the level of contamination on the samples by XPS.
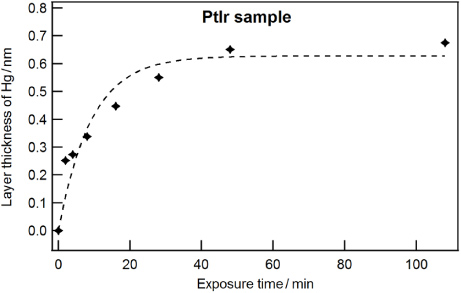
Figure 4 shows the rapid increase in mercury layer thickness with time. Within a few minutes the intensity of the photoelectron lines of mercury increased dramatically. The speed of contamination is unexpectedly fast and initial saturation is reached after 1 h with a layer thickness of (0.63 ± 0.05) nm.
Figure 4. The layer thickness of mercury on a PtIr sample is increased as a function of time due to the exposure to mercury vapour. The initial contamination rate was found to be 0.07 nm min−1.
Download figure:
Standard imageSurprisingly, the mercury contamination on the AuPt alloy was much lower, and it starts with a delay of 1 h as shown in figure 5. The reason for this delay is not yet understood, but might be caused by natural contamination with carbon and oxide compounds. Surface reactions are quite complex and impurities can play an important role, as shown for Au [29–32]. For other materials such as Pd it is known that oxygen can promote or prevent chemical reaction [33, 34].
Figure 5. After 1 h of exposure to mercury vapour in a glass exsiccator, no contamination was found (dotted line). But after 2 h, a significant increase in the photoelectron lines Hg 4f7/2 and Hg 4f5/2 was detected (dashed line).
Download figure:
Standard imageIt is generally accepted that the growth of natural contamination by carbon and oxygen compounds on a clean mass standard is initially very rapid for the first few days and weeks, but then slows down and further accumulation of contaminants is small. A rich summary of observation and results can be found elsewhere [35]. Very recently, a simple two-step model was presented which can explain the short-term as well as the long-term growth of contaminants [20]. It is the first model that is based on physical principles, following concepts of Langmuir [36]. For the natural contamination with carbon and oxygen compounds it is in good agreement with numerous observations [35], and furthermore, as we will show in the following, it can also be applied to the mercury contamination. Molecules of mercury vapour have a certain probability S (e.g. S = 1) to stick on a clean metal surface. The amount of material accumulated on the surface in time, δm/δt, and the loss of clean surface, δAc/δt, are consequently proportional to the clean surface Ac itself.

One solution to this differential equation is an exponential function in time (5). Integration over time delivers the total mass Δmtot of the saturated contamination layer (6):


Since the clean surface area is limited and decreasing, the gain in mass for this first step of contamination is self-limited. The dashed line in figure 4 shows the best fit to our data using (6). The initial and self-limited growth model is in fair agreement with our data. Once the surface is covered, the sticking probability for mercury on mercury is completely different from the initial sticking factor of mercury on metal, but does not further change with time in the simplest case. Hence, this second step gives rise to an additional but small gain in mass, which never stops, and which is superimposed on the initial growth of the first layer. Under this assumption the sticking probability is unchanged upon increasing layer thickness; the multilayer growth would be linear in time. As reported earlier [27] the first layer of mercury forms intermetallics. Therefore, it is most likely that the sticking probability for Hg on intermetallics is different from the initial one. Also for further layers it can change with each layer. In this more general case the growth of individual layers Δmi(t) can be described as (7), and the total mass of mercury increases as a series of Langmuir layers as described in (8).
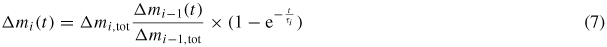

The fraction Δmi−1(t)/Δmi−1,tot represents the coverage factor of the layer (i − 1).
Very recently the short-term adsorption of natural contaminants was studied gravimetrically with 1 kg PtIr artefacts [20] and with a gold-coated surface of a QCM [37]. For both measurements the data are in almost perfect agreement with our exponential model. For the long-term contamination the adsorption is small but uniformly continuous. Cumpson and Seah analysed weighing data of the UK national standard No 18 and found that the mass of the carbonaceous contamination grows as the square-root of time over a period of 60 years [38]. Based on data taken in the period from 1 month to 6 years after cleaning, the same standard shows good agreement with the time-root dependence for the first 15 months, but is in poorer correlation for the long-term data [35, 39]. However, with a thickness-dependent sticking probability also a time-root increase in mass can be well explained by our model.
As explained earlier, the standard C3 was stored in the mass laboratory of METAS in different buildings over the years. The level of mercury contamination is not known, but was most likely not the same at different places. Therefore, it is difficult to determine the long-term growth mechanism and we can only deliver an average value. From our historical standard C3 we can estimate an initial increase in mass of 25 µg right at the beginning of Hg exposure, followed by a long-term average mass gain of roughly (1.3 ± 0.1) µg/year over 120 years, resulting in a total mass of the mercury layer of 200 µg. Since this long-term growth is very small, it is invisible on a short time scale.
In 2003 the mass laboratory moved into a newly built facility which was proven to be completely free of Hg contamination. Two new PtIr standards presented in [20] were stored for 8 years under identical conditions next to C3. By XPS we found no evidence for mercury contamination on these standards. As a matter of fact we found 200 µg Hg on the C3 standard by XPS, after 10 years of storage in this mercury-free environment. This high amount of mercury accreted on the surface is consistent with earlier findings [19], where no asymptotic limit to the mass increase is predicted. Furthermore, it clearly shows that the build-up of a Hg multilayer cannot be described by an equilibrium process, such as outlined in the Brunauer, Emmett and Teller (BET) theory [40], where a rapid loss of Hg would be expected when the exposure to Hg vapour is stopped. In the period from 2003 to 2013 C3 was used as a check-standard for the calibration of primary stainless steel standards, which are not subject to mercury contamination. From these gravimetric measurements we can exclude a loss in mass for C3 with an uncertainty of 12 µg. This is in good accordance with earlier findings [19] on a PtIr sputter-coated QCM, where the Hg mass remains constant also after interruption of exposure to Hg vapour [19]. The authors attribute this to a diffusional process into sputtered PtIr film which exhibits a high density of grain boundaries and dislocations.
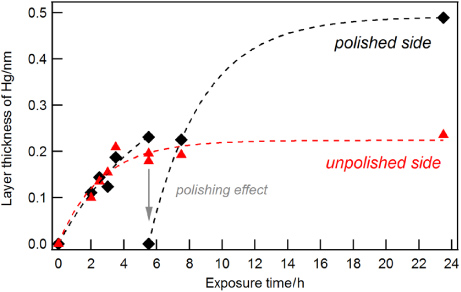
In order to determine the amount of mass accumulated on the surface we exposed a 0.1 mm thick PtIr foil to mercury vapour and studied the increase in mass gravimetrically and the surface chemical state by XPS. The foil was used as rolled, only cleaned by rubbing with ethanol/ether soaked chamois. Surprisingly, the contamination on the foil by mercury is much slower than for the polished samples analysed before. To investigate the influence of the initial surface conditions on the growth of mercury, half of the foil was polished with diamond paste. The entire foil was cleaned again with solvents and the analysis was continued. On the polished area the mercury could completely be removed whereas on the unpolished area it remained unchanged as illustrated in figure 6.
Figure 6. Before polishing, the speed of contamination with mercury was found to be 0.07 nm h−1. By polishing, the mercury contamination could be removed. After polishing, the contamination was twice as fast (0.15 nm h−1) and the saturation level increased.
Download figure:
Standard imageXPS spectra clearly showed that Hg contamination can be removed by polishing. On the other hand, rubbing with ethanol/ether soaked chamois does not, as expected and observed earlier [18], affect the mercury film, but reduces the natural contaminants slightly. However, this reduction of carbon and oxygen compounds does not noticeably influence the further growth of the mercury layer as can be seen on the unpolished area. In contrast, on the polished area the speed of contamination and the level of saturation are increased, showing the same amount of mercury as observed earlier for the polished bulk samples (see figure 4). For the speed of contamination right at the beginning of exposure we calculated (0.15 ± 0.02) nm h−1 for the polished area and (0.07 ± 0.02) nm h−1 for the unpolished area. This clearly indicates that the surface can be activated by polishing, resulting in a much higher speed and saturation level of mercury contamination than for the unpolished surface as rolled. On the foil sample we found no evidence that the natural contamination by species of carbon and oxygen can delay or suppress contamination.
Earlier studies clearly demonstrated that the chemical activity of metal surfaces can be strongly enhanced by mechanical treatment [41, 42] as shown first for steel [43], Ni [44, 45] and Au [41, 46]. The authors attributed this mechanochemical or tribochemical enhanced activity to surface defects, such as kink sites and step edges, which are energetically favourable [41].
Since our first mass determination of the PtIr foil was not successful, the gravimetric measurements were continued with another set of polished small samples with a total surface area of 20 cm2. After 2 h of exposure to Hg vapour we found a gain in mass of (5.0 ± 0.2) µg, and saturation is reached at (7.8 ± 0.2) µg after 18 h. The corresponding evolution of the photoelectron line intensities of Pt4f and Hg4f of the bulk and layer material, respectively, is illustrated in figure 7. Using the gravimetric results above, the total gain in mass can be scaled for a 1 kg PtIr reference standard with a total surface area of 72 cm2 to (18 ± 1) µg after 2 h, and (28 ± 1) µg for a saturated surface. The gravimetric measurements were performed under ambient conditions, where also natural contamination by species of carbon and oxygen takes place. The mass increase for a 1 kg PtIr due to this natural contamination was estimated to be (9.4 ± 1.5) µg [18]. Recent gravimetric measurements reported a much smaller value of (1.63 ± 0.1) µg [20]. Taking this into account the mass of the saturated mercury layer can be estimated to be 19 µg to 26 µg. This amount is in good agreement with earlier studies [19] on a PtIr coated QCM. The authors report about a very rapid initial adsorption of roughly 28 µg extrapolated/corresponding for a PtIr standard, followed by a slow time-root-dependent diffusional growth of mercury on a long term.
Figure 7. Photoelectron lines of platinum and mercury before the exposure to mercury vapour (solid line), after 2 h (dotted line) and after 18 h (dashed line) in total.
Download figure:
Standard imageIn addition, ARXPS measurements were subsequently made of the PtIr sample to verify the layer structure and to determine the layer thickness of mercury and carbon (figure 8). The measurements revealed the same layer structure as for the mass standard C3 with a layer thickness of (0.6 ± 0.1) nm for mercury and (1.2 ± 0.2) nm for carbon which is in good agreement with the result above. The fitted curves using equations (1) to (3) are in good accordance with the measured data, indicating a sharp interface between the individual layers.
Figure 8. ARXPS on a PtIr sample. As the emission angle is increased, the intensity of carbon increases (a). The intensity of mercury increases, reaches a maximum at around 60°, and starts to decrease again by reason of attenuation of Hg photoelectrons in the outermost carbon and oxide compound layer (b). The intensity of platinum (bulk material) continuously decreases (c).
Download figure:
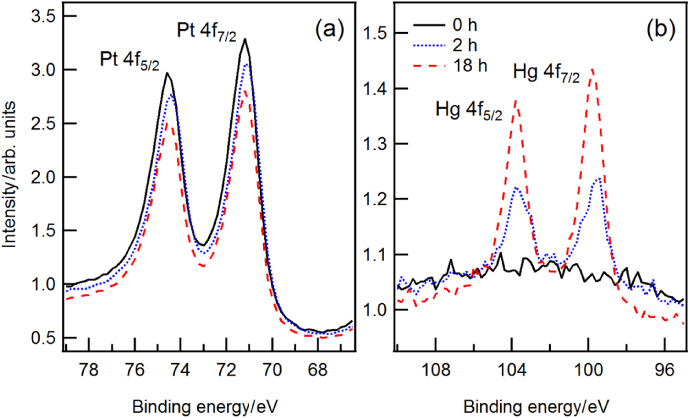
Standard imageIn order to perform ARXPS of the mercury layer itself without any natural contamination, we tried to remove the carbon and oxygen compounds by cleaning the samples for 1 h with hydrogen plasma. Surprisingly, and completely unexpectedly, the mercury concentration on the PtIr and AuPt surfaces decreased upon this treatment. Subsequent steps of hydrogen plasma cleaning clearly demonstrated that mercury can be removed by hydrogen plasma.
3.3. Cleaning by hydrogen plasma
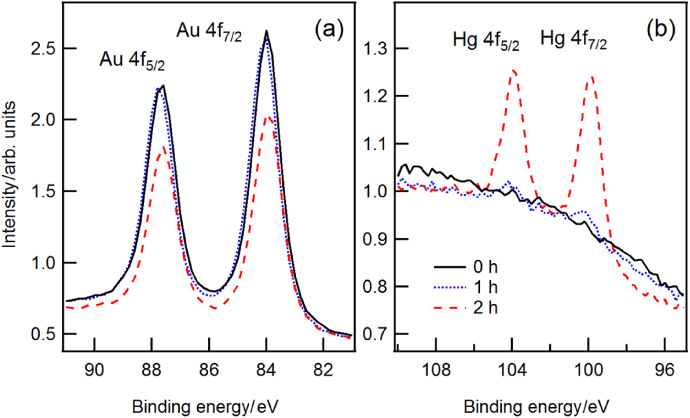
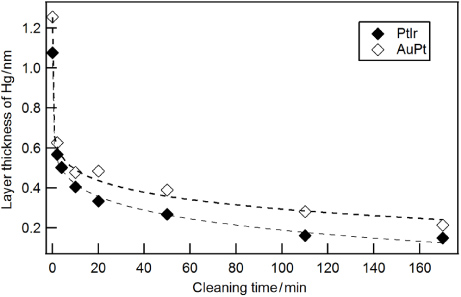
For a more systematic study of the mercury removal by hydrogen plasma we exposed Hg-saturated PtIr and AuPt samples to hydrogen plasma. Figure 9 shows the XPS spectra of the bulk material and mercury layer right after the exposure to mercury vapour (solid black line) and after each individual step of cleaning (dashed coloured lines). The measurements show equal contamination levels of Hg for both materials. Figure 10 shows the decrease in the mercury layer thickness as a function of cleaning time.
Figure 9. XPS spectra of a PtIr sample (top) and AuPt sample (bottom) before cleaning, and after 2 min, 10 min, and 170 min of exposure to hydrogen plasma. After 2 min of cleaning, the intensity of the photoelectron lines Pt4f and Au4f increased. Additional cleaning time could not increase the intensity any further. The photoelectron lines Hg4f decreased upon each treatment with hydrogen plasma.
Download figure:
Standard imageFigure 10. The mercury layer thickness on the PtIr and AuPt samples is reduced due to chemical reaction with hydrogen plasma and the formation of mercury dihydrate. The dashed lines serve as a guide to the eye.
Download figure:
Standard imageHydrogen plasma is well known for its capability to remove organic substances, such as carbon and oxygen compounds, but normally it does not react with inorganic compounds. It is really surprising that it can remove mercury from the surface of metals. Mercury is a hazardous air pollutant and was therefore intensively studied in the past. It is generally accepted that group 12 metals such as Hg, Cd, Zn in their ground state do not react with molecular hydrogen, because large energy barriers exist and the overall reactions are endoergic [20, 21]. But in the presence of an electrical discharge, gaseous HgH2 can be generated from the direct reaction of mercury vapour with molecular hydrogen, as demonstrated previously [20, 21]. The authors used conditions which are almost identical to our hydrogen plasma: in a low-pressure hydrogen atmosphere of 67 Pa to 330 Pa, mercury vapour was synthesized to mercury dihydrate (H–Hg–H) by dc electrical discharge. The cleaning effect of a hydrogen plasma is due to surface chemical reactions [16, 17], since the sputter yield of ionized hydrogen can be neglected [16, 47]. Therefore, we attribute the removal of mercury in our experiment to the chemical reaction with hydrogen and the formation of mercury dihydrate; the generated gaseous HgH2 is pumped away.
4. Outlook and conclusions
For more than 100 years, mercury-containing instruments such as barometers and thermometers were widely used in mass laboratories. But until its discovery, mass metrologists were not aware of this potential source of contamination. The extremely fast speed of contamination found in our study clearly demonstrates that mercury is a severe problem for PtIr primary standards used in mass metrology. Due to the fact that mercury was present in mass laboratories for a long time, we suspect that most of the national standards are contaminated; from QCM measurements over a period of 550 h [19] the authors give an estimate for the mass of mercury adsorbed on a PtIr standard of a few hundred micrograms over a period of 100 years. The historical standard C3 that we analysed in our study fully supports this assumption, and additionally gives clear evidence for further growth of mercury contamination after an initial monolayer is formed.
Cumpson and Seah [18] outlined in their study two different pathways to handle the situation. Since wet cleaning and washing leaves the Hg film intact, the mass remains stable and the work can also be continued with controlled contaminated standards. In this case, additional research needs to be carried out to prove the stability of the Hg film. From the analysis of our historical standard C3 we can conclude that the mercury layer is not stable and slightly increases with time when mercury is present. Furthermore, modern methods for the removal of natural contaminations such as hydrogen plasma also remove mercury. Consequently, this way must be ruled out. The second way is to manufacture new reference standards and to use them in a completely mercury-free environment, as already proposed in [19]. However, it is not yet clear how low a partial pressure is required to attain this.
Our study opens an additional new route: the standards may be cleaned, either by polishing or by 'soft' methods such as hydrogen plasma or thermal desorption, and reused in a contamination-free environment. However, with the use of the hydrogen plasma, weighing experiments will be needed to confirm that no Pt or Ir metal loss occurs during treatment.
In the context of the potential new definition of the kilogram, based on invariant natural constants, the entire dissemination chain is also analysed and reworked [42] in order to improve the stability of reference masses and to enable traceability to fundamental experiments in vacuum. All these research activities are carried out with clean reference standards and samples, among them are PtIr and AuPt. One monolayer of mercury on the surface of these materials completely changes the surface physics, and consequently, the results obtained for pure materials are not applicable to contaminated standards. Furthermore, the complexity of sorption effects on a partially or fully mercury contaminated surface increases, hence to work with a controlled contamination is an emergency solution only. When surface stability and full traceability to vacuum is needed, the surface chemical composition must be well known and be easy to control. Clean and inert noble metal surfaces provide such good conditions. Consequently, mercury-free standards in a contamination-free environment are the most preferable solution. It is therefore recommended that all mercury-containing equipment such as barometers, thermometers and lamps must be removed from mass laboratories. Additionally, the surface chemical state of Pt and Au alloy primary standards must be screened and analysed for contaminations periodically by surface sensitive techniques such as XRF as proposed by [19] or XPS in order to guarantee stable surface conditions.
As demonstrated, mechanical polishing can activate metal surfaces resulting in an increased contamination by mercury but also by carbon and oxygen compounds [36–40]. Freshly polished surfaces can even provoke catalytic processes [40]. On a nano-scale, polishing roughens the surface and leads to defects. With respect to the stability of mass standards, mechanical roughening by polishing should be avoided. As a last step of production, electro-polishing could lead to much smoother surfaces and consequently lead to more stable mass standards. In [19] the authors even propose to remove the outermost damaged layer by electro-polishing or to anneal the standards at temperatures of 400 °C to 500 °C. Further studies are needed to find the best surface preparation technique; this work will be partially carried out within the ongoing EMRP project [48].
Acknowledgments
The authors would like to thank Kevin Auderset for assistance with the contamination equipment and Hans-Anton Ebener for revealing the history of C3. The research leading to the results described in this paper is part of the European Metrology Research Programme (EMRP), which is jointly funded by the EMRP participating countries within EURAMET and the European Union.