ABSTRACT
There are a number of key structures that can be used to reveal the formation and modification history of organic matter in the cosmos. For instance, the susceptibility of organic matter to heat is well documented and the relative thermal stabilities of different isomers can be used as cosmothermometers. Yet despite being an important variable, no previously recognized organic marker of pressure exists. The absence of a pressure marker is unfortunate considering our ability to effectively recognize extraterrestrial organic structures both remotely and in the laboratory. There are a wide variety of pressures in cosmic settings that could potentially be reflected by organic structures. Therefore, to develop an organic cosmic pressure marker, we have used state-of-the-art diamond anvil cell (DAC) and synchrotron-source Fourier transform infrared (FTIR) spectroscopy to reveal the effects of pressure on the substitution patterns for representatives of the commonly encountered methyl substituted naphthalenes, specifically the dimethylnaphthalenes. Interestingly, although temperature and pressure effects are concordant for many isomers, pressure appears to have the opposite effect to heat on the final molecular architecture of the 1,5-dimethylnaphthalene isomer. Our data suggest the possibility of the first pressure parameter or "cosmo-barometer" (1,5-dimethylnaphthalene/total dimethylnaphthalenes) that can distinguish pressure from thermal effects. Information can be obtained from the new pressure marker either remotely by instrumentation on landers or rovers or directly by laboratory measurement, and its use has relevance for all cases where organic matter, temperature, and pressure interplay in the cosmos.
Export citation and abstract BibTeX RIS
1. INTRODUCTION
The cosmos is replete with carbonaceous material. The majority of identified interstellar and circumstellar molecules contain carbon (Henning & Salama 1998), while asteroids and their meteoritic fragments (Sephton 2002) and comets (Sandford 2008) can all contain percentage levels of organic matter. Potential carbon-based records exist, therefore, of the journey from interstellar and circumstellar environments through the protoplanetary disk to planetesimals (Allamandola et al. 1987; Ehrenfreund & Charnley 2000). Direct reading of these records is possible by the chemical analysis of meteorites (Sephton 2002) and comets (Sandford 2008) in the laboratory while indirect reading of these chronicles is possible through remote spectroscopic observation (Sandford 1996).
A tool with great potential for use in astrophysics and astrochemistry is the use of environmentally sensitive aromatic hydrocarbon isomers. It is well known that patterns in the isomer distribution of aromatic hydrocarbons may act as fingerprints of formation environments. Cosmic environments differ widely in their temperatures and pressures (Asphaug et al. 2006; Pierazzo & Chyba 1999; Remington et al. 1997) and markers diagnostic to variations in these two variables are valuable additions to science.
The relative responses of aromatic hydrocarbon isomers to temperature is well documented and commonly used for terrestrial studies (Radke et al. 1982, 1986; Alexander et al. 1984, 1985). Substituted aromatic hydrocarbons can be present in a number of isomeric architectures that represent varying levels of thermal stability (Madison & Roberts 1958). The ratios of thermodynamically favorable to thermodynamically unfavorable forms can be used as maturity indicators (Radke et al. 1986). The less stable isomers are selectively destroyed or their substituents migrated to more stable molecular sites. The maturity parameter concept, therefore, simply makes a ratio of isomers with different thermodynamic stabilities.
Heat is not the only modifier of organic matter in the cosmos. Pressure is also a major agent of change and the variation in pressure in space is extreme. Interstellar environments are close to a vacuum, while the interiors of stars experience extremely high pressures (Burrows & Liebert 1993). More transient phenomena are also associated with high pressures and examples include supernovae that reach maximum pressures of 103–106 GPa (Burrows & Liebert 1993; Remington, et al. 1997) and the near misses or actual impacts of planetesimals and planets that can generate pressures of 3.5 × 103 GPa to 5 × 104 GPa, with the frequency of such instances being dramatically enhanced during periods such as the Late Heavy Bombardment (Asphaug et al. 2006; Melosh 1989; Pierazzo & Chyba 1999; Pierazzo & Melosh 2000).
Despite the clear need for organic indicators of pressure, the effects have not been previously studied in isolation. The neglect of pressure in organic transformation presumably derives from the practical analytical difficulty of isolating its consequences from those of heat. Small aromatic compounds are present in a variety of accessible extraterrestrial materials including interplanetary dust particles, meteorites, and, most recently, samples returned via space missions. The recognition of aromatic hydrocarbon isomers with distinct responses to temperature and pressure would have great scientific value but, to date, such different effects are completely unexplored.
Our objective was to examine a number of methyl substituted naphthalenes, which are known to respond to thermal processing, to determine if the effects of pressure were concordant or discordant with those of temperature. Methyl substituted naphthalenes are ideal targets in the search for indicators of cosmic conditions because this type of organic structure has been implicated as the cause of some unidentified infrared bands (Shan et al. 1991), are present in meteorites (Sephton 2012), and are found in comets (Sandford 2008).
Recently, specific dimethlynaphthalene isomers have been detected as constituents of experimentally produced hydrogenated amorphous carbon and compared to interstellar absorption features in carbonaceous dust in the diffuse interstellar medium and in circumstellar environments (Duley & Hu 2012). Moreover, the dimethylnaphthalenes are relatively easy to analyze by chromatographic and spectroscopic techniques and make ideal representatives of substituted aromatic hydrocarbons for a proof of concept study.
Although the responses of dimethylnaphthalenes to heat have been recognized from nature and in heating experiments in the laboratory (Radke, et al. 1982, 1986; Alexander, et al. 1984, 1985), no data exist on the effects of pressure on dimethylnaphthalenes or any other methyl substituted aromatic compound. Here we have used diamond anvil cell and spectroscopic data from a synchrotron to study the effect pressure has on dimethylnaphthalene isomers. This work has revealed the very important result that the effects of pressure are distinct from those of temperature. This new information provides one tool for recognizing and eventually discriminating between the temperature and pressure histories of organic structures. Our findings also suggest the potential presence of numerous similar environmentally specific indicators within extraterrestrial organic matter that can be used to reconstruct the imprints on organic chemical mixtures from their often lengthy journey through the cosmos.
2. EXPERIMENTAL METHODS
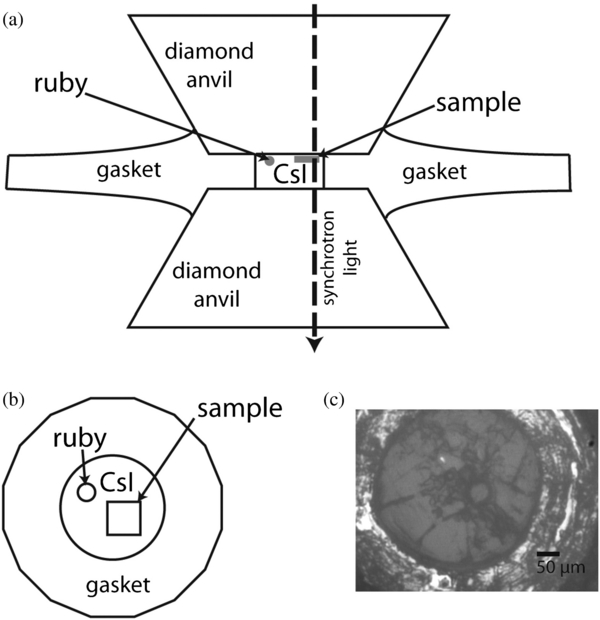
For our experiments, the samples were loaded into a membrane-type diamond anvil cell. The cell contained type II diamonds, 500 μm culets, and a stainless steel gasket. The sample chamber was 200 μm in diameter and had a pre-indented thickness of 30 μm. The sample chamber was filled with a cesium iodide (CsI) window, which is transparent over the wavelength range of interest over the pressures used, and acts as a pressure medium. Figure 1 shows the experimental setup. This facilitates the use of a thin sample, which avoids saturation of any absorbance spectra. The crystalline sample of roughly 100 μm × 100 μm × 15 μm size was positioned in the sample chamber along with a smaller ruby sphere (50 μm diameter) used for pressure measurement. For 1,7-dimethylnaphthalene, which is liquid at room temperature, a CsI window was created, broken, and the liquid loaded into the pore spaces formed.
Figure 1. (a) Schematic of high-pressure synchrotron-source FTIR spectroscopy measurements parallel to the synchrotron beam. Not to scale. (b) Schematic of high-pressure synchrotron-source FTIR spectroscopy measurements in the plane of the sample. Not to scale. (c) Microphotograph of 1,7-dimethylnaphthalene loaded into the diamond anvil cell. The ruby is the sphere toward the center of the sample chamber.
Download figure:
Standard image High-resolution imageFTIR-micro-spectrometry in transmission was performed using synchrotron light at the Swiss Light Source operated in top-up mode as previously documented (Jennings et al. 2010). Additional measurements were carried out in the pressure range 10–20 GPa at the SMIS beamline at SOLEIL (France). Pressure was measured directly by measuring the shift of the R1 pressure-dependent florescence line of ruby as documented above (Mao et al. 1986; Jennings et al. 2010). All samples were measured at increasing and decreasing pressures over a range of 0.5–21 GPa.
Spectra were analyzed by first removing the background and then fitting peaks to a standard Gaussian using Fityk (Wojdyr 2010). Background spectra were taken through an "empty" region of the DAC gasket hole, i.e., CsI window material only, except in the case of 1,7-dimethylnaphthalenes, where backgrounds from similar pressures taken during experimental runs on other solid samples were used.
Although the vibrational assignments of the dimethylnaphthalene family of molecules in the literature are not complete, the out-of-plane C–H vibrations have long been used to distinguish isomers (Constantine & Topsom 1968; Friedman 1966; Hawkins et al. 1957). These peaks fall in the wavelength range of 9.5–14 μm, which is ideally suited to high-pressure work, as they correspond to a transmission window in the diamond spectrum yet are comfortably within the range of the detector.
3. RESULTS AND DISCUSSION
Dimethylnaphthalenes may display substitutions at the thermodynamically stable β positions or the sterically hindered and therefore thermodynamically unstable α positions (Figure 2). The preference of particular substitution positions under progressive thermal stress is relatively well understood (Madison & Roberts 1958). The presence of α-carbon atoms lead to greater thermal reactivity and the ratio of 2,6-(β,β) dimethylnaphthalene and 2,7-(β,β) dimethylnaphthalene to 1,5-(α,α) dimethylnaphthalene is a commonly used parameter to assess the thermal maturity of terrestrial organic matter (Radke et al. 1982).
Figure 2. Various substitution positions on the polycyclic aromatic hydrocarbon naphthalene.
Download figure:
Standard image High-resolution imageThe effects of pressure on substitution position are less well known. Current knowledge of pressure effects on organic transformations implies that pressure significantly retards organic matter metamorphism and the achievement of thermodynamically stable substitution patterns (Price & Wenger 1992). The effects of pressure alone are unexplored and a method is required to recreate high-pressure conditions and monitor the changes induced in molecular architecture. Synchrotron-source FTIR spectroscopy and DAC techniques provide the opportunity to monitor pressure-induced transformations decoupled from those of heat.
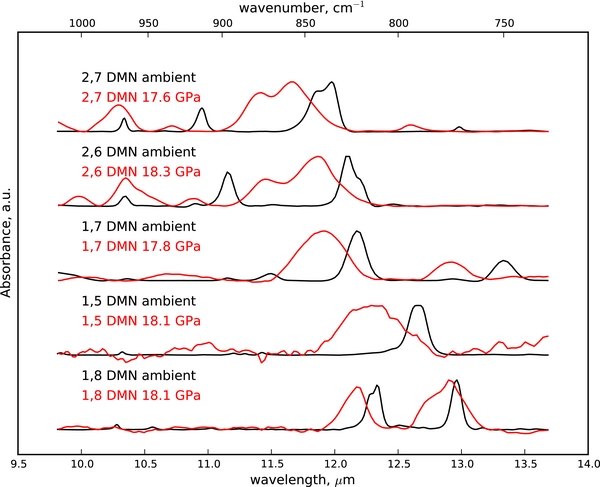
At ambient pressure different isomers produce distinct absorbance bands, allowing discrimination between molecular structures. As shown in Figure 3, this is particularly clear in the CH out-of-plane bending region (700–1000 cm−1; 10–15 μm) of the mid-IR. If the methyl substitution patterns are harmonious, their spectra have bands that are in similar positions. Conversely, differences in band positions imply distinct molecular arrangements. For example, at ambient conditions, β,β substituted 2,7-dimethylnaphthalene and 2,6-dimethylnaphthalene FTIR spectra appear similar, with small differences in peak position reflecting the single difference in methyl position. The remaining isomers studied have distinctly different spectra at ambient conditions (Figure 3).
Figure 3. Spectral data for 1,8-, 1,5-, 1,7-, 2,6-, and 2,7-dimethylnaphthalene. Initial spectra under ambient conditions are given in black and the spectra at high pressures are given in red. Note the formation of a shoulder at 12.9 μm in 1,8-dimethylnaphthalene, coincident with a peak in the high-pressure 1,7-dimethylnaphthalene spectrum. Also, the high-pressure spectra of 2,6- and 2,7-dimethylnaphthalene show similarities in this fingerprint region.
Download figure:
Standard image High-resolution imageComparing infrared responses of various dimethylnaphthalene isomers before and after DAC treatment reveals the effects of pressure. Figure 4 displays the band positions at progressively higher pressures for the various dimethylnaphthalene isomers. The movement, appearance, and disappearance of bands can be used to infer changes in molecular structure. Relatively little change is indicative of a chemical structure that is resistant to pressure.
Figure 4. Compression and decompression peak fit data for 1,8-, 1,5-, 1,7-, 2,6-, and 2,7-dimethylnaphthalene. Each data point represents the center of a peak associated with that material at a single pressure. Particular comparison can be made between the positions and rate of change of 2,6- and 2,7-dimethylnaphthalene at increasing pressures.
Download figure:
Standard image High-resolution imageAt high pressures, all bands are shifted to higher wavenumbers reflecting an increase in energy from compression of nuclei and electrons. All pressure-induced transformations were found to be reversible on decompression with only slight hysteresis. Because of the nature of synchrotron-DAC work, it was not possible to rule out kinetic factors in this reversal through longer timescale experiments (Ceppatelli et al. 2000), but the data has utility as a probe of possible high-pressure polymorphs. In particular, if the dimethylnaphthalene isomers were allowed to relax at high pressure for a long period, as would be the case in a natural system, the transformations observed under short timescales may be irreversible.
Inspection of Figure 4 shows the most striking result is that, in all cases, the dominant spectroscopic features are blue shifted by some .5 to .75 μm (10 to 20 cm−1). Apart from this, the individual molecules show the following: the 2,7- and 2,6-dimethylnaphthalene also show blue shifts of the bands near 11.3 μm (which is associated with the solo CH band), but little or no shift of the band near 10.4 μm. Convergence of 2,6- and 2,7-dimethylene on a common structure owing to different rates of frequency shift with increasing pressure (Figure 4) and the appearance of new peaks at 12.5 and between 10.8–11.1 μm in 2,6-dimethylnaphthalene (Figure 5) reflects the attainment of a similar stable molecular arrangement at pressure.
Figure 5. Evolution of 2,6-dimethylnaphthalene FTIR spectra with increasing pressure. Individual peaks are given. Note the appearance of new peaks between 10.0–11.0 and 11.5–12.9 μm.
Download figure:
Standard image High-resolution image1,7-dimethylnaphthalene shows significant variation in FTIR response with pressure. Notably, pressure causes changes toward the high-pressure 2,6-dimethylnaphthalene and 2,7-dimethylnaphthalene spectrum. Transformation of 1,7-dimethylnaphthalene toward high-pressure 2,7-dimethyl- naphthalene and 2,6-dimethylnaphthalene suggests that migration of the α-methyl substituent to the β position may occur over time. Overall, the spectrum of 1,8-dimethylnaphthalene displays the greatest change with pressure, indicating that it has a structure which is the least stable of the compounds studied here and responds most readily to pressure. With increasing pressure, 1,8-dimethylnaphthalene and 1,7-dimethylnaphthalene develop weak spectral similarities. Hence the β substitutions generally are the most stable under pressure while the α substitutions are the least stable. The data also imply that the migration of methyls from the α to the β positions is one way that the molecules could respond to long periods of time under high pressure. In contrast to the other αα-substituted dimethylnaphthalene, 1,8-dimethylnaphthalene, 1,5-dimethylnaphthalene displays little change up to 18.1 GPa.
Stability in response to pressure is also likely related to the crystal structure of the studied dimethylnaphthalene isomer. Crystal structures are available for 1,8-, 1,5- (neutron diffraction), and 2,6-dimethylnapthalene (X-ray diffraction and complementary computational models) at room or low temperature and ambient pressure (Figure 6; Kaduk & Golab 1999; Wilson 1997; Wilson & Nowell 2000). 2,6-dimethylnaphthalene is known to be an extremely structurally stable arrangement owing to its high interplanar angle herringbone crystal structure. Conversely 2,6-dimethylnapthalene has little void space available for reorganization and its molecular architecture remains relatively unchanged. The changes in the 2,6- and 2,7-dimethylnaphthalene spectra likely stem from the ability of the high-angle herringbone structure to further collapse (Fabbiani et al. 2006), which is reinforced by the potential intramolecular bonds formed between methyls of neighboring molecules.
Figure 6. Crystallographic structure visualization for 2,6-, 1,5-, and 1,8-dimethylnaphthalene at ambient pressure and room/low temperature (details given in Table 1). The difference in interplanar angle likely determines the effect of pressure on these molecules. Left-side structures are viewed looking down the a axis, while right-side structures photos are viewed down the c axis.
Download figure:
Standard image High-resolution imageCrystal structures indicate that 1,8-dimethylnaphthalene has the greatest unit cell volume per molecule and thus the greatest void space available for reorganization. Consequently 1,8-dimethylnaphthalene exhibits significant change with pressure. Although not the most thermodynamically stable of the dimethylnaphthalene isomers at ambient conditions, 1,5-dimethylnaphthalene is very stable at high pressure, complementing observations of crystalline stability approaching the melting point at ambient pressure (Wilson 1997), which is related to its low interplanar angle herringbone structure. The methyl groups of any one 1,5-dimethylnaphthalene sit at nearly a 90° angle to the central rings of neighboring molecules, giving no opportunity for intramolecular bonding. The behavior of 1,5-dimethylnaphthalenes under pressure may reflect their inability to rearrange through migration of methyl groups or reduction in void space and maximization of packing efficiency.
Overall the responses of dimethylnaphthalenes to pressure conform to those observed in response to heat. The β-substituted forms are thermodynamically and barometrically stable relative to the sterically hindered α positions. Yet the response of 1,5 (α,α)-dimethylnaphthalene to pressure is contrary to those changes brought about by heat and reveals that although the molecular consequences of heat and pressure are often the same the two mechanisms are very distinct and can generate opposing effects.
Owing to the use of dimethylnaphthalenes as indicators of the thermal processing of organic matter, the recognition of pressure effects that may be distinct from those observed for heat has important implications. The observed responses offer the opportunity to use the pressure stability but thermal instability of 1,5-dimethylnaphthalene as an indicator of organic synthesis or modification. The ratio of 1,5-dimethylnaphthalene/total dimethylnaphthalenes would be expected to remain constant with pressure but decrease with temperature. The robust nature of aromatic hydrocarbons suggests that this new approach of using pressure-induced effects versus thermal processing could have wide applicability in understanding the history of extraterrestrial organic matter.
4. CONCLUSIONS
Methyl substituted naphthalenes are common constituents of extraterrestrial organic matter. State-of-the-art synchrotron data reveal that dimethylnaphthalane isomers can have distinct responses to pressure. 1,5-dimethylnaphthalene is relatively stable under pressure despite being relatively unstable when heated. The opposing effects of heat and pressure on the molecular stability of dimethylnaphthalane isomers reveals that they are efficient environmental recorders that can discriminate between modifying agents commonly associated with numerous processes in the cosmos. The record contained within dimethylnaphthalenes can be read either remotely by instrumentation on landers or rovers or directly by laboratory measurements. The recognition of this first organic cosmo-barometer suggests that more may exist within the isomeric diversity displayed by extraterrestrial organic matter and a new line of investigation for cosmochemists beckons.
Table 1. Summary of Available Crystallographic Data for Dimethylnaphthalenes (DMN)
| 1,8-DMN | 1,5-DMN | 1,7-DMNa | 2,6-DMN | 2,7-DMN | |
|---|---|---|---|---|---|
| Symmetry | P21/n | P21/c | Liquid | Pbca | Not available |
| Temperature (K) | 200 | 300 | ... | 300 | ... |
| Unit Cell Volume/Molecule | 274.57 Å3 | 227.28 Å3 | ... | 227.79 Å3 | ... |
Download table as: ASCIITypeset image
These high-pressure FTIR measurements were performed on the X01DC beamline at the Swiss Light Source, Paul Scherrer Institut, Villigen, Switzerland, with the assistance of Dr. Ph. Lerche. We also acknowledge SOLEIL for provision of synchrotron radiation facilities (Proposal ID "20120970") for follow-on measurements and we thank Dr. P. Dumas for assistance in using beamline SMIS and Dr. J.-P. Itie for access to the high-pressure facilities at beamline PSICHÉ. The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement No. 312284.