Abstract
The perspectives offered by vertical arrays of nanowires for biosensing applications in living cells depend on the access of individual nanowires to the cell interior. Recent results on electrical access and molecular delivery suggest that direct access is not always obtained. Here, we present a generic approach to directly visualize the membrane conformation of living cells interfaced with nanowire arrays, with single nanowire resolution. The method combines confocal z-stack imaging with an optimized cell membrane labelling strategy which was applied to HEK293 cells interfaced with 2–11 μm long and 3–7 μm spaced nanowires with various surface coatings (bare, aminosilane-coated or polyethyleneimine-coated indium arsenide). We demonstrate that, for all commonly used nanowire lengths, spacings and surface coatings, nanowires generally remain enclosed in a membrane compartment, and are thereby not in direct contact with the cell interior.
Export citation and abstract BibTeX RIS
1. Introduction
The use of arrays of vertical nanowires (NWs) for biological applications, such as electrical measurements and compound delivery, is an attractive field of research with numerous perspectives for cellular investigations [1–3]. For these purposes, arrays of NWs are currently being fabricated using different materials, and with a broad range of geometrical properties (lengths, diameters, spacing between NWs), and surface treatments, e.g. aminosilane or polyethyleneimine (PEI) [1, 4–12].
To validate their intended use with cells, it is crucial to better understand how the NW substrate is interfaced with cells, and ensure that the NWs do not compromise cell health or function. The basic health of cells on NW arrays, including indium arsenide (InAs) NWs used in this study, has so far been evaluated in terms of cell morphology, adhesion and viability. It has been demonstrated that cells spontaneously adhere to numerous types of NW array, where they can grow for up to several weeks, with maintained enzyme activity and membrane integrity [4–7, 9–12]. Furthermore, cellular functions, such as expression of DNA encoding cytosolic protein, intact expression and trafficking of membrane protein, and intact neuronal maturation, have been demonstrated in several cases [4–7, 13].
The interface between cells and NWs has mainly been studied by scanning electron microscopy (SEM), since a high-resolution technique is preferred to image NWs [4–8, 10, 12, 13]. SEM is however limited to visualization of the cell surface of fixed and dried cells, and therefore does not provide information on the intracellular interface. Instead, fluorescence microscopy has been useful for investigating both the exterior and interior of living cells interfaced with NWs, with limited artefacts from sample preparation [4, 6, 8, 10, 13]. Investigations using either technique have revealed a close interface between various types of NW and cell.
The initial observations of a close interface have led many to the assumption that NWs spontaneously penetrate the cell membrane and gain direct access to the cell interior, a feature that is critical to several cellular applications [5, 6, 8]. This hypothesis of spontaneous penetration is supported by the only direct study of cell membrane conformation published so far [8]. However, several publications using NW arrays for molecular delivery and electrical measurements have indirectly suggested the presence of a barrier at the cell–NW interface [6, 7, 10–12]. The cell–NW interface thus remains unclear and requires further investigation with improved methods.
In this communication, we address this question by combining detailed confocal fluorescence imaging with a specific fluorescence labelling strategy for direct visualization of the cell membrane conformation at the NW interface. We take advantage of the regular and controlled topography of InAs NW arrays for systematic imaging of individual NWs interfaced with living HEK293 cells [4]. This novel generic method is used to demonstrate that bare and surface treated NWs, irrespective of their length and spacing, are enclosed in a membrane compartment within the cell, thus confirming the indirect observations made by the community.
2. Materials and methods
All materials were purchased from Sigma-Aldrich unless otherwise stated.
2.1. InAs NW array fabrication
2, 6 and 11 μm long NWs were grown by an Au-assisted vapour–liquid–solid growth mechanism using molecular beam epitaxy (MBE) as previously described [4]. The Au droplets were positioned using electron beam lithography [14] in order to manufacture square lattices with consistent spacing of 3, 4 and 7 μm between NWs for systematic investigations with cells. InAs(111) B substrates were spin coated with a double layer resist consisting of 6% copolymer and 2% polymethyl methacrylate (PMMA), at 4000 rpm. Two systems were used for the exposure, JEOL-JBX9300FS and Raith e-line, resulting in similar and reproducible results for positioned NWs. A layer of 12 nm Au was deposited on the sample by electron beam evaporation, and lift-off was carried out in hot acetone. Right before loading into the MBE system, the sample was plasma etched for 40 s and dipped in 5% hydrofluoric acid for 10 s. NWs were grown at 425 °C with an As2 and In beam equivalent pressure of 1.24 × 10−5 and 3.7 × 10−7 Torr, respectively. For a given set of growth parameters, the NW length is dependent on the growth time, typically in the range 20–60 min, and NW diameter is dependent on the amount of Au in the positioned droplets. Each growth was characterized for mean NW diameter and length using SEM (JEOL 6320F).
2.2. NW array surface chemistry
Three types of NW surface property were used in this study: bare, aminosilane-coated or polyethyleneimine- (PEI-) coated InAs. PEI-coated NWs were functionalized by immersion in 10% PEI for 30 min, and thereafter rinsed in distilled water [6]. For vapour-phase silanization [15], the arrays were rinsed in acetone, isopropanol and 70% ethanol, before immersion in the latter for 30 min on a rotating table. Thereafter, the substrates were rinsed twice in acetone, methanol, and isopropanol, and dried with N2, before they were plasma etched for 1 min, incubated for 1 h in a vacuum chamber with N2 atmosphere with N,N-diisopropylethylamine and 3-aminopropyltriethoxysilane, and finally heated to 115 °C and placed in vacuum overnight. Successful silanization was verified by contact angle measurement on planar InAs [16].
2.3. HEK293 cell culture
Cultures of HEK293 cells (Sigma) were maintained at 37 °C, 5% CO2 and more than 95% humidity. HEK293 cells were cultured in Dulbecco's modified Eagle medium (DMEM/F-12+Glutamax-l, Gibco Invitrogen) with 10% foetal bovine serum (FBS). A stable cell line of HEK293 expressing the kappa-opioid receptor fused to a SNAP-tag (SNAP-KOR) was maintained in medium supplemented with 0.4 mg ml−1 Geneticin.
2.4. Interfacing cells and NW arrays
Before being interfaced with cells, the InAs NW arrays were sterilized in 96% ethanol, washed twice in Milli-Q (MQ) water and equilibrated in DMEM with 10% FBS for a few minutes. HEK293 cells were interfaced with sterilized NW substrates through drop-wise addition of suspended cells, and incubated for two days at 37 °C, 5% CO2 and humidity greater than 95% [4].
2.5. Scanning electron microscopy on cell samples
Cells were cultured on InAs NW arrays for two days, washed three times in phosphate buffered saline (PBS) (Gibco Invitrogen) and incubated in 2% glutaraldehyde in PBS overnight. The sample was critical point dried and sputter coated with 5 nm Au. Imaging was done on a JEOL JSM-6320F SEM, using 30 kV acceleration voltage.
2.6. HEK293 transfection
HEK293 cells were transiently transfected 16–20 h after being interfaced with NW arrays, with 2.5 μg ml−1 plasmid DNA encoding SNAP-KOR (CisBio) using standard calcium phosphate co-precipitation. Four hours after transfection the precipitate was removed by incubation in a prewarmed calcium chelator, ethylene glycol tetraacetic acid (EGTA), for 30 s. EGTA was subsequently replaced with prewarmed DMEM.
2.7. Fluorescence labelling and confocal imaging of membrane conformation
At 48 h after plating, cell cytoplasms of HEK293 on NW arrays were labelled by incubating 30 min at 37 °C in 3 μM calcein acetoxymethyl (AM) (Invitrogen) in DMEM without FBS. Subsequently, the membrane protein SNAP-KOR expressed by the cells was labelled with 5 μM cell-impermeable SNAP-surface 549 (New England BioLabs) for 10 min at less than 20 °C. Fluorescence imaging was performed immediately after labelling on an inverted confocal laser scanning microscope (Leica TCS SP5) using a 63 × magnification, water-immersion objective with numerical aperture 1.2. The fluorophores were excited and emission collected by a photomultiplier tube at ex543:em590–700 (SNAP-surface 549) and ex488:em508–540 (calcein), and reflection was collected at ex633:refl623–643. Complying with the Nyquist rate for optimal imaging conditions based on the SNAP-surface 549 fluorophore, z-stacks were collected with a pinhole of one Airy unit, and a pixel and step size of approximately 56 × 56 nm2 and 200 nm, respectively. Acquisition with different laser lines was done in sequential scans to avoid crosstalk. The scans were re-combined into overlay images using ImageJ software. The interfacing of HEK293 cells with bare, PEI-coated and silanized InAs NWs was repeated at least four times for each. In total 542 NWs in 174 cells were analysed.
3. Results
3.1. Cell culture on ordered InAs NW arrays with defined lengths, diameters and spacings
Since the interface of cells with NW arrays might be affected by the topography of the surface, the study was performed on regular InAs NWs to investigate whether NW geometry affects their ability to penetrate the cell membrane. The precise control of InAs NW growth was exploited to fabricate highly ordered arrays of NWs with defined diameters, lengths and spacings between NWs (figure 1(A)). Square patterned arrays with an NW spacing of 3, 4 and 7 μm were fabricated with a precision of around 10 nm. NWs were grown to lengths of 2, 6 and 11 ± 0.7 μm with diameters of 97, 110 and 91 ± 9 nm respectively ( ± std, N = 59) (figure 1(B)). The small standard deviations demonstrate the homogeneity of the patterning and growth process.
In all experiments, HEK293 cells were gently interfaced with NW substrates through drop-wise addition of suspended cells, a common interfacing method in the community, and allowed to settle down for 48 h prior to analysis [4]. SEM images of fixed and critical point dried HEK293 cells on the NW arrays confirm that the cells spread and form a close interface with the ordered InAs NWs (figure 1(C)). This observation is similar to previous studies on various cell types, NW materials and dimensions [4–7]. The NWs appear to be penetrating the cells, protruding several micrometres from the upper side of cells and creating tent-like deformations on the cell surface (figure 1(D)). At higher magnification, the protruding NWs are often clearly coated (figure 1(E)). The SEM images do however not reveal the nature of the coating and offer no information on the intracellular NW interface.
Figure 1. Cells form a close interface with regular arrays of InAs nanowires (NWs). (A) 11 μm long NWs positioned in a 7 μm square lattice using e-beam lithography, imaged by scanning electron microscopy. Zoom of an individual NW. (B) Histograms showing the distribution of NW length and diameter from n = 59 observations, with Gaussian fit (red line). (C)–(E) HEK293 cells spread out and form a close interface with the NWs. NWs are protruding from the distal side of the cells, and the cell surface is often deformed into tent-like structures around the NWs (D, E). The NWs are often clearly coated (E). Tilting angle: 20° (A), 17° (C)–(E). Scale bars: 10 μm, zoom 500 nm (A), 10 μm (C), 5 μm (D), 1 μm (E).
Download figure:
Standard image3.2. Fluorescence labelling and imaging strategy reveal cell membrane conformation at NW interface
A fluorescence labelling and imaging strategy was optimized for studying the cell membrane conformation around individual NWs and thereby investigating if the NWs gain direct access to the interior of living cells at the single NW level. The membrane was visualized by labelling of a transmembrane protein (a G protein coupled receptor) known to span both leaflets of the lipid bilayer (figure 2(A)(i)). The membrane protein was genetically fused to an extracellular tag (SNAP-tag), which can be labelled specifically at any time point by covalent binding of fluorescent substrate [17]. To investigate if NWs gained stable intracellular access, the SNAP-tagged membrane protein was fluorescently labelled with a membrane impermeable substrate two days after plating the cells on the NW array (figure 2(A)(i)). This specific labelling of transmembrane protein with membrane impermeable fluorophore ensured that only the outer lipid bilayer was labelled, and internal membranes and membrane fragments (figure 2(A)(ii)) remained unlabelled. The absence (figure 2(A)(ii)) or presence (figure 2(A)(iii)) of membrane around individual NWs was then examined using confocal laser scanning microscopy for 3D-like scans of living cells. Cell cytosols were labelled with a fluorophore (calcein) that became fluorescent after modification by intracellular enzymes, only active in living cells [4].
In agreement with our previous results on non-ordered arrays of NWs [4], cells interfaced with the ordered InAs NW arrays remain viable and successfully express the transmembrane protein (figure 2(B)(i)). The regularity of the NW arrays facilitates a systematic analysis of each row of NWs by vertical cross sections and line profiles (figure 2(B)(ii)–(vi)). Individual NWs were directly visualized by laser reflection from their tips (figure 2(B)(iii)) or indirectly as a dark region from which the NW excluded the fluorescent cytosol (figure 2(B)(iv)). In this study, all the NWs within the cell are associated with a signal from the membrane (figure 2(B)(vi)) that appears to cover the full length of each NW (figure 2(B)(v)), indicating that the membrane signal observed at the NWs is an intact continuous lipid bilayer accessible to the extracellular medium. The specificity of the membrane labelling is confirmed by the negligible fluorescence in the cytoplasm and organelles (figure 2(B)(i), (v)), and absence of fluorescence of NWs outside cells (figure 2(B)(ii)–(v), asterisks; 2(B)(vi), black dashed arrows). Hence the outer cell membrane is not penetrated by the NWs, but rather appears to have adapted its conformation to enclose the individual NWs.
Figure 2. Fluorescence labelling and imaging strategy (A) reveals cell membrane conformation at NW interface (B). (A) A transmembrane protein is expressed with an extracellular SNAP-tag ((A)(i), 1). The SNAP-tag is labelled with a fluorescent SNAP-label (red star). The SNAP-label is membrane impermeable ((A)(i), 2) and only SNAP-tagged proteins in the outer lipid bilayer ((A)(i), 3) and no intracellular membranes or membrane fragments ((A)(ii)) are labelled. The method is used to determine the absence ((A)(ii)) or presence ((A)(iii)) of membrane at the cell–NW interface. (B) Fluorescence confocal imaging of a living cell interfaced with an InAs NW array. Horizontal ((B)(i)) and vertical ((B)(ii)–(v)) cross sections of a cell interfaced with arrays of 6 μm long and 4 μm spaced NWs (arrowheads in (i) mark rows of NWs). Cells show maintained viability (calcein, green) and successful expression of the SNAP-membrane protein (red) ((B)(i)). NWs were directly visualized by reflection from their tips ((B)(iii)) or indirectly as a dark region from which the NW excluded the fluorescent cytosol ((B)(i), (iv)). (B)(vi) In a line scan along the dotted line in (B)(v), it is seen that membrane is present at the cell boundaries (grey dashed line) and also at the position of each NW (red dashed arrows). The cell membrane appears to cover the full length of each NW ((B)(v)). The membrane label shows no non-specific binding to NWs outside cells ((B)(ii)–(v), asterisks; (B)(vi), black dashed arrows). Scale bar: 10 μm.
Download figure:
Standard image3.3. Effect of NW length and spacing on cell membrane conformation
To challenge the applicability of the presented method, and to investigate whether NW geometries affect their ability to penetrate cell membranes, cells were interfaced for two days with InAs NW arrays with a range of defined NW lengths and spacings (figure 3). On all lengths and spacings tested here, the cells remain viable and successfully express the transmembrane protein. Cells were interfaced with 11 μm long NWs, a length comparable to cell diameters (figure 3(A)). Even though the long NWs are protruding several μm on the distal side of the cells, they are enclosed in membrane along the entire length of each NW (figure 3(A)(ii)–(v)). This is consistent with the observation made by SEM (figure 1(E)). The higher fluorescence intensity on the section of NWs protruding out of the cell indicates the presence of two bilayers: the basal and the top cell membrane (figure 3(A)(iv)). We also observed NWs enclosed in cell membrane on arrays with a smaller spacing between NWs (3 μm), i.e. more NWs per cell (figure 3(B)), and on arrays with shorter NWs (2 μm) (figure 3(C)). Despite the limit of vertical resolution of our optical setup, all the short 2 μm NWs we could analyse (146 of 166 NWs, 88%), were enclosed in membrane.
These observations show that our method is applicable to NW arrays of different NW lengths and spacings, and that the presence of a cell membrane at the NW interface is generic for a broad range of NW array geometries.
Figure 3. Effect of NW length and spacing on cell membrane conformation. Confocal fluorescence images of cells interfaced with (A) 11 μm long and 7 μm spaced NWs, (B) 11 μm long and 3 μm spaced NWs, and (C) 2 μm long and 7 μm spaced NWs. In all cases, the fluorescence labelling reveals that the cells remain viable (calcein, green) and successfully express SNAP-membrane protein (red) ((i), arrowheads mark rows of NWs). (ABC) Vertical cross sections ((ii)–(iv)) and line profiles (v) show that all NWs are associated with cell membrane. The cell membrane appears to cover the full length of each NW (iv). Grey dashed lines in (v) mark cell boundaries. Scale bars: 10 μm.
Download figure:
Standard image3.4. Effect of NW surface chemistry on cell membrane conformation
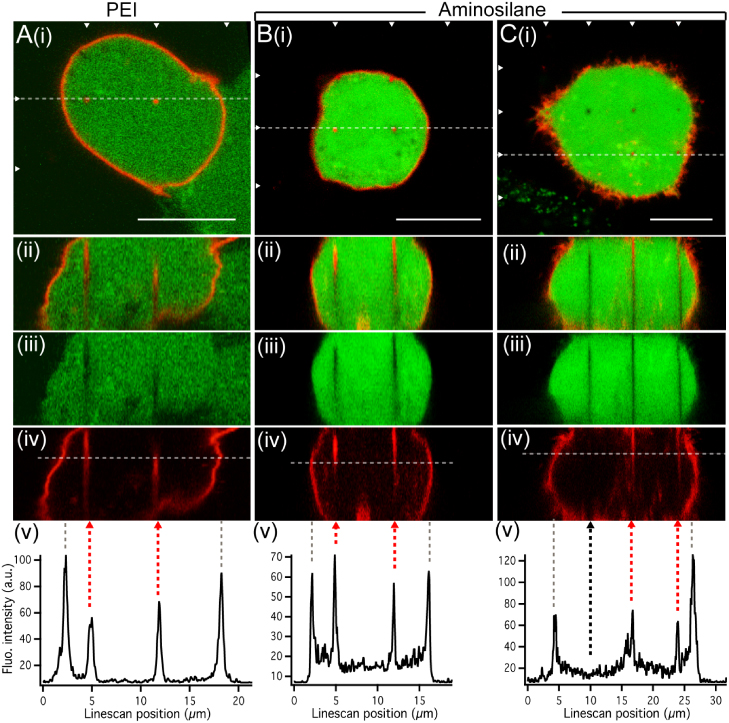
As the next step, the NW surface chemistry was modified to investigate if two of the NW surface modifications commonly used [6, 8] would affect the presence of membrane along the NWs (figure 4). NWs were coated with the cationic polymer polyethyleneimine (PEI), previously used by others on arrays of NWs [6], to optimize their uptake in cells, via interaction with the negatively charged outer leaflet of cell membranes [18, 19]. Cells interfaced with 11 μm long PEI-coated NWs (figure 4(A)) had a maintained viability and successful expression of SNAP-membrane protein (figure 4(A)(i)). Like the bare InAs NWs, the PEI-coated NWs were all enclosed in cell membrane (figure 4(A)(ii)–(v)). The same observation was made on shorter PEI-coated NWs (2 μm length, figure S1(A) available at stacks.iop.org/Nano/23/415102/mmedia).
NWs were also modified with aminosilane [8] to provide a slight positive charge at pH 7.4, and increase the hydrophobicity of the surface. Cells interfaced with 11 μm long aminosilane-coated NWs have maintained viability and successfully express the SNAP-membrane protein (figures 4(B) and (C)(i)). The aminosilanized NWs were almost all enclosed in cell membrane (figure 4(B)(ii)–(v)). However, a few were not associated with a membrane signal (4 of 54 NWs, 7%), suggesting a direct access to the cell interior in that case (figure 4(C)(iii)–(v), black arrow). Shorter aminosilanized NWs of 2 μm length were generally observed to be associated with cell membrane (figure S1(B) available at stacks.iop.org/Nano/23/415102/mmedia).
The results show that coating NWs with cationic PEI does not affect the presence of a membrane at the cell–NW interface, whereas aminosilane coating could improve the probability of having a stable intracellular access.
Figure 4. Effect of NW surface chemistry on cell membrane conformation. HEK293 cells interfaced with arrays of 11 μm long and 7 μm spaced PEI- (A) or aminosilane-coated ((B), (C)) InAs NWs show maintained viability (calcein, green) and expression of the SNAP-membrane protein (red) ((i), arrowheads mark rows of NWs). (A)–(C) Vertical cross sections ((ii)–(iv)) and line profiles (v) show that in most of the cases ((A), (B)), each NW is associated with cell membrane that appears to cover the full length of each NW. A few of the aminosilane-coated NWs were not associated with a membrane signal ((C)(ii)–(iv) and (v), black dashed arrow). Grey dashed lines in (v) mark cell boundaries. Scale bars: 10 μm.
Download figure:
Standard image4. Discussion
For many biological applications of NWs, a direct contact between the NW and the cell interior is crucial. This is for example the case for delivery of non-permeable compounds and for intracellular fluorescence-based biosensing [3, 7, 8, 10–12]. Recent results on electrical access and molecular delivery show that NWs do not always gain direct intracellular access, suggesting a barrier at the cell–NW interface [10–12]. Here we present a generic bioimaging method to determine the presence of a lipid bilayer at the cell–nanomaterial interface, applicable to different cell types, nanomaterials and surface chemistries. The approach is based on fluorescence labelling specific to intact outer cell membrane, and confocal 3D-like imaging allowing analysis of individual NWs. Even though this study takes advantage of highly regular arrays of vertical NWs for a systematic investigation, the method is also applicable to non-vertical nanostructures in non-ordered arrays, as well as single nanostructures. For example, the method has recently been applied to investigate the interface of various cell types with a novel type of non-ordered CuO NWs [22].
Our present study is to our knowledge the first direct study of the cell membrane conformation at the single NW level. Another approach to detect cell penetration by NW arrays, proposed by Shalek et al [8], is based on the detection of the settling of cells down to the NW array surface. Even though the results at the level of single NWs presented here cannot determine if there was a transient penetration at the initial cell contact, they show that cell settling is not a sufficient criterion to evaluate the interface and that cells can settle on the NW array surface without necessarily causing a stable penetration of the cell membrane.
The presented method was applied to NWs with surface chemistries and dimensions representative of previously published studies: with intermediate NW diameter, and spacing and lengths ranging from the standard of a few micrometres to the more extreme. A total of 542 NWs in 174 cells were analysed across the various geometries and coatings, out of which 513 NW (95%) were enclosed in cell membrane. Four of the 11 μm long and 7 μm spaced aminosilane-coated NWs had no apparent membrane. We thus demonstrate that NWs closely interfaced with cells do not necessarily penetrate the cell membrane and access the cell interior, but rather commonly remain enclosed in an intact, continuous cell membrane compartment within the cell. Our results suggest that aminosilane coating might improve the ability of NWs to penetrate the cell membrane. For a small fraction of the NWs (5%), the interface was inconclusive, mostly due to the NWs being too short compared to the resolution in our setup. The fluorescence gradient observed in a few cases along the NWs is not due to an irregular presence of fluorophore at the membrane, but is probably caused by an optical effect of the NWs.
The results of this communication are in agreement with several publications using NW arrays for cellular applications, which have indirectly suggested the presence of a barrier at the cell–NW interface. A barrier like a lipid bilayer could for example explain the low efficiency of NW-mediated transfection of encoding strands of DNA, as seen in our hands (data not shown), as well as being demonstrated by others [6, 7]. In a recent study using hollow nanostraws for intracellular delivery of small molecules, a penetration efficiency of only 1–10% per nanostraw was estimated based on the delivery success per cell and the density of nanostraws [11]. In another study using vertical NW electrodes, an electrical barrier was observed for about half of the HEK293 cells cultured on the electrode array [10]. Short voltage pulses were able to establish a transient electrical access, which is consistent with electroporation of a lipid bilayer. All these results, including the ones presented in this communication and the recent results obtained with CuO [22], suggest that the penetration of nanostructures in cells remains a general challenge and requires improvement of the interfacing methods.
Besides changing NW geometry and surface chemistry, there are other ways of affecting the cell–NW interface. Here we use a gentle way of interfacing to preserve the cell viability, similar to the strategy used in most publications. Interestingly, the transfection efficiency of DNA covalently bound to NWs was increased when cells were forcibly interfaced with the arrays [7]. This could reflect that application of force enables direct intracellular delivery of DNA by promoting NW penetration of the cell membrane. Our generic method is applicable for investigating the effect of force and other potential cell penetrating factors by directly monitoring the cell membrane conformation at the interface with nanomaterials.
5. Conclusion
In summary, we present and use a generic bioimaging approach to determine the presence of a lipid bilayer at the interface between living cells and NWs. The method is applicable to nanostructures of diverse materials, surface chemistries and dimensions, with a minimum length of about 2 μm. We have shown that, irrespective of their length (2, 6 and 11 μm) and spacing (3, 4 and 7 μm), bare, PEI-coated and aminosilane-coated InAs NWs interfaced with cells are generally enclosed in a membrane compartment, and thereby do not spontaneously gain a stable access to the cell interior. The presence of a membrane compartment around NWs is likely to influence not only NW-based molecule delivery, but also the quality of results where NWs are used as electrodes [10, 12, 20, 21]. Therefore, to gain an understanding of the interface between cells and NWs, and how to modify it, is of great value to the continued development and validation of NWs as intracellular biosensors.
Acknowledgments
We thank Rune S Frederiksen, Peter Krogstrup, Pawel Utko and Peter Fuerst for fruitful discussions. For financial support, we thank the Lundbeck Foundation, the Danish Agency for Science Technology and Innovation (The Danish Council for Strategic Research and The Danish Natural Science Research Council), and UNIK Synthetic Biology, funded by the Danish Ministry for Science, Technology and Innovation.