Abstract
Lead (Pb)-free perovskite thin films of the ternary BaTiO3–Ba(Mg1/2Ti1/2)O3–BiFeO3 (BT–BMT–BF) solid solution system with preferential crystal orientation were fabricated and the dependence of their polarization behavior on crystal anisotropy was evaluated. The chemical solution deposition (CSD) technique was utilized for the film deposition while controlling the chemical composition. The films fabricated on single-crystal perovskite substrates of (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3 exhibited preferential (100)pc and (111)pc orientations, respectively, depending on the crystallographic feature on the substrate surface. Excess Bi addition into the precursor solution to compensate for the volatile Bi component enhanced the polarization behavior of the resulting films heat-treated at 750 °C to induce crystallization. The (111)pc-oriented BT–BMT–BF film had a larger remanent polarization (Pr) of approximately 35 µC/cm2 than the (100)pc-oriented film (Pr = approximately 20 µC/cm2).
Export citation and abstract BibTeX RIS
1. Introduction
Perovskite-type metal oxides that possess ferroelectricity are attractive materials for application to various electronic devices, such as nonvolatile memories, piezoelectric sensors/actuators, and energy harvesters. Solid solutions of lead (Pb)-based perovskite oxides [e.g., PbZrO3–PbTiO3 (PZT), Pb(Mg1/3Nb2/3)O3–PbTiO3 (PMN–PT), Pb(Zn1/3Nb2/3)O3–PbTiO3 (PZN–PT), etc.] are presently utilized for actual application to ferroelectric devices because of their enhanced spontaneous polarization and piezoelectric displacement as well as their high Curie temperature, which are strongly required for constructing high-performance devices. On the other hand, Pb-free ferroelectric materials have also been explored widely in recent research because of environmental issues related to toxic Pb components. Various candidate Pb-free materials have been proposed as alternatives to Pb-based ferroelectrics.
The ternary solid-solution system of BaTiO3–Bi(Mg1/2Ti1/2)O3–BiFeO3 (BT–BMT–BF) is one candidate Pb-free material that can exhibit excellent spontaneous polarization (Ps) and piezoelectric response.1–5) An important issue is its phase transition behavior; the crystallographic symmetry of the perovskite unit cell varies with the chemical composition. In particular, the composition of 0.3BT–0.1BMT–0.6BF is located near the phase boundary between the BF-rich rhombohedral phase and the intermediate pseudocubic phase neighboring the BT-rich tetragonal phase. Fujii and co-workers.1,2) found this phase boundary and then reported a remanent polarization (Pr) of ∼30 µC/cm2 and the small-field piezoelectric constant (d33) of 94 pC/N for sintered bulk materials, which are significantly larger than those of the BF-rich rhombohedral materials. Also, the Curie temperature (Tc) of the 0.3BT–0.1BMT–0.6BF bulk was measured to be ∼470 °C, which is significantly higher than that of Pb-free BaTiO3 (120 °C) and comparable to that of Pb-based PbTiO3 (495 °C). These results suggest that BT–BMT–BF solid solution with the phase-boundary composition could be a prospective candidate for the Pb-free ferroelectric material. Furthermore, our group has recently reported the phase transition behavior and dielectric properties of polycrystalline BT–BMT–BF thin films on a silicon wafer with various chemical compositions towards their application to high-k dielectric capacitors.6) These films exhibited a maximum dielectric permittivity of approximately 800 for a chemical composition of x/(x + z) = 0.56–0.67 in zBT–0.1BMT–xBF, near the phase boundary.
To further enhance the ferroelectric properties of the BT–BMT–BF materials, the crystal orientation should be controlled in order to align the polar axis along a certain direction. Among the many techniques for controlling the crystal orientation, the epitaxial growth of ferroelectric thin films is the most popular method, because analogous approaches have been reported for many other perovskite-type ferroelectrics, such as BF7,8) and PZT,9–11) resulting in the significant improvement of the polarization behavior. Also, epitaxial films are utilized for determining the polar-axis direction of the perovskite unit cell, by comparing the ferroelectric properties of the objective films with those for a different crystal orientation.
Here, we fabricated epitaxial thin films of ternary BT–BMT–BF solid solution on a single-crystal perovskite electrode/substrate and then evaluated their ferroelectric and dielectric properties along different crystallographic directions to confirm the essential polarization behavior of the BT–BMT–BF. A chemical solution deposition (CSD) process was utilized for the film deposition with the aim of precisely controlling the complicated chemical composition of BT–BMT–BF.
2. Experimental procedure
BT–BMT–BF thin films were fabricated using the CSD technique. The precursor solution of BT–BMT–BF utilized for coating was prepared by mixing individual solutions of BT, BMT, and BF. The BT solution was prepared using (CH3COO)2Ba and Ti(O-n-C4H9)4 as starting materials and a mixture of 2-methoxyethanol and acetic acid (with volume ratio of  ) as a solvent.12–14) The BMT solution was prepared using Bi(O-t-C5H11)3, Mg(OC2H5)2, and Ti(O-n-C4H9)4 as starting materials and 2-methoxyethanol as a solvent. The BF solution was prepared using Bi(O-t-C5H11)3 and Fe(acetylacetonate)3.15) The BT solution was prepared with a stoichiometric composition, whereas both stoichiometric and Bi-excess (+5%) solutions were prepared for BMT and BF to compensate for Bi evaporation during the crystallization process. The nominal composition of the BT–BMT–BF solution was fixed at
) as a solvent.12–14) The BMT solution was prepared using Bi(O-t-C5H11)3, Mg(OC2H5)2, and Ti(O-n-C4H9)4 as starting materials and 2-methoxyethanol as a solvent. The BF solution was prepared using Bi(O-t-C5H11)3 and Fe(acetylacetonate)3.15) The BT solution was prepared with a stoichiometric composition, whereas both stoichiometric and Bi-excess (+5%) solutions were prepared for BMT and BF to compensate for Bi evaporation during the crystallization process. The nominal composition of the BT–BMT–BF solution was fixed at  (in molar ratio), corresponding to the phase boundary where enhanced ferroelectric properties were confirmed. The concentration of the solution was approximately 0.1 mol/dm3. For the epitaxial crystal growth of the BT–BMT–BF films, two kinds of single-crystal substrates with perovskite electrodes, i.e., (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3, were utilized for the film deposition.
(in molar ratio), corresponding to the phase boundary where enhanced ferroelectric properties were confirmed. The concentration of the solution was approximately 0.1 mol/dm3. For the epitaxial crystal growth of the BT–BMT–BF films, two kinds of single-crystal substrates with perovskite electrodes, i.e., (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3, were utilized for the film deposition.
The precursor solution of BT–BMT–BF was spin-coated on the substrate with a rotation speed of 3000 rpm for 50 s to prepare the precursor film. The film was dried at 150 °C for 3 min, followed by pyrolysis at 400 °C for 3 min, on hot plates. These processes were performed eight times to deposit a precursor film with a thickness of approximately 100 nm. Then the precursor film was heat-treated at 600–750 °C for 5 min [rapid thermal annealing (RTA) with a heating rate of approximately 30 °C/s] in air to induce crystallization. All of these processes were performed three times to fabricate crystallized BT–BMT–BF films with a thickness of approximately 300 nm. After the CSD processing, circular Pt top electrodes with diameters of ∼0.2 mm were deposited on the film surface by electron-beam evaporation.
The crystalline phase and crystal orientation of the films were confirmed by X-ray diffraction (XRD) analysis. The thickness and microstructure of the films were observed by scanning electron microscopy (SEM). The dielectric properties of the films were evaluated using an Agilent 4194A impedance analyzer at an oscillation voltage of 100 mV. The ferroelectric properties were evaluated using Toyo FCE-1 and FCE-3 ferroelectric test systems with a measurement frequency of 10 kHz. The evaluation of dielectric and ferroelectric properties was performed at room temperature.
3. Results and discussion
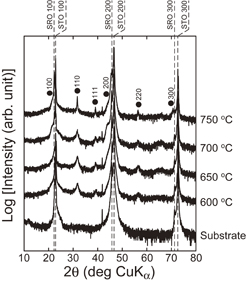
Firstly, the crystallization behavior of CSD-derived BT–BMT–BF films on the surface of a perovskite (100) plane was evaluated for the samples fabricated at various crystallization temperatures on (100)SrRuO3//(100)SrTiO3 substrates. Here, the films were derived from the precursor solution with a stoichiometric composition and no excess Bi. Figure 1 shows XRD patterns (2θ–ω scan) of the resulting films fabricated at the crystallization temperatures of 600–750 °C on (100)SrRuO3//(100)SrTiO3. Diffraction peaks derived from a single perovskite phase were confirmed for the film samples in addition to (100)SrRuO3 and (100)SrTiO3 diffraction peaks from the substrate. Presently, we have to say that the symmetry of the perovskite phase can be pseudocubic, due to the absence of peak splitting of perovskite (100)pc diffraction. The intensity of the XRD peaks from the perovskite-type crystalline phase increased with the crystallization temperature. The diffraction peaks of perovskite (100)pc were confirmed from the films preferentially, indicating the {100}-oriented crystal growth of perovskite-type films on the (100)SrRuO3//(100)SrTiO3 substrate. However, other minor peaks such as those originating from (110)pc and (111)pc planes also appeared, indicating the presence of randomly oriented domains in the resulting films. For reference, the Lotgering factor of (h00)pc planes was 0.30 for the film fabricated at 750 °C.
Fig. 1. XRD patterns (2θ–ω scan) of BT–BMT–BF films on (100)SrRuO3//(100)SrTiO3 substrates fabricated at various crystallization temperatures. The films were derived from the precursor solution with a stoichiometric composition.
Download figure:
Standard image High-resolution imageFerroelectric evaluation was performed for the BT–BMT–BF films fabricated at various crystallization temperatures. Figure 2 shows polarization–electric field (P–E) curves measured for the resulting films. For the film crystallized at 600 °C, a linear relationship between P and E values was observed in the electric-field range between ±1700 kV/cm. Irreversible P–E hysteresis curves were obtained for the films crystallized at 650 °C and above, together with the increase in Pr with the crystallization temperature. These results indicated that the ferroelectric phase of BT–BMT–BF was successfully grown with increasing crystallization temperature, whereas the temperature of 600 °C resulted in insufficient crystal growth, which prevented the ferroelectric polarization. The largest Pr value of the BT–BMT–BF films was approximately 12 µC/cm2, which is significantly smaller than those of the bulk materials of BT–BMT–BF with a random crystal orientation. The reasons for the lower Pr values of the resulting films are assumed to be (i) lattice defects formed by the evaporation of volatile components during the heat treatment and/or (ii) the inappropriate crystal orientation of the BT–BMT–BF films. Regarding the defect formation, we have confirmed that the Bi component was evaporated gradually at heat-treatment temperatures of approximately 600 °C and above as in the CSD process for Bi-based perovskite films.16–20) Also, regarding the control of the crystal orientation, the polar axis of the BT–BMT–BF unit cell with the present composition (i.e.,  ) can be along the 〈111〉 or 〈110〉 direction, not along the 〈100〉 direction.
) can be along the 〈111〉 or 〈110〉 direction, not along the 〈100〉 direction.
Fig. 2. P–E loops of BT–BMT–BF films on (100)SrRuO3//(100)SrTiO3 substrates fabricated at various crystallization temperatures. The films were derived from the precursor solution with a stoichiometric composition.
Download figure:
Standard image High-resolution imageNext, the compositional compensation of Bi evaporation was performed by the CSD process using a precursor solution with excess Bi. The amount of excess Bi (+5%) was determined from the previous data for CSD experiments on other Bi-based perovskite films.16,17) Also, the film deposition was performed on the (111)SrRuO3//(111)SrTiO3 substrate, as well as on the (100)SrRuO3//(100)SrTiO3 substrates to evaluate the effect of the crystal orientation. Figures 3(a) and 3(b) show XRD patterns (2θ–ω scan) of the films fabricated on (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3 substrates, respectively, using the solution with excessive Bi. For the resulting films fabricated at 700 and 750 °C, the preferential growth of perovskite (100)pc and (111)pc planes was successfully confirmed on (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3, respectively, although small minor peaks still remained. A trace amount of Bi2O3 was detected for the film on (100)SrRuO3//(100)SrTiO3 [Fig. 3(a)], which was derived from the excess Bi in the precursor solution. The Lotgering factor of the (h00)pc planes was enhanced to 0.98 for the (100)pc-oriented film fabricated at 750 °C.
Download figure:
Standard image High-resolution imageFig. 3. XRD patterns (2θ–ω scan) of BT–BMT–BF films on (a) (100)SrRuO3//(100)SrTiO3 and (b) (111)SrRuO3//(111)SrTiO3 substrates fabricated at various crystallization temperatures. The films were derived from the precursor solution with +5% Bi excess composition.
Download figure:
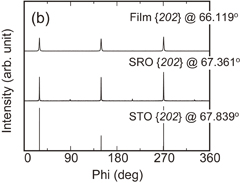
Standard image High-resolution imageThe in-plane crystal orientations of the resulting films were evaluated by XRD analysis. Figures 4(a) and 4(b) show ϕ-scan XRD data of the films on (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3 substrates, respectively. All scans were measured using the (202)pc diffraction. Fourfold symmetric peaks of the film, SrRuO3, and SrTiO3 were observed at the same angle ϕ in the (100)pc-oriented film on the (100)SrRuO3//(100)SrTiO3 substrate. Also, threefold symmetric peaks of the film, SrRuO3, and SrTiO3 were observed at the same ϕ in the (111)pc-oriented film on the (111)SrRuO3//(111)SrTiO3 substrate. These results suggest that the films and SrRuO3 layers were epitaxially grown on the SrTiO3 substrate in both samples.21–28)
Download figure:
Standard image High-resolution imageFig. 4. ϕ-scan XRD data of BT–BMT–BF films on (a) (100)SrRuO3//(100)SrTiO3 and (b) (111)SrRuO3//(111)SrTiO3 substrates in Fig. 3. The data were measured using the (202)pc diffraction of the BT–BMT–BF film, SrRuO3, and SrTiO3.
Download figure:
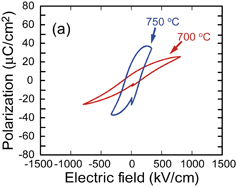
Standard image High-resolution imageP–E hysteresis loops were also measured for the ferroelectric evaluation of the (100)pc- and (111)pc-oriented BT–BMT–BF films derived from the precursor solution with excess Bi. The measurement data of the films fabricated on (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3 substrates are shown in Figs. 5(a) and 5(b), respectively. For the films fabricated on (100)SrRuO3//(100)SrTiO3 [Fig. 5(a)], the Pr values were larger than those derived from the precursor solution with stoichiometric Bi, for example, Pr for the film crystallized at 750 °C was approximately 20 µC/cm2. However, excess Bi might degrade the insulating property of the films, lowering the maximum applied field and distorting the P–E hysteresis curves. Meanwhile, for the films fabricated on (111)SrRuO3//(111)SrTiO3 [Fig. 5(b)], enhanced Pr values greater than those for the films fabricated on (100)SrRuO3//(100)SrTiO3, with well-saturated P–E hysteresis loops, were observed successfully. Pr for the film fabricated at 750 °C was approximately 35 µC/cm2. These data suggest that the polar axis of the BT–BMT–BF unit cell is along the 〈111〉 direction, resulting in the enhanced polarization of the (111)pc-oriented film compared with that of the (100)pc-oriented film. Similar tendencies of the ferroelectric anisotropy have also been observed for Pb-based perovskite thin films such as PZT and PT–PMN with morphotropic phase boundary (MPB) compositions.9,10,29) However, the resulting Pr value of approximately 35 µC/cm2 is not particularly large compared with the experimental results of bulk BT–BMT–BF materials [Pr = approximately 30 µC/cm2 (Refs. 1 and 2)]. A non-ferroelectric domain due to insufficient crystallization or defect pinning might still be present in the resulting film, as indicated by the relatively low squareness of the P–E hysteresis loop in Fig. 5(b).
Download figure:
Standard image High-resolution imageFig. 5. P–E loops of BT–BMT–BF films on (a) (100)SrRuO3//(100)SrTiO3 and (b) (111)SrRuO3//(111)SrTiO3 substrates fabricated at various crystallization temperatures. The films were derived from the precursor solution with +5% Bi excess composition.
Download figure:
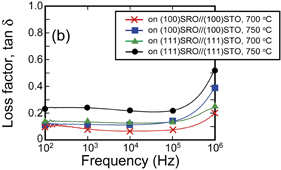
Standard image High-resolution imageThe dielectric properties were evaluated by the measurement of relative dielectric constant (εr) with changing oscillation frequency (f). Figure 6 shows the relationship between εr and f for the films fabricated on (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3 substrates, respectively, using the precursor solution with excess Bi. Note that all of these films exhibited dielectric relaxation, i.e., dielectric degradation with increasing oscillation frequency, similarly to the bulk materials.1,2) The εr value of the (111)pc-oriented film was larger than that of the (100)pc-oriented film, although the loss factor (tan δ) of the (111)pc-oriented film was also higher than that of the (100)pc-oriented film; the εr values of the (111)pc- and (100)pc-oriented films fabricated at 750 °C were 1750 and 880 at 1 kHz, respectively. A similar phenomenon, i.e., larger dielectric permittivity for (111)pc-oriented films than for (100)pc- or (110)pc-oriented ones, has been confirmed for epitaxial PZT films within the compositional range of Zr/(Zr + Ti) = 0.4–0.6 (with coexisting tetragonal and rhombohedral symmetries).9,10,30–32)
Download figure:
Standard image High-resolution imageFig. 6. Relative dielectric constant (εr) and loss factor (tan δ) as a function of oscillation frequency measured for BT–BMT–BF films on (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3 substrates fabricated at various crystallization temperatures. The films were derived from the precursor solution with +5% Bi excess composition.
Download figure:
Standard image High-resolution image4. Conclusions
Epitaxially grown thin films of ternary BT–BMT–BFO solid solution were fabricated by the CSD technique. The films deposited on (100)SrRuO3//(100)SrTiO3 and (111)SrRuO3//(111)SrTiO3 substrates were grown with the crystal orientation of the perovskite (100)pc and (111)pc planes normal to the substrate surface, respectively. Enhanced ferroelectric behavior was confirmed for the (111)pc-oriented BT–BMT–BFO film: the Pr values for the (111)pc-oriented films were significantly larger than those for the (100)pc-oriented films. These results imply the crystallographic anisotropy of the polarization behavior, i.e., the polar axis of the BT–BMT–BFO unit cell is along the 〈111〉 direction.