Abstract
We investigated novel organic/inorganic hybrid thermoelectric materials prepared using several metal-polymer complexes, binders (insulating polymers), and inorganic semiconductor nanomaterials. It was found that the three-component hybrid thermoelectric materials, which consisted of nanodispersed poly(nickel 1,1,2,2-ethenetetrathiolate) (Ni-PETT), cellulose acetate (CA), and carbon nanotubes (CNTs), showed high thermoelectric performance. Ni-PETT had a large negative Seebeck coefficient of −42 µV K−1 and was an n-type semiconducting polymer complex. Ni-PETT sufficiently dispersed p-type CNTs in N-methyl-2-pyrrolidone. The charge transfer interaction between Ni-PETT and CNTs could provide a strong contact. Good films could be obtained by using CA as a binder. In addition, the electrical conductivity of the three-component hybrid films was increased by methanol treatment. The Seebeck coefficient, electrical conductivity, and power factor of Ni-PETT/CA/CNT films normalized on the basis of the CNT mass were 1.9, 5.2, and 2.8 times higher than those of the CNT sheets.
Export citation and abstract BibTeX RIS
1. Introduction
Thermoelectric materials can directly convert low-quality waste heat into usable electric energy.1,2) Therefore, thermoelectric energy conversion technologies are expected to play an important role in solving energy problems. The efficiency of a thermoelectric material depends on the dimensionless figure of merit, ZT,3) which is expressed by ZT = S2σT/κ, where S is the Seebeck coefficient (V K−1), σ is the electrical conductivity (S m−1), T is the absolute temperature (K), and κ is the thermal conductivity (W m−1 K−1). If materials have similar thermal conductivities, the parameter power factor PF4) (= S2σ) is used to characterize thermoelectric performance characteristics. Thus, good thermoelectric materials exhibit a large power factor and a low thermal conductivity.
Most of the practical thermoelectric materials are inorganic materials; they, however, have disadvantages such as rarity, toxicity, poor processability, and high cost of manufacture.5) To overcome these issues, instead of inorganic thermoelectric materials, research activities on organic thermoelectric materials6–8) are on the rise because of the abundance of the required elements and the environmentally friendliness, flexibility, light weight, easy processability, and low cost of these organic materials.9) However, compared with the thermoelectric properties of inorganic materials, the thermoelectric performance of organic materials is not very practical. Conducting polymers,7,10–13) which have π-conjugate systems, were investigated for their use as thermoelectric materials. Among numerous conducting polymers, poly(3,4-ethylenedioxythiophene) (PEDOT)14–21) has often been used as an organic thermoelectric material. Although the devices fabricated using PEDOT-based films show high performance of thermoelectric materials, the instability in an ambient environment is a serious disadvantage that limits the practical use.
Recently, hybrids of organic and inorganic materials have been studied as next-generation thermoelectric materials. The properties of novel hybrid materials based on organic thermoelectric materials are different from those based on inorganic thermoelectric materials. It is possible to increase electrical conductivity and decrease thermal conductivity. For example, hybrid organic materials show good thermoelectric performance by incorporating organic materials with carbon nanotubes (CNTs),22–26) nanocrystals,27–31) or nanoparticles32–35) as inorganic materials. We have successfully developed new thermoelectric materials with high thermoelectric property using nanodispersed poly(nickel 1,1,2,2-ethenetetrathiolate) (Ni-PETT), poly(vinyl chloride) (PVC), and CNTs35) without using conducting polymers such as PEDOT-poly(styrene sulfonate). Newly prepared nanoparticles of the n-type semiconducting polymer complex Ni-PETT disperse CNTs well in N-methyl-2-pyrrolidone (NMP). The hybrid films have high thermoelectric performance owing to the effective dispersion of CNTs and the enhancement of the carrier transport between Ni-PETT and CNTs. In this study, organic/inorganic hybrid thermoelectric materials were prepared using several polymer complexes based on metal-PETT, binders, and semiconductor nanomaterials.
2. Experimental methods
2.1. Materials
To synthesize PETTs, we used 1,3,4,6-tetrathiapentalene-2,5-dione (TPD; Tokyo Chemical Industry). Nickel(II) chloride, copper(II) chloride, palladium(II) chloride, zinc(II) chloride, and cobalt(II) chloride were selected as the central metals (Wako Pure Chemical Industry). For binders, cellulose acetate (CA), poly(methyl methacrylate) (PMMA; Wako Pure Chemical Industry), and polyimide (PI) were used. CNTs (Zeon and Aldrich) and Ag nanowires (AgNWs) were used to improve the electric conductivity and mechanical properties of the polymer.
2.2. Preparation of AgNWs
AgNWs were prepared using the procedure reported by Korte et al.,36) Lee et al.,37) and Kuo and Hwang with modification.38) For preparing AgNWs, silver nitrate (AgNO3), potassium nitrate (KNO3), ethylene glycol (EG), and poly(vinyl pyrrolidone) (PVP) were purchased from Wako Pure Chemical Industry. First, 50 mL of KNO3–EG solution (280 mM) was stirred on an oil bath at 424.7 K for 1 h. Then, 400 µL of CuCl2–EG solution (4 mM) was slowly added and stirred. After 15 min, 15 mL of PVP–EG solution (147 mM) was added and stirred for 10 min. Then, 15 mL of AgNO3–EG solution (94 mM) in a separatory funnel was dropped at a rate of ca. 1 mL min−1. After stirring for 3 h, the clear colorless solution was changed to yellow-white, and finally to opaque gray. The products, AgNWs, were cooled to room temperature, filtered, and washed with methanol and water.
2.3. Preparation of metal-PETTs
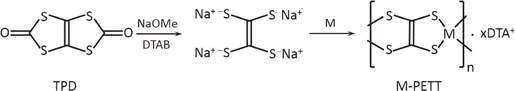
Using some metals as the central metals, metal-PETTs (M-PETTs) (polymer complexes) were synthesized (Fig. 1). M-PETTs were prepared on the basis of previous reports35,39) with some slight modifications including the method reported by Sun et al.40) Dodecyltrimethylammonium bromide (DTAB, 22.2 mmol) and sodium methoxide (NaOMe, 22.2 mmol) were stirred in methanol at room temperature for 12 h to obtain a transparent liquid. TPD (4.8 mmol) was added to the solution and refluxed in an oil bath at 363 K for 12 h. Then, metal chlorides (4.8 mmol) as the central metals and methanol (10 mL) were added and refluxed at 363 K for 12 h. After refluxing, the products were allowed to stand for 12 h to obtain precipitates. The precipitates were filtered and washed with water, methanol, and dimethyl ether. A black powder (M-PETTs) was obtained after drying under vacuum at 313 K overnight.
Fig. 1. Schematic diagram of synthesis of M-PETT. TPD: 1,3,4,6-tetrathiapentalene-2,5-dione, DTAB: dodecyltrimethylammonium bromide. M: Ni, Cu, Pd, Co, and Zn. DTA: dodecyltrimethylammonium.
Download figure:
Standard image High-resolution image2.4. Fabrication of two-component hybrid films: Ni-PETT/polymer
Ni-PETT was added to a NMP solution containing a polymer (CA, PMMA, or PI) and dispersed in an ultrasonic bath for 10 min. The dispersion of Ni-PETT and a polymer was cast on a glass substrate, and dried in air on a hot plate at 333 K for 12 h to obtain a two-component hybrid film. The film was washed with methanol to remove excess Ni-PETT, and then dried on a hotplate at 343 K for 1 h.
2.5. Fabrication of three-component hybrid films: Ni-PETT/CA/CNT
Ni-PETT and CNTs were added to a NMP solution containing CA, and dispersed in an ultrasonic bath for 10 min. The dispersion of Ni-PETT, CA, and CNT was cast on a quartz substrate, and dried in air on a hot plate at 333 K for 12 h to obtain a three-component hybrid film, Ni-PETT/CA/CNT.
2.6. Film characterization
Film thickness was measured with a linear gage (Mitsutoyo LGK-010; resolution, 0.1 µm). The Seebeck coefficients and electric conductivities of M-PETT, M-PETT/polymer, and Ni-PETT/CA/CNT were measured with a thermoelectric evaluation system (Ulvac ZEM-3M8) at 330–380 K under vacuum with helium gas. The PF of each film was calculated using the equation PF = S2σ. The surface morphology of the film was observed by field emission scanning electron microscopy (FE-SEM; Hitachi S-4800 Type2).
3. Results and discussion
3.1. Thermoelectric properties of M-PETTs
Elemental analysis and inductively couple plasma atomic emission spectrometry revealed that Ni-PETT is an oligomer doped with DTA ions at an Na/DTA/Ni molar ratio of  .20) For Ni-PETT, the Seebeck coefficient is −42 µV K−1 at 340 K. Ni-PETT exhibits the n-type thermoelectric property. For Cu-PETT and Pd-PETT, the Seebeck coefficients are 18.4 and 12.8 µV K−1 at 340 K, respectively, and their PETTs are p-type materials. Although the reason is not clear, the Seebeck coefficients of Co-PETT and Zn-PETT are nearly zero. On the other hand, the electrical conductivities of Ni-PETT, Cu-PETT, and Pd-PETT are 538, 1.5, and 0.56 S cm−1, respectively. The absolute values of the Seebeck coefficient and electric conductivity of Ni-PETT are higher than those of the other M-PETTs. Although we do not have the reason to determine the n- or p-type, Sun et al.40) considered that either central metal atoms or ligand bridges might be selectively oxidized, and the oxidation reaction depended on the species of central metal atoms. In addition, the values of the power factor of Ni-PETT are ca. 400–3000 times higher than those of other M-PETTs according to calculation using the values of S and σ. The large electron delocalization in the direction of the polymer chain and the easy charge transfer from chain to chain may contribute to the more excellent electrical characteristics of Ni-PETT.41) The thermoelectric properties of Co- and Zn-PETT could not be measured owing to overload. Therefore, Ni-PETT as a complex of two-component hybrid films was used.
.20) For Ni-PETT, the Seebeck coefficient is −42 µV K−1 at 340 K. Ni-PETT exhibits the n-type thermoelectric property. For Cu-PETT and Pd-PETT, the Seebeck coefficients are 18.4 and 12.8 µV K−1 at 340 K, respectively, and their PETTs are p-type materials. Although the reason is not clear, the Seebeck coefficients of Co-PETT and Zn-PETT are nearly zero. On the other hand, the electrical conductivities of Ni-PETT, Cu-PETT, and Pd-PETT are 538, 1.5, and 0.56 S cm−1, respectively. The absolute values of the Seebeck coefficient and electric conductivity of Ni-PETT are higher than those of the other M-PETTs. Although we do not have the reason to determine the n- or p-type, Sun et al.40) considered that either central metal atoms or ligand bridges might be selectively oxidized, and the oxidation reaction depended on the species of central metal atoms. In addition, the values of the power factor of Ni-PETT are ca. 400–3000 times higher than those of other M-PETTs according to calculation using the values of S and σ. The large electron delocalization in the direction of the polymer chain and the easy charge transfer from chain to chain may contribute to the more excellent electrical characteristics of Ni-PETT.41) The thermoelectric properties of Co- and Zn-PETT could not be measured owing to overload. Therefore, Ni-PETT as a complex of two-component hybrid films was used.
3.2. Electrical conductivity of two-component hybrid M-PETT/polymer
It is important to select an optimal polymer, because the polymer added as a binder affects film properties. Two-component hybrid thermoelectric materials were prepared using Ni-PETT and several polymers. The concentrations of the polymers were investigated in the range of 5 to 50 wt %. The thickness of the film increased with the increase in the concentration of each polymer, and the thickness of each film ranged from ca. 10 to 15 µm. Because PI exhibits high heat resistance, it was employed as a binder. When PI was used as the polymer, PI did not act as a binder and no film was obtained at any concentration of polymer except for 50 wt %. In addition, PMMA was employed as a binder, because PMMA has shatter-resistance. As a result, the films of Ni-PETT/PMMA were very fragile after methanol treatment. On the other hand, two-component hybrid films of Ni-PETT/CA exhibited good film-forming property and electrical conductivity. CA is often employed in photographic films and magnetic tapes, and has the high capability of forming films. Following methanol treatment, it shows markedly enhanced electrical conductivity. This enhancement of electrical conductivity was considered to be due to the alignment of the polymer and the removal of the excess binder polymer that acted as an insulator due to the treatment. The electrical conductivities of several films at each concentration of the polymer are summarized in Table I.
Table I. Electrical conductivity of Ni-PETT/polymer before and after methanol treatment.
| Sample | Treatmenta) | Concentration of polymer (wt %) | |||
|---|---|---|---|---|---|
| 15 | 20 | 30 | 50 | ||
| (S cm−1) | |||||
| Ni-PETT/CA | Pristine | 2.3 | 3.4 | 0.7 | 0.4 |
| Treated | 7.9 | 7.4 | 0.7 | 0.3 | |
| Ni-PETT/PMMA | Pristine | 3.4 | 1.8 | 1.0 | 0.5 |
| Treated | 4.0 | 2.7 | 0.9 | 0.5 | |
| Ni-PETT/PI | Pristine | — | — | — | 0.7 |
| Treated | — | — | — | 0.5 | |
a) Methanol treatment.
3.3. Thermoelectric properties of three-component hybrid Ni-PETT/CA/CNT
To enhance the electrical conductivity, AgNWs and CNTs were added to Ni-PETT/CA, and three-component hybrid thermoelectric materials were prepared. SEM images of the prepared AgNWs are shown in Fig. 2. No aggregation of AgNWs was observed and uniform AgNWs were obtained. The AgNWs had a length of 9.3 ± 5.3 µm and a diameter of 71.4 ± 27.2 nm. The mass ratio of the components in Ni-PETT/CA/AgNW was  and the NMP solution containing three components was cast on a quartz substrate to form the hybrid film. Although the prepared AgNWs have an electrical conductivity of approximately 6000 S cm−1, the hybrid Ni-PETT/CA/AgNW film shows nonconductance. We think that the structural change of AgNWs made the properties of the hybrid film poor. Therefore, to confirm the change in the structure for AgNWs, structural analysis was carried out by X-ray diffraction. Unfortunately, no evidence of the structural change of AgNWs was obtained, and details regarding the poor properties of the Ni-PETT/CA/AgNW hybrid film were unclarified. Instead of AgNWs, CNTs were used to prepare the three-component hybrid thermoelectric materials. The CNTs had a nominal length of 1 µm and a nominal diameter of 1.4 nm. The mass ratio of the components in Ni-PETT/CA/CNT was
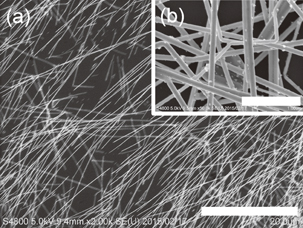
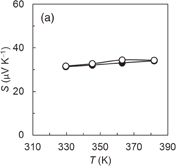
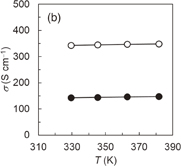
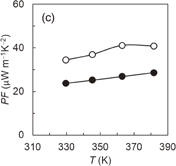
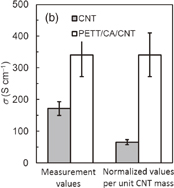
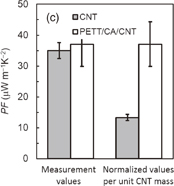
and the NMP solution containing three components was cast on a quartz substrate to form the hybrid film. Although the prepared AgNWs have an electrical conductivity of approximately 6000 S cm−1, the hybrid Ni-PETT/CA/AgNW film shows nonconductance. We think that the structural change of AgNWs made the properties of the hybrid film poor. Therefore, to confirm the change in the structure for AgNWs, structural analysis was carried out by X-ray diffraction. Unfortunately, no evidence of the structural change of AgNWs was obtained, and details regarding the poor properties of the Ni-PETT/CA/AgNW hybrid film were unclarified. Instead of AgNWs, CNTs were used to prepare the three-component hybrid thermoelectric materials. The CNTs had a nominal length of 1 µm and a nominal diameter of 1.4 nm. The mass ratio of the components in Ni-PETT/CA/CNT was  . Figures 3(a)–3(c) show the effects of temperature on the Seebeck coefficient, electrical conductivity, and power factor in Ni-PETT/CA/CNT, respectively. The Seebeck coefficient, electrical conductivity, and power factor do not depend on temperature, and a stable thermoelectric performance can be obtained. In addition, the methanol treatment can enhance the electrical conductivity similarly to the two-component hybrid thermoelectric system. SEM images of the Ni/PETT/CNT surface before and after the methanol treatment are shown in Fig. 4. From the SEM images, the removal of the excess polymer covering CNTs by methanol treatment is clearly observed. At the concentrations of CNTs from 20 to 60 wt %, the thermoelectric performance of Ni-PETT/CA/CNT is almost independent of the concentration of CNTs. As shown in Fig. 5, the electrical conductivity and power factor of the Ni-PETT/CA/CNT film are higher than those of the CNT sheet except for the Seebeck coefficient. CNT by itself is often used as a p-type thermoelectric material, for comparison of its thermoelectric performance with that of a hybrid material; each obtained value was normalized against CNT mass. All the thermoelectric properties (Seebeck coefficient, electrical conductivity, and power factor) of the Ni-PETT/CA/CNT film normalized on the basis of CNT mass are much greater than those of the CNT sheet. The Seebeck coefficient, electrical conductivity, and power factor of the Ni-PETT/CA/CNT film normalized on the basis of CNT mass are respectively 1.9, 5.2, and 2.8 times higher than those of the CNT sheet. The thermoelectric properties of composite films at 340 K are summarized in Table II. This remarkable enhancement of the electrical conductivity of the three-component film might be due to the charge transfer interaction between p-type CNTs and n-type Ni-PETT. Moreover, the reduction in the average diameter of the CNT bundles promotes the dispersion of CNTs in NMP and increases the number of contact points between CNTs, resulting in the improved electrical conductivity. We do not understand well why there was a difference in thermoelectric performance between CNT-system and AgNW-system materials. We interpreted this as follows. The film could not be obtained at a CA concentration lower than 30 wt % in the two-hybrid film (Ni-PETT/CA). On the other hand, with CNTs as an inorganic material, a good film could be formed in the three-hybrid film (i.e., Ni-PETT/CA/CNT) at the CA concentration of 15 wt %. These results indicate that CNT possesses the capability to form films (for example, CNT buckypapers). In contrast, because AgNWs do not have the high film-forming property, good three-hybrid films (Ni-PETT/CA/AgNW) cannot be obtained. The capability of forming films might underlie the difference in thermoelectric performance between Ni-PETT/CA/CNT and Ni-PETT/CA/AgNW.
. Figures 3(a)–3(c) show the effects of temperature on the Seebeck coefficient, electrical conductivity, and power factor in Ni-PETT/CA/CNT, respectively. The Seebeck coefficient, electrical conductivity, and power factor do not depend on temperature, and a stable thermoelectric performance can be obtained. In addition, the methanol treatment can enhance the electrical conductivity similarly to the two-component hybrid thermoelectric system. SEM images of the Ni/PETT/CNT surface before and after the methanol treatment are shown in Fig. 4. From the SEM images, the removal of the excess polymer covering CNTs by methanol treatment is clearly observed. At the concentrations of CNTs from 20 to 60 wt %, the thermoelectric performance of Ni-PETT/CA/CNT is almost independent of the concentration of CNTs. As shown in Fig. 5, the electrical conductivity and power factor of the Ni-PETT/CA/CNT film are higher than those of the CNT sheet except for the Seebeck coefficient. CNT by itself is often used as a p-type thermoelectric material, for comparison of its thermoelectric performance with that of a hybrid material; each obtained value was normalized against CNT mass. All the thermoelectric properties (Seebeck coefficient, electrical conductivity, and power factor) of the Ni-PETT/CA/CNT film normalized on the basis of CNT mass are much greater than those of the CNT sheet. The Seebeck coefficient, electrical conductivity, and power factor of the Ni-PETT/CA/CNT film normalized on the basis of CNT mass are respectively 1.9, 5.2, and 2.8 times higher than those of the CNT sheet. The thermoelectric properties of composite films at 340 K are summarized in Table II. This remarkable enhancement of the electrical conductivity of the three-component film might be due to the charge transfer interaction between p-type CNTs and n-type Ni-PETT. Moreover, the reduction in the average diameter of the CNT bundles promotes the dispersion of CNTs in NMP and increases the number of contact points between CNTs, resulting in the improved electrical conductivity. We do not understand well why there was a difference in thermoelectric performance between CNT-system and AgNW-system materials. We interpreted this as follows. The film could not be obtained at a CA concentration lower than 30 wt % in the two-hybrid film (Ni-PETT/CA). On the other hand, with CNTs as an inorganic material, a good film could be formed in the three-hybrid film (i.e., Ni-PETT/CA/CNT) at the CA concentration of 15 wt %. These results indicate that CNT possesses the capability to form films (for example, CNT buckypapers). In contrast, because AgNWs do not have the high film-forming property, good three-hybrid films (Ni-PETT/CA/AgNW) cannot be obtained. The capability of forming films might underlie the difference in thermoelectric performance between Ni-PETT/CA/CNT and Ni-PETT/CA/AgNW.
Fig. 2. SEM images of prepared AgNWs. (a) A low-magnification SEM image shows uniform nanowires. The bar denotes 20 µm. (b) The inset illustrates a higher-magnification SEM image and the bar denotes 1 µm. The average length and diameter of AgNWs are 9.3 ± 5.3 µm and 71.4 ± 27.2 nm, respectively.
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageFig. 3. Temperature dependence of the thermoelectric performance of Ni-PETT/CA/CNT before (closed circles) and after (open circles) methanol treatment. (a) Seebeck coefficient (S), (b) electrical conductivity (σ), and (c) power factor (PF). Mass ratio: Ni-PETT/CA/CNT = 10/8/3.
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageFig. 4. SEM images of the films of Ni-PETT/CA/CNT before (a) and after (b) the methanol treatment.
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageFig. 5. Comparison of thermoelectric performance characteristics between CNTs and Ni-PETT/CA/CNT. (a) Seebeck coefficient (S), (b) electrical conductivity (σ), and (c) power factor (PF). The left and right bars denote the measurement values and the normalized values per unit CNT mass, respectively.
Download figure:
Standard image High-resolution imageTable II. Thermoelectric properties of composite films at 340 K.
| Sample | S (µV K−1) | σ (S cm−1) | PF (µW m−1 K−2) |
|---|---|---|---|
| CNT sheet | 45.2 | 172.0 | 35.0 |
| Ni-PETT/CA/CNTa) | 33.0 | 341.0 | 37.0 |
a) Mass ratio: Ni-PETT/CA/CNT = 10/8/3.
4. Conclusions
We have developed three-component hybrid organic materials of Ni-PETT/CA/CNT with high thermoelectric properties. The Seebeck coefficient and electrical conductivity of Ni-PETT/CA/CNT were 33.0 µV K−1 and 341.0 S cm−1, respectively. The materials exhibit a high PF of 37.0 µW m−1 K−2 at 340 K. The organic material PETT shows a good thermoelectric performance with Ni as the central metal. The addition of CA allows for the formation of better Ni-PETT films. The high thermoelectric efficiency can be obtained by using CNTs with high electrical conductivity as inorganic materials. Compared with the CNT sheets, the thermoelectric performance of the Ni-PETT/CA/CNT films normalized on the basis of the CNT mass was higher than that of the CNT sheets. Moreover, it is revealed that the methanol treatment markedly enhances the thermoelectric performance of Ni-PETT/CA/CNT films. The developed three-component hybrid thermoelectric materials are expected to be novel hybrid organic/inorganic materials for future thermoelectric devices.
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 15K04613) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT).