Abstract
The concentration changes of nickel-related species after thermal annealing in Schottky electrode-formed (EL-formed) and electrode-free (EL-free) p-type silicon samples diffused with nickel were measured by deep-level transient spectroscopy. The nickel donor center began to decay at approximately 100 °C with the activation energies of 1.06 and 0.26 eV for the EL-formed and EL-free samples, respectively, which were analyzed as the required energies for the center to form complexes with interstitial nickel (Nii) and hydrogen, respectively. These complexes evolved into extended complexes by further bonding of Nii at higher annealing temperatures. All the complexes above disappeared by evolving into precipitates within temperatures lower than 400 °C without recovering the nickel donor center. The transformation reactions of the complexes progressed at lower temperatures and shorter times in the EL-formed samples than in the EL-free samples because of the electric neutralization of the nickel-related species in the space-charge region of the electrode.
Export citation and abstract BibTeX RIS
1. Introduction
Since nickel is the most detrimental element for silicon devices even at trace concentrations of 1010–1011/cm3,1,2) the solubility,3–6) diffusivity,7,8) and precipitation behavior9,10) of this element in crystalline silicon have long been investigated. According to these investigations together with related reviews,2,11,12) the dominant part of nickel dissolves in silicon as an interstitial species (Nii) and a minor part resides as a substitutional species (Nis). While the former species dissolved at high temperatures easily outdiffuses to form precipitates at sample surfaces during cooling, the latter species can remain in the bulk of samples after the cooling because of its lower diffusivity. In p-type silicon diffused with nickel at high temperatures, this species has generally been observed by deep-level transient spectroscopy (DLTS)13–23) as the donor center characterized by an energy level of Ev + 0.17 ± 0.02 eV (Ev: the top energy of the valence band). Since DLTS is a sensitive and species-discriminating probe, it is useful to assess the trace contamination of nickel in silicon, as demonstrated in a previous study.24) It is also important to investigate the thermal behavior of the center and other nickel-related species after the diffusion of nickel for the actual assessment of nickel contamination. Several measurements reporting the annealing behavior of the center in heavily nickel-diffused samples15,17–19) and in a melt-doped sample20) were reported. However, they are fragmentary and not always consistent; while one group showed strong stability of the center after the annealing above 600 °C,17–19) other groups showed easy decay of the center after the annealing below 250 °C.15,20,23)
In this study using p-type silicon diffused with dilute nickel, changes in the DLTS spectrum of the samples after long-time storage at room temperature and annealing at different temperatures were thoroughly measured. The Schottky electrode-formed (EL-formed) and electrode-free (EL-free) samples were used to investigate the various reactions of nickel-related species in the space-charge region (SCR) and in the ordinary bulk, respectively. Differently from the findings reported in several papers,17–19) the nickel center began to decay at low temperatures of approximately 100 °C by forming complexes with hydrogen and Nii with different activation energies in different types of sample. The successive transformations of the complexes into other species and the disappearance of all the nickel-related complexes by precipitation after further annealing at different temperatures lower than 400 °C were observed for different types of sample. Hydrogen and Nii were suggested to play the most important roles in the transformation reactions of nickel-related complexes.
2. Experimental methods
The starting samples were 100-mm-diameter, 550-µm-thick (100) float-zone-grown (FZ) Si wafers doped with boron at (8.5 ± 1.0) × 1014/cm3 (p-type) and mirror-polished on one side. Following the method employed by Hourai et al.,25) nickel-contaminated samples were prepared: the wafers were first immersed in a 1-NH4OH/1-H2O2/5-H2O solution warmed at 75–80 °C for 10 min to remove organic and metal contaminants and then immersed in a 5% HF aqueous solution for 3 min at room temperature to remove the native silicon oxide. Since this process was the same as that for the ordinary hydrogenation of wafers26) and the wafer surfaces were hydrophobic after the immersion, it was clear that they were completely covered or bonded with hydrogen similarly to hydrogenated samples.26) Next, the wafers were immersed in a 1-HCl/1-H2O2/6-H2O solution warmed at 75–80 °C for 10 min. Since the surface of the wafer was hydrophilic at this stage, the wafer was assumed to be covered with a thin silicon oxide. After the wafer was mounted on a spinning machine, 5 ml of aqueous solution containing 10 ppm nickel [Ni(NO3)2] was poured on the surface of the wafer until the solution spread to the entire wafer. This state was maintained for 1 min to have the metal ion homogeneously adsorbed in the oxide. Then, the residual solution on the wafer was spun off by the spinning machine. The contamination level of nickel on the wafers before diffusion was (2.1 ± 0.2) × 1013/cm2 measured using a commercially available total reflection X-ray fluorescence spectrometer (Rigaku TREX610T). The wafers were diffused at 525 °C for 10 min in a quartz furnace under flowing clean, dry nitrogen gas. Then, they were cooled to room temperature by drawing them at a constant speed of 100 mm/min to the mouth of the furnace. After the cooling, the wafers were cut into many small plates (8 × 20 mm2). These samples were divided into two groups. After rinsing one part of the samples with 0.5% HF aqueous solution, 1-mm-diameter, 100-nm-thick Ti Schottky barrier electrodes were formed on the diffused side of the samples by evaporation, and a 1-µm-thick Al metal layer was deposited on each Ti electrode by evaporation (EL-formed samples). These samples together with the samples without electrodes (EL-free samples) were stored at room temperature and annealed in air in a temperature-controlled furnace after the storage. After the storage or annealing, the Schottky electrodes were formed on the EL-free samples by the same method as that for the EL-formed samples. All the preparation processes of the samples mentioned above were performed in a class 100 clean room. Before performing DLTS measurements, capacitance–voltage (C–V) measurements were always carried out. The temperature scan DLTS measurement was performed using a commercially available spectrometer (SEMILAB DLS-83D). The bias voltage was −5.0 V, and the forward pulse voltage determined from the bias voltage was +4.0 V. The lock-in frequency and pulse width were 244 Hz and 20 µs, respectively. The analytical depths estimated under these conditions were within 3.0 µm from the surface.27) The concentrations of the nickel center and its related species measured by DLTS were always calibrated using the dopant concentration measured by the C–V method.
3. Results
3.1. Spectrum change after long-time storage at room temperature
Before measuring the DLTS spectrum, the electric characteristics of the Schottky barrier diodes were examined. Since sound diodes were always formed without etching the surfaces of the samples, no nickel precipitate was considered to form on the surfaces of the samples; all the contaminating nickel was dissolved in the bulk and no outdiffusion of the element occurred during the cooling of the samples. As reported in a previous paper,24) the depth distribution of the nickel center was almost homogeneous throughout the depth (from 2.0 × 1013 near the surface to 1.5 × 1013/cm3 deep in the bulk). Since the bulk concentration of nickel is 3.8 × 1014/cm3 when all the contaminating nickel homogeneously dissolves, the concentration fractions of the nickel center to the dissolved nickel are estimated to be from 4 to 5%.
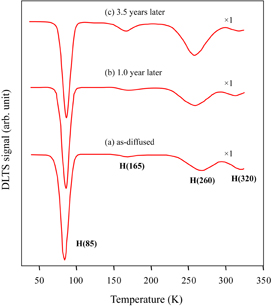
Both EL-formed and EL-free samples were stored for a long time in a clean room at room temperature. For the EL-free samples, no appreciable change in the spectrum was observed after storage for 3.5 years. Figure 1 shows the change in the DLTS spectrum of the same EL-formed sample with cumulative storage time. For the as-diffused sample, four peaks were clearly observed at approximately 85, 165, 260, and 320 K, which are denoted as H(85), H(165), H(260), and H(320), respectively, after their peak temperatures. The largest peak H(85), whose activation energy was 0.17 ± 0.01 eV as determined by thermal emission measurement,28) corresponds to the nickel donor center.13–23) The species H(165) and H(260), whose activation energies were 0.27 ± 0.02 and 0.45 ± 0.02 eV, respectively, correspond to the hydrogenated nickel centers20,23) that appeared after wet chemical etching (hydrogenation). The species H(320), whose activation energy was 0.61 ± 0.05 eV, has been assigned as the nitrogen-related defect originally included in the crystal,29) although its structure has not yet been identified. The spectral feature shown in Fig. 1(a) resembles the features in the samples diffused with deposited nickel film at high temperatures (≥950 °C) reported earlier.15–18) The highest temperature peak observed by Lemke15) at 300K (E1 denoted by him), however, does not appear in this study and other reports.16–18) After 1.0 year of storage, while the intensity of H(260) slightly increased, the intensities of H(85) and H(165) negligibly changed. After 3.5 years of storage, a further increase in the intensity of H(260), a slight decrease (<10%) in that of H(85), and a slight increase in that of H(165) were observed. Note that the increase in the concentration of H(260) is larger than the decrease in the concentration of H(85) after 3.5 years of storage.
Fig. 1. Change in DLTS spectrum with storage time at room temperature for an EL-formed sample. The cumulative storage times (years) are shown in the figure.
Download figure:
Standard image High-resolution image3.2. Spectrum change by annealing
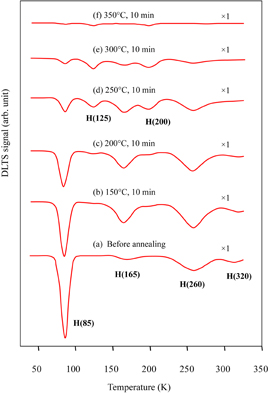
Figure 2 shows the change in the typical DLTS spectrum by annealing for the EL-free samples after 3.5 years of storage at room temperature. After annealing at 150 °C for 10 min, the decrease in the concentration of H(85) and the increases in the concentrations of both H(165) and H(260) were observed. After annealing at 200 °C, while the H(85) concentration further decreased, both H(165) and H(260) concentrations began to decrease. Two new peaks, H(125) and H(200), whose activation energies are 0.20 ± 0.02 and 0.39 ± 0.02 eV, respectively, appeared at this temperature. The appearance of these peaks was not reported in previous papers.15–23) After annealing at 350 °C for 60 min, all the peaks disappeared.
Fig. 2. Change in DLTS spectrum after annealing for EL-free samples. The annealing conditions and magnifications are shown in the figure.
Download figure:
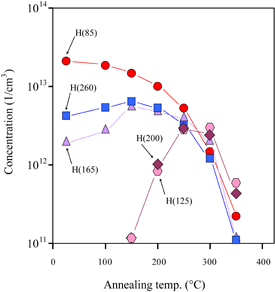
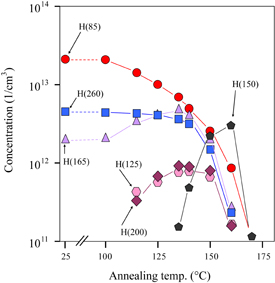
Standard image High-resolution imageThe detailed concentration changes of the peaks by isochronal (10 min) annealing are shown in Fig. 3. It is clearly seen that while the H(85) concentration continuously decreased with increasing annealing temperature, the concentrations of H(165) and H(260) independently changed. No recovery of the H(85) concentration after annealing was observed. Since the concentrations of H(125) and H(200) identically changed with annealing temperature, suggesting that they originate from the same defect, they are denoted as H(125/200). Since H(125/200) began to appear after the decays of H(165) and H(260), the former species is suggested to be the extended product of either of the latter species. Since no DLTS peak appeared after annealing at higher temperatures than 350 °C, insoluble species such as nickel precipitate is inferred to evolve above this temperature.
Fig. 3. Change in concentration of each peak with isochronal (10 min) annealing temperature for EL-free samples.
Download figure:
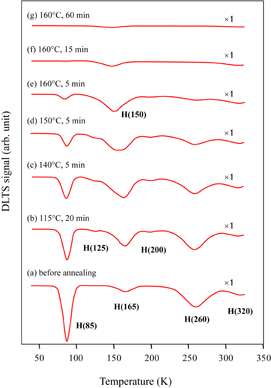
Standard image High-resolution imageFigure 4 shows the change in the DLTS spectrum by annealing of the EL-formed samples. After annealing at 115 °C for 20 min, while the H(85) concentration decreased, the H(165) concentration considerably increased. The small pair peaks H(125/200) appeared after this annealing. The species H(150) (activation energy: 0.20 ± 0.02 eV), which did not appear in the EL-free samples, appeared after annealing at 140 °C. This peak formed a maximum after annealing at 160 °C for 5 min and gradually decreased to disappear by annealing for a long time at this temperature. The concentration changes of these peaks after isochronal (5 min) annealing are shown in Fig. 5. The concentration change of each peak progressed within lower annealing temperatures and shorter times in the EL-formed samples than in the EL-free samples. All the peaks disappeared after annealing at 170 °C for 5 min. Note that the maximum intensities of H(125/200) were much smaller in the EL-formed samples than in the EL-free samples.
Fig. 4. Change in DLTS spectrum after annealing in EL-formed samples. The annealing conditions and magnifications are shown in the figure.
Download figure:
Standard image High-resolution imageFig. 5. Change in concentration of each peak with isochronal (5 min) annealing temperature for EL-formed samples.
Download figure:
Standard image High-resolution image3.3. Decay time constants of the nickel center
Since the concentration (N) of the nickel center showed an exponential decay with annealing time (t) by isothermal annealing (not shown) as in the case of the copper center,27) the decay time constant τ of the center is given by the equation
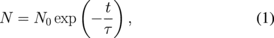
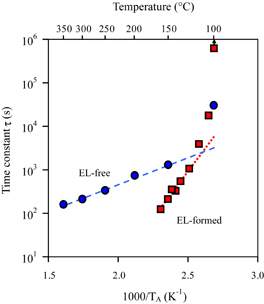
in which N0 is the initial concentration of the center before annealing. Figure 6 shows the Arrhenius plots of τ's for the EL-free and EL-formed samples, in which TA is the absolute annealing temperature. For the EL-free samples, the τ values in log-scale vary linearly with reciprocal temperature between 150 and 350 °C. The activation energy obtained from the slope between these temperatures as shown by the broken line is 0.26 ± 0.01 eV. For the EL-formed samples, τ has a threshold at approximately 100 °C, sharply decreases with increasing temperature, and linearly decreases with reciprocal temperature above 125 °C. The activation energy obtained from the linear slope shown by the dotted line is 1.06 ± 0.02 eV, indicating that the decay reaction of the nickel center in the SCR is different from that in the bulk.
Fig. 6. Arrhenius plots of the dissociation time constants of the nickel center for the EL-free and EL-formed samples. The arrow at 100 °C for the EL-formed sample indicates that τ is larger than 106 s.
Download figure:
Standard image High-resolution image4. Discussion
4.1. Transformation reactions of nickel-related species
A possible explanation of the annealing behavior of the nickel-related species observed in the different types of sample is discussed. Since H(165) and H(260) are hydrogenated substitutional nickel complexes (NisHx, x: integers) according to the analyses by Shiraishi et al.20) and Scheffler et al.,23) there is no doubt that hydrogen is included in the as-diffused samples. Because the wafer surfaces were covered or bonded with hydrogen after their immersion in the HF aqueous solution as stated in Experimental Methods, it is inferred that a considerable amount of hydrogen has been introduced into the bulk during the cleaning and diffusion processes of the wafers. A part of the neutral hydrogen is known to reside at the tetrahedral (T) interstitial site and diffuses between adjacent T sites with a small diffusion barrier (<0.2 eV), while most of the hydrogen resides at the bond center (BC) site in p-type Si.30–32) Since all the contaminating nickel dissolves in the bulk and the total fraction of the measured nickel-related species of the dissolved nickel is small (<10%) as described earlier, a considerable fraction of the diffused nickel is assumed to be accumulated as Nii in the bulk at room temperature. Nii has no level in the bandgap and diffuses as a neutral species from a T site to adjacent T sites with the diffusion barrier of 0.21 eV estimated by first-principles calculation33,34) (0.15 eV measured in a recent experiment35)). Accordingly, hydrogen and Nii are the main impurities that react with the nickel center and other nickel-related species during annealing. Since the Fermi level of the sample in this study is low (Ev + 0.24 eV) at room temperature estimated from the ordinary semiconductor theory,36) a considerable amount of the nickel center is assumed to be positively charged (Nis+) in the EL-free samples.
First, the decay of the nickel center in the EL-free samples is considered. By comparing the low activation energy of the decay of the center obtained in this study with the binding energy of the center (2.68–2.60 eV) estimated by first-principles calculation,33) the direct dissociation of the center (Nis → Nii + V, V: vacancy) is difficult. The center is assumed to decay owing to the actions of hydrogen and Nii by annealing as

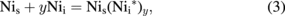
in which y takes integers and Nii* denotes the bonding state of Nii, although its actual structure is not specified in this study. Since the increase in the total concentration of H(165) and H(260) from room temperature to 150 °C is comparable to the decrease in the concentration of the nickel center between these temperatures (Fig. 3), the decay of the center is assumed to be caused by its hydrogenation [Eq. (2)]. The decay activation energy of the nickel center obtained in this study (0.26 eV) is accordingly attributed to the required energy to bond free hydrogen to the nickel center. Since H(165) and H(260) began to decay at 200 °C with the continuous decay of the nickel center, its hydrogenation and the successive production of new species to annihilate NisHx are considered to occur. H(125/200) can be assumed to be one of these new products. Since the energy levels of this species do not correspond to any known levels of the hydrogenated species,20,23) the former species should form by the bonding of Nii to NisHx. The newly produced species are then formally expressed as NisHx(Nii*)z, in which z takes several integers. When H(125/200) grows to electrically inactive products, such as precipitates, by the further bonding of Nii at higher temperatures, the disappearance of all the nickel-related species after annealing at or above 350 °C is explained. Since the nickel center is considered to be immobile at the examined annealing temperatures, the formation of complexes containing several Nis seems difficult.
Next, the concentration changes in the nickel-related species in the EL-formed samples are considered. Note that all the nickel-related species in the SCR tend to become neutral owing to the bending of the electronic bands of the Schottky diode37) and then the nickel center easily forms complexes with Nii at low temperatures. Since the increase in the concentration of H(165) from room temperature to 135 °C is as small as approximately 1/5 of the decrease in the concentration of the nickel center between these temperatures (Fig. 5), the major part of the center decays owing to the reaction (or reactions) other than that given by Eq. (2). When Nis(Nii*)y is assumed to be electrically inactive for small values of y, the decay of the center is mostly attributed to the reaction given by Eq. (3). The activation energy obtained in this study is therefore attributed to the required energy to bond free Nii to the center. The formation of a small amount of H(125/200) between 115 and 140 °C is suggested to be caused by the small decrease in the H(260) concentration, i.e., the former species is the product of the latter species and Nii. H(165) is considered to become electrically inactive after bonding Nii. Since H(150) appears only in the EL-formed samples, the species is produced independently of the hydrogen-containing species. When the electrically inactive Nis(Nii*)y becomes electrically active by further bonding several Nii to the species, H(150) can be regarded as this product. All the nickel-related species are considered to precipitate by the further bonding of Nii at or above 170 °C.
4.2. Reactions of nickel-related species at room temperature
The increase in the concentration of H(260) without a corresponding decrease in the concentration of the nickel center in the EL-formed samples (Fig. 1) cannot be explained by the reaction given by Eq. (2). A probable explanation is that the formation of Nis and its subsequent hydrogenation easily take place at room temperature in the SCR through the action of a special impurity complex (or complexes), which contains V (or V's). Approximately 1016/cm3 carbon and 1015/cm3 oxygen are generally included in as-grown FZ silicon crystals.38) Since carbon is tightly bonded at the lattice site with a smaller volume than a silicon atom,39) it is generally accepted that the formation of a carbon–V complex is difficult (a carbon-self-interstitial complex is easy to form40)). Oxygen (O)–V complexes, such as OV and O2V, are estimated to accumulate at a maximum concentration of approximately 1012/cm3 in commercial Czochralski (CZ) crystals (oxygen concentration ∼1018/cm3) at room temperature.40) Since the concentrations of O–V complexes are estimated to be several orders lower in FZ crystals than in CZ crystals owing to the lower concentrations of oxygen in the former crystals, the concentrations of the complexes are insufficient to explain the measured concentration of the nickel center (∼1013/cm3). Accordingly, it is also difficult to assume that O–V complexes take part in the formation of Nis in the studied samples.
As reported in a preceding paper,24) the inclusion of approximately 1013/cm3 nitrogen complex was always observed in the sample used in this study. Several nitrogen defects are considered to contain a vacancy,40–45) which assists in the formation of Nis from Nii, as suggested in previous papers.23,24) Since the diffusion barrier of Nii is very low,33–35) the diffusivity of this species is estimated to have a sufficient value to have the species reach a nitrogen complex at room temperature. The neutralization of all the species including the nitrogen defects in the SCR can facilitate the transformation reaction of Nii and hydrogenation, although the structure of the relevant defect and the path to transform Nii to Nis are not determined yet.
5. Conclusions
The concentration changes in the nickel center and its related species by annealing EL-formed and EL-free samples diffused with dilute nickel were measured by DLTS. In the EL-free samples, the center began to decay at low temperatures of approximately 100 °C by forming hydrogenated nickel species with the activation energy of 0.26 ± 0.1 eV. After annealing at higher temperatures, these species evolved to extended products, such as H(125/200) presumably by the bonding of Nii. All the nickel-related species disappeared to form precipitates without recovering the nickel center after further annealing at temperatures lower than 400 °C. In the EL-formed samples, the center decayed with the activation energy of 1.06 ± 0.02 eV, presumably owing to the bonding of a few Nii to form electrically inactive complexes Nis(Nii*)y. In addition to H(125/200), a new complex H(150), suggested to be one of the extended products of Nis(Nii*)y, appeared by further bonding of Nii. All the nickel-related species above disappeared to evolve into precipitates by the further bonding of Nii within temperatures lower than 175 °C. The easy precipitation at very low temperatures in the EL-formed samples is attributed to the electric neutralization of all the nickel-related species in the SCR. The reason for the evolution of the hydrogenated nickel center during the storage at room temperature is suggested to be the special action of a nitrogen defect (or defects) to transform Nii to Nis and the subsequent bonding of mobile hydrogen. The structure of the nitrogen defect and the path to transform Nii to Nis remain to be elucidated in the future.
Acknowledgements
The authors would like to thank the members of Dr. N. J. Kawai's group of Renesas Technology Corporation for preparing a part of the nickel-contaminated samples.