Abstract
Implementation of shape-memory polymer (SMP) sheet-based microvalves into plastic-based microfluidic devices has been studied toward the use in disposable and mass producible micro total analysis devices. Poly(ε-caprolactone) (PCL) and poly(methyl methacrylate-co-styrene) (MS) were used as SMP and main substrate materials, respectively. Bonding between PCL sheets and MS plates was the critical issue in the practical implementation. We found the pristine PCL sheet has relatively rough surface with Ra of 85.14 nm, which is the cause of poor bonding. Hence, by introducing the post-anneal treatment with sandwiched between two flat glass plates, the PCL surface could be smoothed to Ra of 12.50 nm, and tight bonding could be obtained. Consequently, microfluidic devices consisting of plastic/PCL/plastic layers were successfully fabricated and therein the actuation of SMP valves without any leakage was demonstrated. The present technology is expected to be applicable to disposable microfluidic devices as required for point-of-care testing.
Export citation and abstract BibTeX RIS
1. Introduction
In the last few decades, significant progress has been seen in the field of micro total analysis system (µTAS) and/or lab-on-a-chip (LOC). Chip-format devices that are integrated with micrometer-sized reactors and fluidic channels on a single substrate can integrate a total sequence of lab processes to perform chemical/biochemical analysis.1–6) One of the most promising applications of such technologies is a compact and disposable diagnostic device for point-of-care testing (POCT): testing at or near the site of patient care wherever that medical care is needed.7,8) A microvalve is an essential element to construct and control the complicated microfluidic network. So far, various types of microvalves have been designed and developed with several action principles that include electromagnetic, electrostatic, piezoelectric, bimetallic, pneumatic, and stimuli-responsive polymer actuation.9–18) However, there still remain challenging issues in the development of practical microvalves that should satisfy various requirements such as robust and reliable actuation, high withstanding pressure, short response time, mass producibility, low cost, and compactness including the external control and driving system.
To fulfill the requirements of a microfluidic valve for practical use in disposable integrated microfluidic devices, we proposed and developed a shape-memory polymer (SMP) microvalve that uses the solid–solid phase transition of poly(ε-caprolactone) (PCL).19,20) The SMP microvalves connect or disconnect two adjacent microchannels formed on a separate substrate by the thermally triggered recovery of the PCL surface structure that was memorized in advance. Since the prestored energy in the PCL is used for valve actuation, peripheral systems for operating SMP microvalves can be made compact, unlike the case of pneumatic microvalves, which usually require relatively large and elaborate equipment or facilities to supply and control the compressed air. The response time and withstanding pressure of SMP microvalves were evaluated to be 300–500 ms and (1.1 ± 0.3) × 102 kPa, respectively, and are sufficient values for practical use. Such performance of the SMP microvalves is considered to be dominated by material properties of PCL concerning thermal and mechanical characteristics. In the previous reports, poly(dimethylsiloxane) (PDMS) was used as a substrate material of microfluidic channel, and tight bonding between PDMS and PCL sheets was obtained easily by oxygen plasma treatment on their surfaces.
When considering the practical use of microfluidic devices for a POCT purpose, the use of plastics as the main substrate material is desirable to satisfy the requirements of low cost, disposability, and mass producibility.21–24) Then, we attempted to implement our SPM microvalve technology into plastic-based microfluidic devices instead of PDMS-based ones by the same method as previously reported. However, unlike the case of PDMS, it turned out that we could not obtain good bonding between PCL sheets and plastic substrates. The most likely cause of this problem was speculated to be the rigidity of the plastic substrate and the rough surface of a PCL sheet.
In this paper, to implement PCL sheet-based SMP microvalves into plastic-based microfluidic devices, we attempted the modification of the surface roughness of PCL sheets by post-anneal treatment and explored high-strength bonding capability with the plastic substrate. Furthermore, we report the fabrication of simple microfluidic devices consisting of plastic/PCL/plastic layers and show the feasibility of implementation of SMP microvalves into plastic-based microfluidic devices.
2. Experimental methods
2.1. Fabrication of the PCL sheet
The fabrication process of the PCL sheet is shown in Figs. 1(a)–1(g). First, PCL macromonomer 4b50-m (0.8 g) and benzoyl peroxide (BPOs; Wako Pure Chemical Industries, 0.08 g) as the initiating reagent were mixed and dissolved in xylene (1.2 g) as the solvent (a). PCL macromonomer 4b50-m was synthesized following the method described in the literature.25) Then, the mixture was poured into a mold made of glass (b), and was cured in an oven (TGK VT210P) at 80 °C at atmospheric pressure for 3 h to cross-link the polymer (c). Next, the cross-linked polymer sheet was removed from the mold, and the residual solvent was exchanged by soaking the sheet into the mixture of acetone (50 vol %) and methanol (50 vol %) (d). This process is carried out for the following two reasons: (i) to remove the unreacted compounds, and (ii) to prevent the anisotropic contraction. Although the PCL sheet soaked with pure acetone (good solvent) tends to contract heterogeneously during the following drying step, addition of methanol (poor solvent) allows the isotropic contraction. Finally, the solvents were dried in a vacuum at room temperature for 12 h (e). During the drying process, the PCL sheet was sandwiched between two poly(tetrafluoroethylene) (PTFE) sheets and fixed by two clips to prevent the curling. As the PCL sheet after the drying process maybe somewhat strained because of the load of the clips, the strain of the sheet was removed by the subsequent post-anneal treatment. The fabricated sheet is shown in (f). The thickness and size of the sheet was approximately 200 µm and 30 × 30 mm2, respectively. Since the PCL is the semicrystalline polymer, it looks opaque at room temperature.
Fig. 1. Fabrication process of the PCL sheet [(a)–(g)] and bonding process of PCL sheet with PDMS/MS [(h) and (i)]. (a) 4b50-m, BPO and xylene were mixed. (b, c) The mixture was poured into a glass mold, and was cured in an oven at 80 °C for 3 h. (d) The solvent was exchanged by soaking the sheet into the mixture of acetone (50 vol %) and methanol (50 vol %). (e) The solvents were dried in a vacuum at room temperature for 12 h. PTFE sheets were used to prevent the PCL sheet from curling. (f) Photo of the fabricated PCL sheet. It is opaque in room temperature. (g) The PCL surface roughness was decreased by post-anneal treatment in the air or with a flat glass plate on the PCL. (h) The surfaces of the PCL sheet and PDMS/MS plate were exposed to O2 plasma (25 Pa, 100 W) for 10 s before bonding. (i) PDMS and MS plates were press-bonded to the PCL sheet at room temperature for 30 min with the applied pressure of 0.05 and 5 MPa, respectively.
Download figure:
Standard image High-resolution imageSurface roughness of the fabricated PCL sheet was decreased by additionally conducting the post-anneal treatment, that is, heating the PCL sheet at 80 °C (higher than the melting temperature Tm of PCL) on a hot plate, keeping it for 1 min, and gradual cooling down to room temperature in the air. Moreover, much smoother surface could be obtained by placing a flat glass plate on the PCL during the post-anneal treatment. PCL sheets subjected to the post-anneal treatment without and with placing the glass plate were denoted as #1 and #2, respectively, as shown in Fig. 1(g).
2.2. AFM observation of PCL sheet surfaces
The surfaces of prepared PCL sheets were observed using an atomic force microscope (AFM; JPK Instruments NanoWizard II). The cantilever used in this study was an Olympus BioLever mini with the stiffness of 0.1 N/m and the resonance frequency of 110 kHz. The field of 30 × 30 µm2 was measured using contact mode with scanning speed of 60 µm/s. The root mean square roughness (Rrms), the arithmetic mean roughness (Ra) and the maximum roughness (Rt) were measured in three different points for each sample.
2.3. Bonding of PCL/PDMS and PCL/MS
Bonding capability of prepared PCL sheets (#1 and #2) was examined for PDMS and MS [poly(methyl methacrylate-co-styrene); JSP]. MS is a plastic material that exhibits the excellent properties of both poly(methyl methacrylate) and polystyrene, namely, high transparency for visible light, high surface hardness, high workability, low water absorption, and so on. The surface of MS plate was very smooth with the Ra of 0.54 nm. PDMS was prepared by mixing a PDMS prepolymer (Dow Corning Sylgard 184) and cross-linker (catalyst of Silpot 184) at the volume ratio of  , and curing at 85 °C for 2 h. The bonding procedure is shown in Figs. 1(h) and 1(i). First, both materials to be bonded were exposed to oxygen plasma (25 Pa, 100 W) for 10 s. Next, they were press-bonded at room temperature for 30 min. The pressure applied for PCL/PDMS and PCL/MS bonding was 0.05 and 5 MPa, respectively. It should be noted that the pressure used for PCL/PDMS bonding was much lower than that used for PCL/MS bonding because of the high deformability of PDMS.
, and curing at 85 °C for 2 h. The bonding procedure is shown in Figs. 1(h) and 1(i). First, both materials to be bonded were exposed to oxygen plasma (25 Pa, 100 W) for 10 s. Next, they were press-bonded at room temperature for 30 min. The pressure applied for PCL/PDMS and PCL/MS bonding was 0.05 and 5 MPa, respectively. It should be noted that the pressure used for PCL/PDMS bonding was much lower than that used for PCL/MS bonding because of the high deformability of PDMS.
2.4. Cross-sectional observation of bonded PCL/PDMS
Cross sections of bonded PCL/PDMS samples were observed using a scanning electron microscope (SEM; JEOL JSM-6400). The bonded samples were cut carefully using a surgical scalpel. To avoid charging during the SEM observation, an osmium thin film was coated over the cross sections and low-energy electron beam of 1.0 kV was used.
2.5. Implementation of SMP microvalves into the plastic-based microfluidic device
A plastic-based microfluidic device integrated with microvalves as shown in Fig. 2 was fabricated. A PCL sheet was sandwiched between two MS plates, and microvalves were integrated into the microchannel. The fabrication procedure is almost the same with the method described in the literature,19) except for adding the post-anneal treatment of PCL for smoothing its surface. The smoothed PCL sheet and MS plate were treated in an oxygen plasma, and then press-bonded together. Next, concave structures that are necessary for constructing normally-open SMP valves were formed on the top surface of the attached PCL sheet using hot embossing machine (Nanonics NanoimPro Type 210). The mold used here had five round protrusions with height of 45 µm and diameter of 300 µm, which were microfabricated on a glass plate by photolithography using a dry film photoresist TMMF2045 (Tokyo Ohka Kogyo). The pressure of 1 MPa was applied at 80 °C (>Tm) for 3 min, and the pressure was removed after the PCL sheet was cooled below 30 °C. Finally, another MS plate, on which a four-turn serpentine-shaped fluidic channel with a rectangular cross section of 250 µm in width and 100 µm in depth and inlet/outlet holes with 2 mm diameter was engraved using a computer numerical control (CNC) milling machine (Roland DG MDX-540), was bonded onto the PCL sheet after the oxygen plasma treatment. To check whether there is any leakage due to poor bonding, a sulforhodamine B solution was introduced into the fabricated microfluidic device. The actuation of microvalves was examined by heating the device up to 80 °C on a hot plate.
Fig. 2. Schematic diagrams of the plastic-based microfluidic device integrated with SMP microvalves. (a) Single view drawing of the device and a microvalve. (b) Cross section of the normally-open SMP microvalve. The PCL sheet with hot-embossed concave features on its surface is sandwiched between two MS substrates. By heating the PCL sheet, the prestored elastic energy is released, resulting in the shape recovery to its original flat surface. The microvalve actuates from open state to close state.
Download figure:
Standard image High-resolution image3. Results and discussion
Figure 3 shows AFM surface profiles of three types of PCL sheets prepared in this study. Also, Rrms, Ra, and Rt of each sample are summarized in Table I. These roughness values are the average of those measured at three different points. The sheet before post-anneal treatment was the roughest, with Rrms of 104.7 nm and Ra of 85.14 nm. The rough surface is attributed to the semicrystalline nature of the PCL.26,27) By conducting the post-anneal treatment in the air, Rrms and Ra were decreased a little to 63.73 and 50.77 nm, respectively (#1). Moreover, when a flat glass plate was placed on the PCL sheet during the post-anneal treatment, significant roughness decrease was observed; Rrms and Ra were 15.32 and 12.50 nm, respectively (#2). The result is considered to be due to the transfer of the flat structure of the glass plate during the cooling.
Fig. 3. AFM surface profiles of PCL sheets. #1 and #2 show PCL sheets after post-anneal treatment in the air and with a flat glass plate on the PCL, respectively.
Download figure:
Standard image High-resolution imageTable I. Surface roughness of the PCL sheets used in this study (in nm).
| Before post-anneal | #1 | #2 | |
|---|---|---|---|
| Rrms | 104.7 | 63.73 | 15.32 |
| Ra | 85.14 | 50.77 | 12.50 |
| Rt | 879.7 | 574.9 | 97.34 |
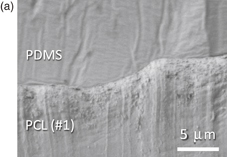
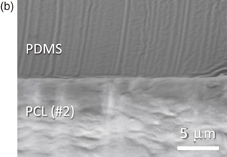
Next, we attempted to bond the PCL sheets to PDMS and MS plates. As a result, both PCL sheets of #1 and #2 could bond with PDMS. It is known that Si–OH groups are generated on the PDMS surface by the oxygen plasma treatment, which can form covalent bonding with Si–OH groups on glass and COOH/COH groups on polymer materials.28–30) However, the surface should be in contact with each other to form the chemical bonding. The reason that even the PCL sheet with relatively large surface roughness (#1) could bond to PDMS can be confirmed from the SEM cross-sectional photos of PCL/PDMS as shown in Fig. 4. Namely, the surface of smooth PCL sheet (#2) is in contact with PDMS undoubtedly (b). In addition, the surface of the relatively rough PCL (#1) is also in contact with PDMS because of the deformation of PDMS (a). Since PDMS is a soft material with young modulus of 2.5 MPa, it can deform along the PCL surface, resulting in good bonding.
Download figure:
Standard image High-resolution imageFig. 4. Cross-sectional SEM photos of bonded PCL/PDMS. Both PCL sheets of #1 and #2 are in contact with PDMS. Since PDMS is a soft material with young modulus of 2.5 MPa, it can deform along the PCL surface, resulting in good bonding even with relatively rough surface of PCL (#1).
Download figure:
Standard image High-resolution imageIn the case of PCL bonding to MS, only the smooth PCL sheet of #2, which was obtained by the post-anneal treatment with the glass plate, could bond tightly with MS. The bonding could endure the applied pressure of 1.0 × 102 kPa. In contrast, the rough PCL sheet of #1 could not form bonding with MS at all. This fact means that the surface roughness of #1, though decreased by post-anneal treatment to some extent, was still not enough for bonding. Young's modulus of MS is 72.5 MPa and is much stiffer than that of PDMS. Therefore, the rough PCL surface critically obstacles the direct contact with the surface of MS, which is essential for forming chemical bonds.
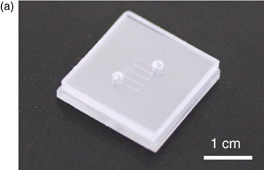
Since it is now possible to fabricate the surface-smoothed PCL sheet bondable to MS plate, the SMP sheet-based microvalves were integrated into plastic-based microfluidic device. Figure 5(a) shows the photo of the prototype device with five microvalves. Figure 5(b) shows fluorescence microscope photographs to demonstrate the operation of SMP microvalves fabricated using PCL sheets of #1 (left) and #2 (right). Here microchannels and microvalves were filled with sulforhodamine B solution for the visual convenience. The photos in the upper and lower rows show the microvalves in open state and closed state, respectively. Thus, leakage was observed for the device fabricated with PCL sheet #1. On the other hand, there was no leakage in the device fabricated with the sheet #2, and all the five microvalves could turn to the close state without any leakage.
Download figure:
Standard image High-resolution imageFig. 5. (a) A photo of the fabricated plastic-based microfluidic device integrated with SMP microvalves. The PCL sheet smoothed by post-anneal treatment with a glass plate on it was sandwiched between two MS plates. (b) Fluorescence microscope images of the SMP microvalves integrated into the device made of PCL sheet (#1) (left) and (#2) (right). Successful actuation of a microvalve from open to close state without any leakage was observed for the case of #2.
Download figure:
Standard image High-resolution imageIn this study, the whole device was heated to trigger the valve actuation. However, it is possible to form the microheater on MS plate using semiconductor microfabrication technique such as sputtering and photolithography. Thus, microvalves can be actuated by local heating of PCL sheet. Furthermore, it is also possible to fabricate the microheater using cheaper method such as screen printing. Therefore, it is suggested that the SMP microvalve can be integrated into the plastic-based microfluidic device with small peripheral equipment including actuation system.
4. Conclusions
Implementation of PCL sheet-based SMP microvalves into MS plastic-based microfluidic devices was studied. The PCL sheet can be easily implemented in PDMS-based microfluidic devices just by oxygen plasma activation of bonding surfaces. However, it is not straightforward to obtain tight bonding between PCL sheets and MS plastic substrates. The difficulty is mostly originated from the rough surface of the PCL sheet prepared by casting from organic-based solvents. Indeed the Ra and Rt of the PCL sheet before post-anneal treatment showed relatively large values of approximately 85 and 880 nm, respectively. Some approaches of the post-anneal treatment were attempted to reduce the surface roughness, and it was found that the much smoother surface with Ra ∼ 12 nm and Rt ∼ 100 nm can be obtained by placing a flat glass plate on the PCL sheet during the post-anneal treatment. Hence, bonding between PCL and MS, and consequently the implementation of PCL sheet-based SMP microvalves into plastic-based microfluidic devices were successfully achieved. Plastic-based microfluidic device is desirable to satisfy the requirements of low cost, disposability, and mass producibility. Therefore, the present technology is expected to be useful when applying the microfluidic device technology for the medical purpose such as POCT in the near future.
Acknowledgment
This research was supported by the COI STREAM (Center of Innovation Science and Technology based Radical Innovation and Entrepreneurship Program) from the Japan Science and Technology Agency, JST.