Abstract
The highest efficiency of 24.4% for the solar-to-hydrogen (STH) energy conversion was obtained in an outdoor field test by combining concentrator photovoltaic (CPV) modules with InGaP/GaAs/Ge three-junction cells and polymer-electrolyte electrochemical (EC) cells. The high efficiency was obtained by using the high-efficiency CPV modules (∼31% under the present operation conditions) and the direct connection between the CPV modules and the EC cells with an almost optimized number of elements in series. The STH efficiency bottleneck was clarified to be the efficiency of the CPV modules, the over-potential of the EC cells, and matching of the operation point to the maximal-power point of the CPV modules.
Export citation and abstract BibTeX RIS
Conversion of solar energy to the free energy of chemical substances is highly demanded because it allows us to level temporal fluctuations of solar irradiance by using long-term energy storage and to overcome spatially non-uniform irradiance by using long-distance energy transport. The simplest and the most widely explored chemical substance for the energy storage is hydrogen, which can be generated by water splitting. The free energy of hydrogen, which is obtained by converting from the solar energy, can be used for generating electricity by using a fuel cell. Hydrogen transport is also possible by using chemical carriers such as methylcyclohexane1,2) and ammonia.3) In addition, hydrogen generated by sunlight can reduce CO2 into methane, which is a commonly used transportable fuel.4,5) In order for hydrogen to play a major role as an artificial, sunlight-derived, fuel, methods are needed for high-efficiency and low-cost production of hydrogen from water by sunlight irradiation.
Before describing the details on the world record efficiency of 24.4% for solar-to-hydrogen (STH) energy conversion, we will briefly review the history of research on improving the STH efficiency. In order to split water into hydrogen and oxygen, researchers have studied both photo-electrochemical water splitting and the combination of photovoltaic (PV) and electrochemical (EC) cells. In 1972, Honda and Fujishima6) proposed to use photo-electrochemical water splitting. The approach of combining PV and EC cells can be traced back to 1977.7) Following this pioneering work, the STH efficiency has been steadily improved8) and the highest up to date STH efficiency has been 18.3%, even though theoretical studies predict the possibility of obtaining values above 25%.9–12)
Following the work by Honda and Fujishima, substantial efforts have been made to develop a material with adequate alignment between the band edges and the redox potentials of two key reactions: hydrogen and oxygen evolution. However, the wide band gaps of these component materials, which necessitate UV illumination, have limited the STH efficiency by one-absorber approach to values on the order of a few percent.
A dramatic STH improvement was achieved by using tandem semiconductor electrodes. Khaselev and Turner employed an InGaP/GaAs two-junction as a photo-cathode for hydrogen evolution reaction, which is a well-known tandem structure for PV cells, except that the top InGaP contains a p-layer alone.13) The electrode at the p-GaAs surface was connected to a Pt electrode for oxygen evolution, and the STH efficiency was 12.4% under illumination with an intensity equivalent to 11 suns. This is the highest efficiency achieved by using semiconductor photo-electrodes, in spite of a variety of materials and configurations that have been tested so far.
The approach that uses semiconductor photo-electrodes is challenging because it requires: 1) substantial sunlight absorption, 2) appropriate band alignment to the redox potentials, and 3) material durability in both acidic and alkaline electrolytes. Thus, an alternative approach, combining PV devices at the outside of an electrolyte and electrodes, has been proposed. A device integrating an AlxGa1−xAs/Si PV cell and an electrochemical cell using both a RuO2 anode and a Pt-black cathode with an HClO4 aqueous electrolyte achieved a STH efficiency of 18.3% under AM0 illumination.14) Similar to the PV modules, sunlight concentration optics was introduced and a concentrator (500 sun) PV + EC system was developed: a GaxIn1−xP/GayIn1−yAs double-junction PV cell was incorporated into a receiver module that contained an EC cell that in turn consisted of a Pt cathode, an Ir anode, and a polymer electrolyte.15) The system achieved a STH efficiency of 15.1%, which is a recalculated value, based on the hydrogen free energy of 1.23 V.
In the above, a non-conventional two-junction structure was used for obtaining good matching between the maximal-power voltage of the PV cell and the operation voltage of the EC cell. Such a circuit connection is of particular importance for maximizing the STH efficiency and it can be more easily optimized by simply wiring PV and EC modules, provided that the wiring ohmic heating is negligible. Thus, significant research has been performed on PV–EC direct connection systems.16–28) In this approach, the key factor underlying the high STH efficiency is the use of high-efficiency PV modules. A wired combination of commercially available InGaP/GaAs/Ge triple-junction PV cells and polymer electrolyte EC cells (PEEC) achieved a STH efficiency of 15.1% by using a series connection of two PV and three EC cells, under a 10-sun-equivalent solar simulator.29) Subsequently, a field test of connecting the EC cells and the concentrator PV (CPV) modules with ×800 sunlight concentration on a sun tracker yielded a STH efficiency of 17.1% in Miyazaki, Japan.30)
After the PV–EC circuit connection optimization, there are two remaining factors limiting the STH efficiency: the efficiencies of the CPV modules and the EC cells. The CPV module efficiency in the previous work30) was as small as 23%. Here, we employed improved CPV modules, with efficiencies of ∼31%, and obtained a STH efficiency of 24.4%, which is the highest value up to date.
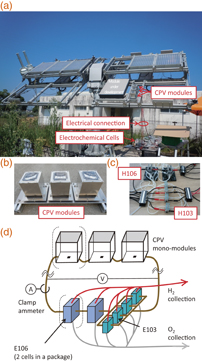
Hydrogen production by connecting CPV modules and EC cells was attempted in the outdoor test field at Miyazaki University, Japan, as shown in Fig. 1(a). A CPV mono-module [Sumitomo Electric Industries; Fig. 1(b)] had a 57 mm2 light-receiving area, and a 2.5 mm2 InGaP/GaAs/Ge three-junction cell was placed at the sunlight focus. Two types of polymer-electrolyte EC cells, E103 and E106 [h-tec Education; Fig. 1(c)] were connected to the CPV modules by copper wiring. The EC cells contained platinum-loaded carbon paper electrodes with a proton exchange polymer membrane, and pure water was fed into the cells. Here, three configurations of PV–EC direct connections were examined for clarifying the impact of matching the operation point, as will be described later. As shown in Fig. 1(d), two or three CPV modules were connected to the EC cells, with the number of cells connected in series varying from three to five. A package of E106 contains two EC cells in series, each with the membrane area of 16 cm2, while a package of E103 contains one EC cell with the area of 4 cm2. In the series of experiments, therefore, a set of four E103s in parallel was treated as being equivalent to one EC cell of E106, because their overall membrane areas were the same; a setup of five (three) EC cells in series contained two (one) E106 packages in series and a set of four-parallel E103 as an additional cell in series, as shown in Fig. 1(d).
Fig. 1. (a) A view of the experimental setup used in the outdoor test field at Miyazaki University, Japan. (b) A more detailed view of the CPV modules. (c) A more detailed view of the EC cells with peripheral wiring. (d) Schematic of the direct connection between the CPV modules and the EC cells. In some configurations, the parenthesized module and cell were not included in the circuit. Table I lists the number of CPV modules and EC cells used in different configurations.
Download figure:
Standard image High-resolution imageHere, we define the series connection ratio S representing the number of the PV and the EC cells in series: S = (the number of the EC cells in series)/(the number of the PV modules in series). For all connections, the volume of produced hydrogen linearly increased with time, as shown in Fig. 2. The production rate of hydrogen depended on the number of the EC cells, yet the STH efficiency depended on the amount of solar energy that was used to obtain a certain hydrogen production rate, as will be discussed in detail below. The volume of oxygen produced was also measured, but the collection of oxygen from the EC cells was not as stable as in the case of hydrogen, because oxygen bubbles tended to adhere to the cell walls. Nevertheless, the averaged volumetric production rate of oxygen was half of the hydrogen production rate, indicating that hydrogen was indeed produced by water splitting and no substantial gas leakage existed.
Fig. 2. Temporal evolution of the amount of hydrogen collected from the EC cells. The number S = x/y represents the series connection configuration in Table I. The open symbols are the experimental data and the dotted lines are the predicted hydrogen generation, based on the current to the EC cells, Iop. The gap between the predicted and data points corresponds to the Faraday efficiency ηF. For the series S = 5/3 and 3/3, the value of ηF is close to unity and the dotted lines overlap with the symbols.
Download figure:
Standard image High-resolution imageThe STH efficiency is defined as the ratio of the free energy stored in hydrogen molecules (ΔG° = 237 kJ mol−1) to the energy of sunlight used to generate hydrogen:
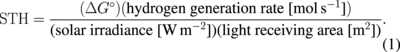
Solar irradiance was measured on site by using a standard setup (EKO Instruments). Here, direct normal irradiance was used, because CPV modules utilize only direct irradiance. The rate of hydrogen generation was calculated from the data in Fig. 2. The STH efficiency depended on the connection configuration, and the maximal value was 24.4%, as shown in the last column of Table I. A different experiment, using the same connection, yielded a value of 24.3%, confirming the measurement reliability. A large error existed for the readout of gas volume in a hydrogen-storage cylinder, resulting in the STH efficiency error range as in Table I.
Table I. Number of the CPV modules and the EC cells connected in series, for different experimental configurations. For each configuration, the experiment was performed twice for checking reproducibility. The values of direct normal irradiance (DNI), the rate of hydrogen collection, and the resultant STH efficiency are summarized. The elementary efficiencies are defined in the text.
| Series connection ratio | Symbol in Figs. 2–4 | Number of cells in series | DNI (W/m2) | H2 collection rate (µmol/s) | Elementary efficiency | STH efficiency | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| PV | EC | ηPV 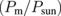 |
ηop 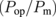 |
ηEC 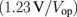 |
ηF 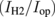 |
|||||
| S = 3/2 | □ | 2 | 3 | 782.1 | 4.79 | 0.310 | 0.96 | 0.809 | 0.93 | 0.223 ± 0.003 |
| S = 3/2 | NA | 2 | 3 | 780.3 | 4.94 | 0.310 | 0.92 | 0.808 | 1.00 | 0.231 ± 0.003 |
| S = 5/3 | △ | 3 | 5 | 766.8 | 7.66 | 0.305 | 1.00 | 0.814 | 0.98 | 0.243 ± 0.002 |
| S = 5/3 | NA | 3 | 5 | 761.2 | 7.63 | 0.312 | 1.09 | 0.817 | 0.87 | 0.244 ± 0.002 |
| S = 3/3 | ○ | 3 | 3 | 750.5 | 4.53 | 0.305 | 0.63 | 0.814 | 0.94 | 0.147 ± 0.002 |
| S = 3/3 | NA | 3 | 3 | 723.0 | 4.42 | 0.314 | 0.61 | 0.813 | 0.96 | 0.149 ± 0.002 |
Let us now discuss the process of the STH efficiency maximization by directly connecting the CPV modules and the EC cells. Using the relationship:

where F (= 96500 C mol−1) is the Faraday constant and 2 is the number of electrons necessary for converting two H+ ions into a hydrogen molecule, the STH efficiency in Eq. (1) can be represented as the product of four elementary efficiencies:
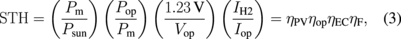
where Psun is the sunlight power received by the PV modules, Pm is the maximal power output of the PV modules, and Pop is the actual power applied to a set of the EC cells. Pop is the product of the operation voltage Vop and the operation current Iop, both of which were measured as shown in Fig. 1(d). IH2 is the total charge per unit time received by the hydrogen molecules in the EC cells. The elementary efficiencies can be intuitively interpreted as follows: ηPV is the PV module efficiency; ηop is the matching efficiency between the maximal-power point of the PV modules and the actual operation point  ; ηEC is the energy loss owing to the over-potential of the EC cells; ηF is the Faraday efficiency, capturing the quantum efficiency of the electrons transfer from the electrode to the protons in an electrolyte. These values are summarized in Table I for the three different connection configurations. For each configuration, Table I lists the results for two experimental runs, for checking reproducibility. Note that, in some measurements, the Iop values suffer from the zero point drift, resulting in a large ηop and a small ηF. Nevertheless, the product of these two efficiencies remains constant, because they contain Iop in the numerator and the denominator, respectively.
; ηEC is the energy loss owing to the over-potential of the EC cells; ηF is the Faraday efficiency, capturing the quantum efficiency of the electrons transfer from the electrode to the protons in an electrolyte. These values are summarized in Table I for the three different connection configurations. For each configuration, Table I lists the results for two experimental runs, for checking reproducibility. Note that, in some measurements, the Iop values suffer from the zero point drift, resulting in a large ηop and a small ηF. Nevertheless, the product of these two efficiencies remains constant, because they contain Iop in the numerator and the denominator, respectively.
It is apparent that ηPV and ηEC are independent of the connection mode; these are the efficiencies determined by the PV modules and the EC cells, respectively, with a moderate dependence on the operation temperature. It is, of course, necessary to use high-efficiency PV modules and EC cells with small over-potential, for obtaining the maximal STH; here, the major reason for obtaining the highest STH so far was the high efficiency of the CPV modules. The over-potential of the EC cells was not an issue in these experiments. A typical upper limit on ηEC is the ratio of the standard free energy to the standard enthalpy of hydrogen generation from water. The value at 298 K is 0.83, and the values of ηEC quite close to this value were obtained. This was owing to a small current density under the experimental conditions (∼0.02 A cm−2). For practical applications, because a small current density in the EC cells necessitates large membrane area and increases the associated cost, a typical current density is ∼1 A cm−2. Such a large current density tends to increase the operation voltage of the EC cells, reducing ηEC. Further development of EC cells is necessary for reducing the internal resistance, i.e., the slope of the I–V curve. The value of ηF is normally close to unity when conventional EC cells are employed for water splitting, but we have observed that a very low current density tends to reduce ηF (data not shown). This is probably owing to the existence of parasitic reactions at the electrodes that are not negligible under such a small current density.29)
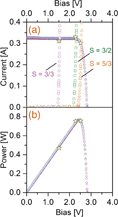
The operation-point matching efficiency ηop is of primary importance for the STH efficiency maximization with given PV modules and EC cells. As shown in Table I, the ηop is close to unity for the S = 3/2 and 5/3 configurations, and it is far below unity for the S = 3/3 configuration. The difference becomes clear when we examine the overlap between the I–V curves for the CPV modules and the EC cells, as shown in Fig. 3(a). In this figure, the lateral voltage axis was normalized so that the curve for a single CPV module is shown equivalently: i.e., the voltage for the series-connected sets of the CPV modules and the EC cells was divided by the number of the CPV modules in series. Here, the I–V curves for the series-connected CPV modules were measured when they were disconnected from the EC cells just prior to the experiment of hydrogen production. The I–V curves for the set of the EC cells were measured in a laboratory environment as disconnected from the CPV modules. In Fig. 3, a relationship is clearly visible between the maximal-power point of the CPV modules and the system operation point, i.e., the cross-point of the two I–V curves for the CPV modules and the EC cells. The distance between these two points, i.e., the value of ηop, depends on the connection configuration.
Fig. 3. (a) I–V curves for the CPV modules and the EC cells. The lateral voltage axis is normalized so that the curve for a single CPV module is shown equivalently: i.e., the voltage for the series-connected sets of the CPV modules and the EC cells was divided by the number of the CPV modules in series. The number S = x/y represents the series connection configuration in Table I. The stars represent the operation point (Vop and Iop) measured in the course of hydrogen production. (b) Power–voltage curves for the data in (a).
Download figure:
Standard image High-resolution imageClearly, variation in the series connection ratio S allows us to maximize ηop, and it is possible to predict the STH efficiency as a function of S given the I–V curves for the PV modules and the EC cells. The detailed methodology will be presented elsewhere. Figure 4 shows such a prediction by assuming ηF = 1. The optimized configuration with S = 1.64 yields a STH efficiency of 25.6%. The two configurations S = 3/2 and 5/3 were very close to this optimized condition, and it was reasonable to obtain a STH efficiency of 24.4% by considering that ηF is smaller than unity in reality.
Fig. 4. The predicted STH efficiency as a function of the series connection ratio S, by assuming the I–V curves for the CPV modules and the EC cells as shown in Fig. 3. The Faraday efficiency ηF was assumed to be unity. The open marks are the measured STH efficiencies for each configuration, from Table I.
Download figure:
Standard image High-resolution imageIf the fluctuation of sunlight irradiance is accounted for, the I–V curve of the CPV modules changes: the current is proportional to the irradiance and the open-circuit voltage logarithmically decreases with the irradiance. The operation point to the right of the maximal-power point of the CPV modules is quite susceptible to such a variation of the I–V curve.29) The connection S = 5/3 is more susceptible to the fluctuation of solar irradiance than the other connections, even though it yields the highest STH under the high-irradiance condition. In the future, it will be useful to introduce a dynamic control of the series connection ratio by introducing a DC/DC converter with high energy transmission efficiency.
To emphasize that combining highly-efficient CPVs and EC cells can represent a real solution for hydrogen production from sunlight, not only in terms of efficiency but also in terms of cost, let us perform a naive estimation of the hydrogen production cost. The cost of hydrogen production by water electrolysis depends linearly on the cost of electricity, with an intercept corresponding to the capital cost of an electrolyzer divided by its lifetime. Assuming an electrolyzer with an electricity-hydrogen conversion ratio of 47 kW h/kg-H2, which corresponds to the operation voltage of 1.7 V, a capital cost of 1.0 $/W and a lifetime of 7 years, the hydrogen cost of 4 $/kg, which is the DOE target price, requires the electricity cost of 0.07 $/(kW h). Assuming the operation in a region with high solar irradiance, such as Australia, the value 0.07 $/(kW h) is well within the target of high-efficiency CPV development. Even though the estimation here does not contain any profit or interest, we believe that hydrogen production combining high-efficiency PV modules and EC cells is one of the most realistic approaches to renewable hydrogen production.
In conclusion, we demonstrated that high-efficiency CPV modules connected with EC cells achieve a STH efficiency of 24.4%. The key factors were: 1) the use of high-efficiency CPV modules (∼31% under the present operation conditions) and 2) the direct connection between the CPV modules and the EC cells, with an almost optimized number of elements in series. The over-potential of the EC cells was not an issue, owing to a small current density (∼0.02 A cm−2), but a reduction in the over-potential for a typical current density (1–2 A cm−2) is still required. This, as well as further improving the CPV module efficiency and the dynamic control of operation-point matching, are the future research and development directions for solar-powered hydrogen production.
Acknowledgments
The authors thank Takashi Iwasaki, Rui Mikami, and Makoto Inagaki of Sumitomo Electric Industries, Ltd. for supplying the CPV modules.