Abstract
We demonstrate that a sodium-ion secondary battery (SIB)-type thermocell consisting of two types of Prussian blue analogue (PBA) with different electrochemical thermoelectric coefficients (SEC ≡ ∂V/∂T; V and T are the redox potential and temperature, respectively) produces electrical energy during heat cycles. The device produces an electrical energy of 2.3 meV/PBA per heat cycle between 295 K (= TL) and 323 K (= TH). The ideal thermal efficiency (η = 1.0%), which is evaluated using the heat capacity (C = 4.16 meV/K) of ideal Na2Co[Fe(CN)6], reaches 11% of the Carnot efficiency (ηth = 8.7%). Our SIB-type thermocell is a promising thermoelectric device that harvests waste heat near room temperature.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
The thermoelectric device, which can convert heat into electricity and vice versa, is a fascinating technology for a smart society. In the development of thermoelectric semiconductors, the Seebeck coefficient [S ≡ ΔV/ΔT, where ΔV (ΔT) is the voltage (temperature) difference between the hot and cold electrodes] is a significant material parameter. Bi2Te3 (S = 0.2 mV/K1) at room temperature) and PbTe (= 0.12 mV/K2) at 300 K) are prototypical semiconductors and exhibit high dimensionless figures of merit (ZT ≡ S2/ρκ T, where T, ρ, and κ represent the temperature, resistivity, and thermal conductivity, respectively). Moreover, they have practical use for Peltier cooling and power generation in space vehicles.3) These devices, however, require a high-grade heat source of several hundred Kelvins to achieve a thermal efficiency of ∼10%.4)
On the other hand, several thermocells, which consist of hot and cold electrodes of identical type and solvable redox couples, were proposed in the 1950s and 1960s. The thermocell converts ΔT into ΔV between the electrodes through the electrochemical thermoelectric coefficient (SEC ≡ ∂V/∂T, where V and T are the redox potential and temperature, respectively). For example, a thermocell using [Fe(CN)6]3+/[Fe(CN)6]4+ 5) was demonstrated to convert ΔT into ΔV between the two electrodes. The thermocell, however, needs a pump to transfer the accumulated species from the electrode region, which results in the loss of a major advantage, the absence of moving parts. Kobayashi et al.6) proposed a sodium-ion secondary battery (SIB)-type thermocell, whose configuration is the same as that of a SIB, except that the anode and cathode materials are the same. They fabricated a CR2032-type thermocell made of layered oxides, for example, Na0.99CoO2 and Na0.52MnO2, and confirmed that ΔV changes linearly with ΔT. Compared to the aforementioned thermocell, the SIB-type thermocell utilizes the SEC value of a redoxable solid, and hence has no moving parts.
If the thermocell is made of two types of redox material with different SEC, the heating/cooling of the thermocell changes the cell voltage (Vcell) between the anode and cathode. In other words, such a thermocell produces electrical energy during heat cycles, in sharp contrast with the thermoelectric devices described above. Lee et al.7) and Yang et al.8) demonstrated that this idea is feasible using solvable redox couples,9) for example, [Fe(CN)6]3+/[Fe(CN)6]4+ and Cu+/Cu2+, as the anode and Prussian blue analogues (PBAs) as the cathode. These thermocells, however, are bulky and heavy because the electrolyte contains redoxable ions. To overcome this drawback, we propose a SIB-type thermocell that consists of two types of redoxable solid with different SEC. It is possible to minimize the amount of electrolyte used in this thermocell. This type of thermocell extends the use of so-called battery materials from energy storage to energy conversion.
PBAs, whose chemical formulas are LixM[Fe(CN)6]y and NaxM[Fe(CN)6]y (where M is a transition metal), are promising candidates for use as the cathode materials in lithium ion batteries and SIBs.10–21) For example, a thin film of Li1.6Co[Fe(CN)6]0.92.9H2O shows a high capacity of 132 mAh/g with good cyclability.12) PBAs have face-centered cubic structure ( ; Z = 4). They consist of a three-dimensional (3D) jungle-gym-type host framework and guest Li+ ions, which are accommodated in the nanopores of the framework. Importantly, the host framework, Fe–CN–M–NC–Fe, is robust against Li+/Na+ deintercalation and concomitant oxidization of M and Fe. In fact, the host framework of Li1.6Co[Fe(CN)6]0.92.9H2O is stable even if we remove all of the Li+ from the framework.12) Recently, Magnússon et al.22) systematically investigated the SEC23) value of LixCo[Fe(CN)6]y for various x and y.
; Z = 4). They consist of a three-dimensional (3D) jungle-gym-type host framework and guest Li+ ions, which are accommodated in the nanopores of the framework. Importantly, the host framework, Fe–CN–M–NC–Fe, is robust against Li+/Na+ deintercalation and concomitant oxidization of M and Fe. In fact, the host framework of Li1.6Co[Fe(CN)6]0.92.9H2O is stable even if we remove all of the Li+ from the framework.12) Recently, Magnússon et al.22) systematically investigated the SEC23) value of LixCo[Fe(CN)6]y for various x and y.
In this study, we fabricated a SIB-type thermocell with a thin film of NaxCo[Fe(CN)6]0.713.6H2O (NCF71) as the anode and a thin film of NaxCo[Fe(CN)6]0.92.9H2O (NCF90) as the cathode. The SEC values are 0.53 and 1.32 mV/K for NCF71 at x = 0.51 and NCF90 at x = 0.71, respectively. Owing to the difference in SEC, the device produces an electrical energy of 2.3 meV/NCF90 per heat cycle between 295 K (= TL) and 323 K (= TH). The ideal thermal efficiency (η = 1.0%) reaches 11% of the Carnot efficiency (ηth ≡ 1 − TL/TH = 8.7%).
The NCF71 and NCF90 films were synthesized by means of electrochemical deposition on indium tin oxide transparent electrodes. Details of the synthesis conditions are described in the literature.24,25) The chemical compositions of the films were determined by the inductively coupled plasma method and CHN organic elementary analysis (PerkinElmer 2400 CHN Elemental Analyzer). Both the compounds show face-centered cubic structure ( ; Z = 4) with lattice constants (a) of 10.3 Å (NCF71) and 10.4 Å (NCF90). The film thickness is ≈1.0 µm, as determined by a profilometer (Ulvac DEKTAK3030). The typical area of a film is 1.0 cm2. The mass of each film was evaluated from the thickness, area, and ideal density.
; Z = 4) with lattice constants (a) of 10.3 Å (NCF71) and 10.4 Å (NCF90). The film thickness is ≈1.0 µm, as determined by a profilometer (Ulvac DEKTAK3030). The typical area of a film is 1.0 cm2. The mass of each film was evaluated from the thickness, area, and ideal density.
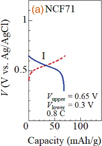
Figures 1(a) and 1(b) show the charge and discharge curves of the NCF71 and NCF90 films, respectively, in an aqueous solution of 10 mol/L NaClO4. The electrochemical properties of the films were investigated using a potentiostat (HOKUTO DENKO HJ1001SD8) with a beaker-type cell in the three-pole configuration. The working, reference, and counter electrodes were the PBA films, a standard Ag/AgCl electrode, and Pt, respectively. In NCF71 [Fig. 1(a)], the discharge curve shows a single plateau (plateau I) at ≈0.55 V versus Ag/AgCl, which is ascribed to the reduction reaction24) Na0.13Co2+[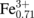
 (CN)6]0.71 + 0.71Na+ → Na0.84Co2+[Fe2+(CN)6]0.71. The discharge capacity is 68 mAh/g, which is close to the ideal value (= 72 mAh/g). In the discharge process, Na+ is inserted into the framework, which causes the reduction of Fe3+ to maintain charge neutrality. In NCF90 [Fig. 1(b)], the discharge curve shows two plateaus (plateaus II and III) at ≈1.0 and ≈0.53 V. Plateau II (x ≤ 0.6) at ≈1.0 V is ascribed to the reaction16) Co3+[
(CN)6]0.71 + 0.71Na+ → Na0.84Co2+[Fe2+(CN)6]0.71. The discharge capacity is 68 mAh/g, which is close to the ideal value (= 72 mAh/g). In the discharge process, Na+ is inserted into the framework, which causes the reduction of Fe3+ to maintain charge neutrality. In NCF90 [Fig. 1(b)], the discharge curve shows two plateaus (plateaus II and III) at ≈1.0 and ≈0.53 V. Plateau II (x ≤ 0.6) at ≈1.0 V is ascribed to the reaction16) Co3+[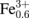
 (CN)6]0.9 + 0.6Na+ → Na0.6Co3+[Fe2+(CN)6]0.9. Plateau III (x ≥ 0.6) at ≈0.53 V is ascribed to the reaction16,25) Na0.6Co3+[Fe2+(CN)6]0.9 + Na+ → Na1.6Co2+[Fe2+(CN)6]0.9. The discharge capacity is 115 mAh/g, which is close to the ideal value (= 132 mAh/g). We use plateau I of the NCF71 film and plateau III of the NCF90 film in the SIB-type thermocell because their redox potentials are almost the same.
(CN)6]0.9 + 0.6Na+ → Na0.6Co3+[Fe2+(CN)6]0.9. Plateau III (x ≥ 0.6) at ≈0.53 V is ascribed to the reaction16,25) Na0.6Co3+[Fe2+(CN)6]0.9 + Na+ → Na1.6Co2+[Fe2+(CN)6]0.9. The discharge capacity is 115 mAh/g, which is close to the ideal value (= 132 mAh/g). We use plateau I of the NCF71 film and plateau III of the NCF90 film in the SIB-type thermocell because their redox potentials are almost the same.
Download figure:
Standard image High-resolution imageFig. 1. Charge (red broken curve) and discharge (blue solid curve) curves of (a) NaxCo[Fe(CN)6]0.71 (NCF71) and (b) NaxCo[Fe(CN)6]0.9 (NCF90) films measured in aqueous solution of 10 mol/L NaClO4. For convenience of explanation, we defined plateaus I, II, and III in the discharge curves. Vupper and Vlower are the upper and lower cutoff voltages, respectively.
Download figure:
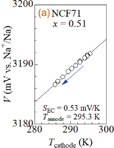
Standard image High-resolution imageTo determine the SEC values of the NCF71 (plateau I) and NCF90 (plateau III; x ≥ 0.6) films, we fabricated a specially designed two-pole cell whose anode (Tanode) and cathode (Tcathode) temperatures are independently controlled by Peltier elements. The cathode, anode, and electrolyte were the thin film, Na metal, and propylene carbonate containing 1 mol/L NaClO4, respectively. We carefully measured V against Tcathode with Tanode fixed at 295.3 K. Figures 2(a) and 2(b) show the V values of NCF71 at x = 0.51 at plateau I and of NCF90 at x = 0.71 at plateau III, respectively, against Tcathode. We evaluated SEC by least-squares fittings with primary functions, as indicated by the solid straight lines. Thus, the SEC values are determined to be 0.53 and 1.32 mV/K for NCF71 at x = 0.51 (plateau I) and NCF90 at x = 0.71 (plateau III), respectively. The difference (= 0.79 mV/K) in SEC between NCF71 and NCF90 is significant.
Download figure:
Standard image High-resolution imageFig. 2. Redox potential (V) of (a) NCF71 at x = 0.51 and (b) NCF90 at x = 0.71 against temperature (Tcathode) of cathode. Temperature (Tanode) of anode was fixed at 295.3 K. x in NCF71 (NCF90) was evaluated from the moved charge under the assumption that x = 0.84 (1.6) in the discharged state and 0.13 (0.0) in the charged state. Solid straight lines are results of least-squares fitting.
Download figure:
Standard image High-resolution imageWe fabricate a SIB-type thermocell (two-pole beaker-type cell) whose anode, cathode and electrolyte are the as-grown NCF71 film, a pre-oxidized NCF90 film, and an aqueous solution of 10 mol/L NaClO4, respectively (inset of Fig. 3). The NCF90 film was pre-oxidized at Vupper = 0.65 V against Ag/AgCl in an aqueous solution of 10 mol/L NaClO4. Here, we defined TL (= 295 K) and TH (= 323 K) as the lowest and highest temperatures, respectively. The as-prepared device, which showed a finite Vcell (= 0.19 V), was discharged to 0 V under a constant current condition (I = 7.3 µA). Then, we slowly increased the temperature (T) of the device from TL to TH. Figure 3(a) shows Vcell against T in this heating process. As expected, Vcell increases linearly with T at a rate of 0.96 mV/K. The increase in Vcell is reasonably ascribed to the difference in SEC between the anode and cathode. Moreover, the observed value (= 0.96 mV) is close to the difference (= 0.79 mV) in SEC between NCF71 and NCF90. At TH, the device shows a finite Vcell (= 26 mV). Figure 3(b) shows the first discharge process at TH at I = 2.9 µA. Vcell decreases linearly to 0 V in proportion to the moved charge. The final moved charge is 0.09 e per NCF90 unit. The electric work done in this discharge process was 1.2 meV/NCF90.
Download figure:
Standard image High-resolution imageFig. 3. (a) Vcell of the SIB-type thermocell against T. TL (= 295 K) and TH (= 323 K) are the lowest and highest temperatures, respectively. Inset shows schematic picture of the thermocell. (b) First discharge process at TH under a constant current condition (I = 2.9 µA). The moved charge was normalized by that of NCF90.
Download figure:
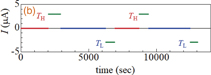
Standard image High-resolution imageThe heat cycle of the SIB-type thermocell consists of four processes, as shown schematically in Fig. 4: (i) heating from TL to TH, (ii) discharge at TH, (iii) cooling from TH to TL, and (iv) discharge at TL. Figures 5(a) and 5(b) show the variation in Vcell and current (I), respectively, during the first and second heat cycles. In the heating process [(i)], Vcell gradually increases with time and eventually reaches Vcell = 26 mV at TH. The discharge process [(ii)] at TH produces an electrical energy of 1.2 meV/NCF90 under a constant current condition (I = 2.9 µA). In the cooling process [(iii)], Vcell gradually decreases with time and eventually reaches Vcell = −25 mV at TL. Like process (ii), the discharge process [(iv)] at TL produces electrical energy. Note that the signs of both the current and the voltage are opposite for processes (ii) and (iv).
Fig. 4. Heat cycle of SIB-type thermocell against Vcell and T. The heat cycle consists of four processes: (i) heating from TL to TH, (ii) discharge at TH, (iii) cooling from TH to TL, and (vi) discharge at TL. The heating and cooling processes are performed without current (I = 0).
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageFig. 5. (a) Vcell and (b) I in the SIB-type thermocell against time in the first and second heat cycles. The lowest (TL) and highest (TH) temperatures are 295 and 323 K, respectively. The discharge processes were performed under a constant current condition (I = 2.9 µA).
Download figure:
Standard image High-resolution imageNow, let us discuss the thermal efficiency (η ≡ W/Q, where W and Q are the output work and input thermal energy, respectively) of the SIB-type thermocell. The output work (W) is expressed as W = WH + WL, where WH and WL are the electrical work during the discharge processes at TL and TH. W = 2WH (= 2.3 meV/NCF90), because WL is essentially the same as WH. The input thermal energy is Ctot(TH − TL), where Ctot is the sum of the specific heats of the anode (Canode) and cathode (Ccathode). Here, let us use the calculated CPBA (= 4.16 meV/K) of ideal Na2Co[Fe(CN)6] in the Dulong–Petit law. Then, Q (= 233 meV) is easily evaluated considering Ctot = Canode + Ccathode = 2CPBA. Thus, we obtained η = 1.0%. Strictly speaking, we should include the heat capacity of the electrolyte in the thermal efficiency. The amount of electrolyte, however, is minimized in the SIB-type thermocell because the thermocell utilizes redoxable solids. In this sense, η (= 1.0%) is an ideal value. Incidentally, the Carnot efficiency (ηth ≡ 1 − TL/TH) is 8.7% between TL (= 295 K) and TH (= 323 K). Thus, the experimentally obtained η (= 1.0%) is 11% of the ideal value (ηth = 8.7%). We will bring η closer to ηth by further development of anode and cathode materials with high |SEC| and flatter discharge curves. The flatter the discharge curve becomes, the more charge can be moved.
In conclusion, we demonstrate that a SIB-type thermocell consisting of two types of PBA with different SEC values can harvest waste heat near room temperature. The device produces an electrical energy of 2.3 meV/NCF90 per heat cycle between 295 and 323 K. The ideal thermal efficiency (η = 1.0%) reaches 11% of the Carnot efficiency (ηth = 8.7%). We emphasize that our SIB-type thermocell can be easily formed into a sheet or a large device at a low cost, because it has the same device structure as SIBs.
Acknowledgments
This work was supported by the Yazaki Memorial Foundation for Science and Technology and the Nippon Sheet Glass Foundation for Materials Science and Engineering. This work was also supported by JSPS KAKENHI (Grant Number JP17H0113). T.S. and W.K. were supported by the Nanotech Research Professional (NRP) course of the Nanotech Career-up Alliance in Nanotech (CUPAL) project. The elementary analyses were performed at the Chemical Analysis Division, Research Facility Center for Science and Engineering, University of Tsukuba.