Abstract
The diffusion of hydrogen atoms on dust grains is a key process in the formation of interstellar H2 and some hydrogenated molecules such as formaldehyde and methanol. We investigate the adsorption and diffusion of H atoms on pure solid CO as an analog of dust surfaces observed toward some cold interstellar regions. Using a combination of photostimulated desorption and resonance-enhanced multiphoton ionization methods to detect H atoms directly, the relative adsorption probabilities and diffusion coefficients of the H atoms are measured on pure solid CO at 8, 12, and 15 K. There is little difference between the diffusion coefficients of the hydrogen and deuterium atoms, indicating that the diffusion is limited by thermal hopping. The activation energies controlling the H-atom diffusion depend on the surface temperature, and values of 22, 30, and ∼37 meV were obtained for 8, 12, and 15 K, respectively.
Export citation and abstract BibTeX RIS
1. Introduction
Hydrogen is the most abundant element in space, and is a common component of complex molecules. In a cold interstellar cloud, various molecules are produced in or on an ice mantle of nanometer-sized inorganic grains at low temperatures (for a review, see Watanabe & Kouchi 2008; Hama & Watanabe 2013). For example, although H2 molecules can form in the gas phase via a sequential reaction of radiative attachment and the associative detachment of H− and H, the formation rate is insufficient to explain the observed abundance of cosmic H2 molecules (Gould & Salpeter 1963). Therefore, it has been suggested that H2 molecules form more efficiently on the surface of cosmic dust particles. Because H–H recombination is a radical–radical barrierless reaction, the diffusion of H atoms at a low coverage on dust surfaces is the main rate-determining process for H2 formation (Hollenbach & Salpeter 1971). In addition to H2 formation, H atoms play an important role in the formation of H2CO and CH3OH by the successive hydrogenation of CO on dust. These formation processes proceed via tunneling reactions, of which the rates always compete with the diffusion and desorption rates of the H atoms. Therefore, to understand the formation of not only these molecules but also the complex molecules that evolved from them, the diffusion of H atoms on the various types of dust surfaces must be clarified. In cold dense clouds, dust grains are generally covered with amorphous solid water (ASW) that may include other molecules such as CO, CO2, and CH3OH. In addition, the solid phase of pure CO has been detected toward young stellar objects, and is thus considered to exist on the surface of dust particles (Pontoppidan et al. 2003; Pontoppidan 2006). Therefore, as an elementary astrochemical process in cold interstellar regions, the diffusion of H atoms on solid CO, as well as ASW, need to be better understood.
For ASW, the activation barriers of H-atom surface diffusion were first estimated by the thermal-programmed desorption method (TPD), which assumes thermal hopping (Manicò et al. 2001; Perets et al. 2005; Matar et al. 2008). Later, using a combination of photostimulated desorption (PSD) and resonance-enhanced multiphoton ionization (REMPI) methods (Watanabe et al. 2010; Hama et al. 2012), the mechanism of H-atom diffusion, whether thermal hopping or tunneling diffusion, was found to depend on the morphology of the considered ice surface and the diffusion length (Kuwahata et al. 2015). For porous ASW, the diffusion was found to be dominated by thermal hopping as previously suggested (Pirronello et al. 1997). Based on the direct detection of H and D adatoms by the PSD + REMPI technique, it has been demonstrated that ASW surfaces contain various potential sites that can be categorized in at least three groups: very shallow-, middle-, and deep-potential sites, with thermal hopping activation energies of ≤18, 22, and ≥30 meV, respectively (Hama et al. 2012), which overcomes previous contradictory results (Perets et al. 2005; Matar et al. 2008). In contrast to ASW, little is known about diffusion on pure solid CO. The activation barrier of H-atom diffusion on crystalline CO (110) was theoretically estimated to be in the range from 70 to 170 K (∼6–15 meV) by the nudged elastic band method (Fuchs et al. 2009). However, the distribution of the adsorption potential depth on dust should differ significantly from that of crystalline CO. Therefore, experiments are needed to directly determine the activation barriers of H-atom diffusion on solid CO, which is more relevant to realistic dust.
In this Letter, we clarify the H-atom diffusion mechanism on pure solid CO (i.e., thermal hopping or tunneling) and derive the activation energies of the diffusion process.
2. Experimental Procedure
The experiments were performed using a specially designed apparatus Reaction Apparatus for Surface Characteristic Analysis at Low-temperature (RASCAL) at the Institute of Low Temperature Science, Hokkaido University (Watanabe et al. 2010; Hama et al. 2012). RASCAL consists of a main vacuum chamber, a triply differentially pumped atomic source, and two laser systems for the photodesorption of surface adsorbates and the analysis of desorbed H atoms.
Thirty monolayers of pure solid CO were vapor-deposited at a rate of 0.08–0.11 monolayers s−1 through a capillary plate (Hidaka et al. 2007) onto an aluminum substrate at 8 K in the main chamber, which was evacuated down to 1.5 × 10−8 Pa by two turbo-molecular pumps. The H atoms were produced by a microwave discharge source and were cooled to 100 K (kinetic temperature) by passing through a cold aluminum pipe. After the pipe, the H atoms traveled to the substrate through three circular orifices of 1–2 mm diameter in differentially pumped free-flight regions of ∼700 mm. Before reaching the solid CO, the atomic beam was chopped by a thin aluminum disk with four 1.5 mm slits that rotated at 150 rpm in the differentially pumped chamber. Each H-atom pulse had a duration of 0.73 ms; thus, an accumulation time of 1 s corresponds to an actual beam operation time of approximately 7.3 ms. The incidence angle was fixed at 45° to the substrate. During the atomic source operation, the typical pressure of the three parts separated by the orifices and the main chamber were 5 × 10−3, 2 × 10−5, 6 × 10−7, and 2 ×10−8 Pa, respectively. Using the experimental method described previously (Hama et al. 2012), the flux of the H beam was estimated to be 5 × 1011 to 1 × 1012 cm−2 s−1 and dissociation fraction of H/(H+H2) was about 70%.
The experimental basis and procedure of the PSD + REMPI method are the same as in previous works (Watanabe et al. 2010; Hama et al. 2012). After the accumulation of H atoms via the rotating chopper disk, adsorbed H atoms on the solid CO were detected by the procedure shown in Figure 1. A small fraction of the adsorbed H atoms was sequentially (10 Hz) sublimated by radiation from a weak photodesorption YAG (PSD) laser (532 nm) and selectively ionized by (2+1) REMPI at approximately 1.5 mm above the CO surface and detected using a time-of-flight method. Laser radiation with a wavelength of ∼243.067 nm excites an H atom from the 1S to the 2S state by the two-photon process, which is further ionized by a single additional photon. The delay time of the REMPI laser after the PSD laser irradiation was set to coincide with the moment when the H signal reached a maximum value. Any photodesorption that does not result from a chemical process may instead be due to phonons emitted from the aluminum surface (Hama et al. 2012). It was observed that the PSD laser caused little evaporation and/or structural changes. In fact, the solid CO is transparent to photons at 532 nm. Furthermore, the IR spectra of the solid CO did not change upon laser irradiation. We also confirm that the experimental results are not sensitive to whether the solid CO was pre-processed with a laser. The signal intensity of H, IH, which is proportional to the surface density of H atoms, was obtained from the recorded REMPI spectrum (Watanabe et al. 2010). All PSD + REMPI measurements were performed with both the quadrupole mass analyzer and the vacuum gauge turned off, because these devices can create undesired H atoms. We confirm that no H atoms were detected without H-atom deposition, even after the longest waiting time.
Figure 1. Timing chart of the experiments. (a) Deposition of H. (b) Photodesorption of H by YAG laser at 532 nm. (c) Excitation of H by two-phonon absorption in the REMPI laser and ionization of exited H by the REMPI laser. (d) Detection of H by the time-of-flight method.
Download figure:
Standard image High-resolution image3. Results and Discussion
3.1. Adsorption Coefficients of H Atoms on the Solid CO Surface
When H atoms are deposited onto the surface, the time evolution of the number density of the H atoms and of the H signal are determined by a competition between the deposition, monoatomic desorption, and H–H recombination following the H-atom diffusion on the surface of the solid CO, and is therefore described by the following equation:
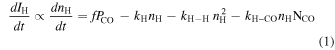
where nH (cm−2) is the surface number density of the H atoms, f is the flux of the H atoms, Pco is the adsorption coefficient of the H atoms onto the pure solid CO, kH is the atomic desorption rate, and kH–H is the H–H recombination-rate constant. The Pco may change by the coverage of undissociated H2 provided from the source. However, this effect was found to be minor in the present low-flux experiment (Kuwahata et al. 2015, see the supplemental material). The final term on the right-hand side corresponds to a pseudo first-order approximation reaction of H and CO for a constant CO number density, because it is known that H atoms react with CO to produce HCO via tunneling on pure solid CO (Hidaka et al. 2007). As the H atoms were deposited on the solid CO surface, IH, the intensity of the H-atom REMPI signal (Figure 2) increased linearly and then became saturated, as shown in Figure 3. The observed linear increase indicates that the first term on the right-hand side of Equation (1) dominates the number density of H atoms on the surface. During the linear phase, all of the terms that correspond to H-atom losses at the surface are negligible when compared with fPco. This implies that the slope of the linear part in Figure 3 (IH versus time) is strongly dependent on the Pco value at a given temperature.
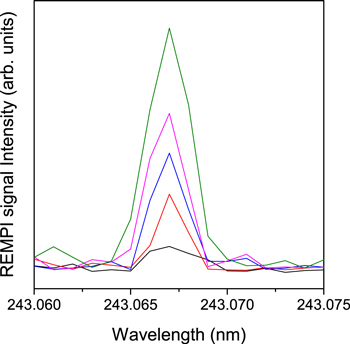
Figure 2. REMPI spectra of H atoms desorbed from CO solid at 8 K. The spectra from lower to upper correspond to H-accumulation time of 90, 145, 259, 565, and 2413 s, respectively. The accumulation time of 1 s corresponds to an actual beam operation time of ∼7.3 ms.
Download figure:
Standard image High-resolution imageFigure 3. Signal intensities of the photodesorbed H from the pure solid CO at (a) 8, (b) 12, and (c) 15 K during the accumulation of pulsed H atoms. The accumulation time, τ, of 1 s corresponds to an actual beam operation time of ∼7.3 ms. The solid lines are linear fits to the duration of the proportionally increasing IH. The corresponding best-fit equation is given in each panel.
Download figure:
Standard image High-resolution imageIn the time evolution of the REMPI signal for a substrate temperature of 8 K, saturation starts after about 300 s (Figure 3). Assuming that the value of Pco is unity at 8 K, then the relative adsorption coefficients can be derived as 0.068 and 0.005 at 12 and 15 K, respectively, from the slopes of the linear parts in Figures 3(b) and (c). The temperature dependence of the adsorption coefficient appears to be much larger for CO than for ASW, where the relative coefficients are 1, 0.4, and 0.2 at 8, 15, and 20 K, respectively (Watanabe et al. 2010).
When the H signal starts to saturate, H-atom losses due to processes such as recombination become efficient, and finally (when the coverage is high enough) balances with the supply via deposition; the number density of H atoms on the surface remains constant.
3.2. Potential Sites on the Surface of Solid CO for H Diffusion
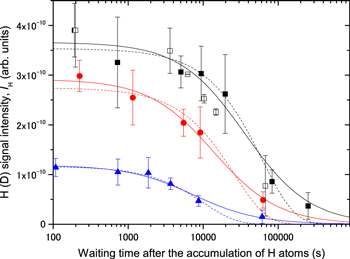
After the H atoms had accumulated on the solid CO surface for a certain amount of time, the gate valve to the atomic source was closed to prevent further H-deposition. After a suitable waiting period the H atoms were then measured. The nH, and hence IH, started to decrease due to monoatomic desorption, recombination, and/or reactions with the CO (Figure 4). The accumulation times of the pulsed H beam were 360, 1800, and 21000 s at 8, 12, and 15 K, respectively. The flux of the H beam, f, is ∼1 × 1012 cm−2 s−1. Assuming an adsorption coefficient of unity for the H atoms at 8 K, and the relative coefficients obtained in Section 3.1, the maximum coverages of H atoms, θ, are ∼2.5 × 10−4, ∼8.6 × 10−5, and ∼7.7 × 10−5 for the experiments at 8, 12, and 15 K, respectively, when the number of sites on the CO surface was 1016 cm−2, such as for ASW. In reality, because H–H recombination may occur even during accumulation, the measured coverages are rather small.
Figure 4. Signal intensities of photodesorbed H from the pure solid CO at 8 K (squares), 12 K (circles), and 15 K (triangles) after the accumulation of the pulsed H atoms. The solid lines are the best-fit curves of the depletion of H by recombination. For reference, the fitting of a single exponential decay is described by dashed lines in case that monoatomic desorption dominates. Open squares represent the measurements of D atoms, for which the intensities are normalized by the highest-intensity H atom. The y-scale is the same as that in Figure 3.
Download figure:
Standard image High-resolution imageAlthough atomic desorption should be minor at 8 K, it was nevertheless evaluated by TPD after the H-atom deposition. During heating the H-atom deposited sample at a rate of 6 K minute−1 from 8 to 30 K, we attempted to detect H-atomic and H2-molecular desorption at a height of about 1.5 mm above the surface by the REMPI method, which is more sensitive and reliable than a quadrupole mass analyzer for H-atom detection. We find that no H atoms were detected regardless of the amount of H-atom deposition, while significant H2 desorption was detected. We repeated the same measurement for the molecular H2 deposition, which was conducted with the same H2-gas flow rate as that used for the H-atom source, but instead this time the microwave was turned off. The detected H2 amount when using the TPD method was equivalent to that obtained for the H-atom deposition case within the difference of 3%. These measurements indicate that atomic desorption is minor, and the accommodation coefficient at 8 K is similar between H and H2, where most of the H atoms were consumed by recombination and remain on the surface as H2. This also shows that the H-atom loss is dominantly caused by recombination.
Further investigations were performed to test for any decrease in the amount of H atoms arising from a tunneling reaction with CO, which has been studied for CO on ASW (Watanabe & Kouchi 2002) and pure solid CO (Watanabe et al. 2004). HCO can be dissociated by photons of the PSD laser at 532 nm (van Ijzendoorn et al. 1983). If HCO exists on the surface, any H atoms photolyzed from the HCO by the PSD laser can be detected by the REMPI. As the tunneling reaction rate between H and CO should be much lower than the diffusion coefficient of H atoms, H-atom consumption by HCO formation should be minor. Nevertheless, for a long period of time after H-atom deposition, the H atoms could finally react with CO. We therefore evaluated the possibility that the detected H atoms result from HCO photolysis. For a waiting time of 6.7 × 104 s at 8 K, the sample was heated at 20 K, and it was subsequently confirmed that the H-atom intensities diminished immediately (Figure 5). This indicates that any remaining H atoms immediately recombined when the sample was heated to 20 K. If the detected H atoms are dominated by the photolysis of HCO rather than physisorbed H atoms, they should be detected even at 20 K, which is lower than the desorption temperature of HCO. Therefore, we conclude that the detected H atoms result solely from physisorbed H atoms.
Figure 5. Signal intensities of photodesorbed H atoms from the pure solid CO at 8 K, which are the same as those plotted in Figure 4 (squares). Star symbols represent the intensity variation when the temperature increases from 8 to 20 K for a waiting time of 6.7 × 104 s.
Download figure:
Standard image High-resolution imageAs described above, H–H recombination mainly dominates over monoatomic desorption and CO hydrogenation after H-atom deposition in the present experiment. Therefore, the rate Equation (1) is reduced to
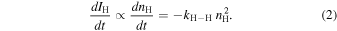
To examine the effect of isotopes on the diffusion, measurements identical to those performed for H atoms were undertaken for deuterium (D) atoms at 8 K. As can be seen in Figure 4, the attenuation behavior of the D atoms does not show any significant difference from that for H atoms, which indicates that the diffusion is dominated by thermal hopping rather than tunneling.
If we analytically integrate Equation (2), we have
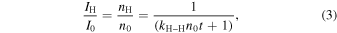
where I0 (arb. units) and n0 (cm−2) are the initial values of IH and nH, respectively, and t is the waiting time after the H-atom accumulation is terminated. The time evolution curves of IH for the three temperatures are shown in Figure 4. Fitting these curves with Equation (3) gives the values of the parameters kH–Hn0. The term kH–Hn0 can be expressed in terms of thermal hopping as follows:
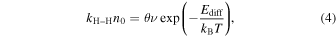
where ν is a frequency factor, 1012–1013 s−1, Ediff is the activation energy for the diffusion of H, kB is the Boltzmann constant, and T is the surface temperature. Using ν = 5 ×1012 s−1 and θ = 2.5 × 10−4 for 1016 sites cm−2 on the surface at 8 K, Ediff is determined to be ∼22 meV. Similarly, we obtain ∼30 and ∼37 meV for Ediff at 12 and 15 K, respectively. When we consider a 10× larger coverage for the assumed 1015 sites cm−2, the activation energies rise by ∼2 meV for each temperature. That is, the uncertainty originating from the coverage is quite small. In this temperature region, the values of the activation barriers dominating thermal hopping increase almost linearly. At higher temperatures, the H atoms tend to be accommodated in deeper potential sites, suggesting that the diffusion of the H atoms is limited by the larger activation energies. It should be noted that a rough surface generally has various potential sites; therefore, our obtained activation energies should be regarded as average values. There must be shallower potential sites that lead to lower activation barriers than the obtained ones. The rapid diffusion for shallower sites should be reflected in the steep decrease of IH just after the termination of H-atom accumulation. From Figure 3, the accumulation times used for 8, 12, and 15 K give IH intensities of about 7 × 10−10, 2 × 10−10, and 2 × 10−10, respectively, while the first plots of IH obtained after 100–250 s in Figure 4 have similar values (within 50%) for each temperature. These small differences indicate that the fraction of shallower potential sites is of the same order or less than those for the obtained activation energies.
The thermal hopping frequency, Ndiff, of the dominant potential site at each temperature can be calculated as follows:
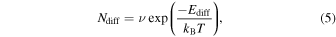
and is found to be 0.07, 1.3, and 1.9 s−1 at 8, 12, and 15 K, respectively. Then, the diffusion coefficient, D, is about 1.8 × 10−17, 3.3 × 10−16, and 4.8 × 10−16 cm2 s−1 at 8, 12, and 15 K, respectively, assuming a two-dimensional random walk expressed as follows:
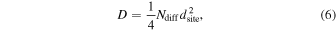
where  is 10−15 cm2, which is a unit area of the adsorption site.
is 10−15 cm2, which is a unit area of the adsorption site.
4. Astrophysical Implications
As pure solid CO covers the surface of dust in some dense, cold interstellar regions, the relevant hydrogen processes need to be better understood in order to accurately describe their chemical evolution. By linearly interpolating the obtained diffusion activation energies at 8 and 12 K, an activation energy of about 23 meV is derived for 10 K, which leads to 13 hops s−1 for an assumed frequency factor of 5 × 1012 s−1. This hopping rate is significant enough to move around on the surface, even at 10 K. We propose that H atoms landing on dust at 10 K immediately diffuse around major sites and may be ultimately trapped in deeper sites. Any H atoms that are accreted at a later time will encounter the trapped H atoms and subsequently recombine if the corresponding hydrogenation reaction has not yet occurred. From simple consideration, H-atom coverage should be larger as the surface temperature is lower. The recombination rate is governed by diffusion rate, kH–H in Equation (1), and the square of H-atom coverage, while CO hydrogenation should have a small rate constant, kH–CO, and the rate proportional to the H-atom coverage. As a result, the ratio in the rates, (H2 recombination/CO hydrogenation), decreases as the increase of temperature. Unfortunately, the absolute value of the ratio cannot be derived without the value of kH–CO, which is not easy to determine experimentally.
We thank T. Hama and H. Hidaka at the Institute of Low Temperature Science and H. Fraser at the Open University for fruitful discussions. This work was supported in part by a JSPS Grant-in-Aid for Specially Promoted Research (JP17H06087).