Abstract
Methyl isocyanate (CH3NCO) was recently found in hot cores and suggested to exist on comet 67P/CG. The incorporation of this molecule into astrochemical networks requires data on its formation and destruction. In this work, ices of pure CH3NCO and of CH3NCO(4%–5%)/H2O mixtures deposited at 20 K were irradiated with a UV D2 lamp (120–400 nm) and bombarded by 5 keV electrons to mimic the secondary electrons produced by cosmic rays (CRs). The destruction of CH3NCO was studied using IR spectroscopy. After processing, the νa–NCO band of CH3NCO disappeared and IR bands corresponding to CO, CO2, OCN−, and HCN/CN− appeared instead. The products of photon and electron processing were very similar. Destruction cross sections and half-life doses were derived from the measurements. Water ice provides a good shield against UV irradiation (half-life dose of ∼64 eV molecule−1 for CH3NCO in water ice), but is not so good against high-energy electrons (half-life dose ∼18 eV molecule−1). It was also found that CH3NCO does not react with H2O over the temperature range 20–200 K. These results indicate that hypothetical CH3NCO in the ices of dense clouds should be stable against UV photons and relatively stable against CRs over the lifetime of a cloud (∼107 yr), and could sublime in the hot core phase. On the surface of a Kuiper Belt object (the original location of comet 67P/CG) the molecule would be swiftly destroyed, by both photons and CRs, but embedded below just 10 μm of water ice, the molecule could survive for ∼109 yr.
Export citation and abstract BibTeX RIS
1. Introduction
The analysis of mass spectrometric measurements from the Cometary Sampling and Composition (COSAC) instrument of Rosetta's Philae lander (Goesmann et al. 2015) indicated that methyl isocyanate was abundant at the surface of comet 67P/Churyumov–Gerasimenko (CG). A ratio of CH3NCO/H2O = 1.3% was estimated from these measurements. The isocyanate functional group is structurally similar to a peptide bond, and chemical routes implying isocyanates have been invoked in models of peptide synthesis under prebiotic conditions (Pascal et al. 2005). In spite of its potential interest for prebiotic chemistry, methyl isocyanate was not included in astrochemical models before the publication of the COSAC results.
Soon after the publication of the work of Goesmann et al. (2015) the presence of CH3NCO was also reported in the interstellar medium (ISM) (Halfen et al. 2015; Cernicharo et al. 2016). The line survey of Orion, carried out with the 30 m IRAM telescope (Tercero et al. 2010, 2012) motivated the complete characterization of CH3NCO in the laboratory. Several groups were involved in this work (see Cernicharo et al. 2016 and references therein). Another group (Halfen et al. 2015) sought the molecule, motivated by its detection in comet 67P/CG. Methyl isocyanate was then detected in two high-mass protostars. Halfen et al. (2015) identified it in SgrB2(N) using data from the 12 m telescope of the Arizona Radio Observatory, and Cernicharo et al. (2016) found it in Orion KL with data from the 30 m IRAM telescope and from the Atacama Large Millimeter Array (ALMA). The analysis of the Orion KL measurements and a re-evaluation of previous SgrB2 data (Cernicharo et al. 2016) showed that the molecule was present in the warm gas of these clouds. Besides, Cernicharo et al. (2016) reported upper limits for the abundance of CH3NCO in cold dark clouds and also for its isomers CH3CNO and CH3OCN, which were not detected towards the two high-mass protostars where CH3NCO was initially discovered. Additional observations of methyl isocyanate in the SgrB2(N2) hot core were published by Belloche et al. (2017). More recently, Ligterink et al. (2017) and Martín-Doménech et al. (2017), using ALMA data, were able to identify CH3NCO in the warm gas of the low-mass solar-type protostar IRAS 16293-2422. In the gas phase of the protostars, the proportion of CH3NCO was found to be much smaller than that reported for the surface of comet 67P/CG. In Orion KL, for instance, a ratio of CH3NCO/H2O = 0.02% was derived by Cernicharo et al. (2016). Abundance ratios of CH3NCO with respect to characteristic N-bearing species such as HNCO and CH3CN are also much lower in the protostars (Cernicharo et al. 2016; Ligterink et al. 2017) than those reported by Goesmann et al. (2015) for the comet's surface. Although the presence of CH3NCO in the ISM is well established at present, the original results from 67P/CG have been questioned. New data have recently been published by Altwegg et al. (2017). These authors could not identify CH3NCO in the coma of the comet with the high-resolution Double Focusing Mass Spectrometer of the Rosetta Orbiter Spectrometer for Ion and Neutral Analysis (ROSINA). In the same work, the COSAC data were revisited and the presence of CH3NCO could not be confirmed.
Gas-phase mechanisms leading to the production of CH3NCO in the ISM were discussed by Halfen et al. (2015). They were based on reactions of HNCO or HOCN with either CH3 or  , but the gas-phase concentrations of HOCN, CH3, and
, but the gas-phase concentrations of HOCN, CH3, and  in the ISM are probably too low (see discussion in Ligterink et al. 2017). Most authors assume that reactions in the bulk or at the surface of the ice mantles covering dust grains are more probable formation pathways for CH3NCO. Specifically, Cernicharo et al. (2016) have proposed the reaction
in the ISM are probably too low (see discussion in Ligterink et al. 2017). Most authors assume that reactions in the bulk or at the surface of the ice mantles covering dust grains are more probable formation pathways for CH3NCO. Specifically, Cernicharo et al. (2016) have proposed the reaction
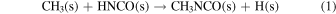
and Belloche et al. (2017) the reaction
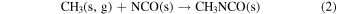
where s and g stand for solid and gas respectively, as sources of CH3NCO in interstellar ices. Experiments by Ligterink et al. (2017), based on the vacuum ultraviolet (VUV) irradiation of CH4/HNCO mixtures at 20 K, suggest that the formation of methyl isocyanate through reactions (1) and (2) is indeed possible. The methyl isocyanate produced in the ices would sublimate with rising temperature in the evolution of the protostar, and that would explain its presence in hot cores (Halfen et al. 2015; Cernicharo et al. 2016; Belloche et al. 2017) and hot corinos (Ligterink et al. 2017; Martín-Doménech et al. 2017). The reactions mentioned in this paragraph have been incorporated into astrochemical models (Belloche et al. 2017; Martín-Doménech et al. 2017; Majumdar et al. 2018; Quénard et al. 2018) that provide a plausible explanation for the presence of CH3NCO in the ISM, but quantitative predictions of column densities, allowing a direct comparison with observations, are difficult due to the absence of kinetic data and to the uncertainty about the concentration of the proposed chemical precursors of methyl isocyanate, both in the ices and in the gas phase.
The production of CH3NCO by reactions (1) and (2) requires the presence of radicals within the ice. The experiments of Ligterink et al. (2017) show that UV photolysis of CH4 could account for the formation of the necessary CH3 radicals. Besides UV photons, cosmic rays (CRs) and thermal heating can induce chemistry in astrophysical ices by producing active species (atoms, radicals, or ions) in the solid medium (Allamandola et al. 1988; Moore et al. 2001; Baratta et al. 2002). However, energetic processing of ices has ambivalent effects. On the one hand it can trigger a chemistry leading to an increase in molecular complexity (Jamieson et al. 2006; Linnartz et al. 2011; Theulé et al. 2013; Öberg 2016), but on the other hand it can destroy complex structures. The occurrence of a given molecule in a particular environment will depend on the balance between these two effects.
In this work we study the destruction of CH3NCO in ices under energetic processing. Ice samples of pure methyl isocyanate and of methyl isocyanate diluted in water ice are irradiated with UV photons and bombarded with high-energy (5 keV) electrons, which mimic the secondary electrons produced by CRs (Kaiser et al. 2013; Mason et al. 2014; Maté et al. 2015, 2016). The decay of the CH3NCO concentration as a function of the energy doses absorbed is derived from these measurements, and the implications for the survival probability of CH3NCO in interstellar and cometary ices are discussed.
2. Experiment
The set-up for the generation, processing, and infrared spectroscopy of ices has been described extensively elsewhere (Maté et al. 2003, 2014; Gálvez et al. 2007) and only the details relevant to this work are given here. It consists of a vacuum chamber with a closed-cycle He cryostat, whose cold finger holds an IR-transparent Si window where ices of the species of interest are condensed from the vapor phase. The temperature of the Si substrate can be varied between 14 K and 300 K with 1 K accuracy. The chamber is coupled through two KBr windows to a Vertex70 Fourier transform IR spectrometer configured for normally incident transmission. A rotatable flange allows the orientation under vacuum of the Si substrate, which in the course of the present experiments faced alternatively the IR beam of the spectrometer or a chamber port provided with a UV lamp or an electron gun. The spectra were recorded with a resolution of 2 cm−1 using an HgCdTe (MCT) detector refrigerated with liquid nitrogen. The gas-phase composition during deposition was monitored by means of a calibrated quadrupole mass spectrometer (QMS) placed in an adjacent chamber (Maté et al. 2017). The base pressure in the vacuum chamber was ∼(0.7–1) × 10−8 mbar. Methyl isocyanate was prepared with a modified version of the synthesis of Han et al. (1989). Silver cyanate (15.0 g, 0.1 mol), dry diethyleneglycol dibutyl ether (25 mL), and iodomethane (7.10 g, 50 mmol) were mixed together in a cell equipped with a magnetic stirring bar and a stopcock. The cell was immersed in a liquid nitrogen bath and degassed. The mixture was heated under stirring at 90°C for 20 hr. The cell was then fitted on a vacuum line (0.1 mbar) equipped with two traps and low boiling compounds were distilled. The first trap was immersed in a bath cooled at −30°C to remove high boiling compounds. The low boiling methyl isocyanate was selectively condensed in the second trap immersed in a liquid nitrogen bath. The yield was 40%. (Caution: methyl isocyanate is a highly toxic, severe lachrymatory agent and should be handled with care.)
Methyl isocyanate vapor was introduced to the chamber by means of a needle valve connecting the chamber with a small Pyrex flask containing approximately 5 ml of liquid methyl isocyanate. For a better handling of the vapor flow to the chamber, the flask was held in an ice bath or in a bath with ice and salt. The liquid samples were subjected to three freeze–pump–thaw cycles for degassing before allowing methyl isocyanate into the vacuum chamber. Deposits were formed at a substrate temperature of 20 K. Deposition pressures in the range ≈10−6 mbar and deposition times of several minutes were typically used. Ice mixtures of CH3NCO and H2O, with typical CH3NCO/H2O ratios in the range 4%–5%, were also co-deposited on the substrate. Separate entrances were used for the H2O and CH3NCO vapors, and the mixture proportion in the ices was estimated from the absorbance ratio of the most intense absorption bands of water: A = 1.9 × 10−16 cm molecule−1 for ν-OH (Mastrapa et al. 2009), and methyl isocyanate: A = 1.3 × 10−16 cm molecule−1 for νa–NCO (Maté et al. 2017).
The deposited CH3NCO and CH3NCO/H2O samples were processed with UV photons and high-energy electrons. A D2 lamp, Hamamatsu L10706, was used for the UV irradiation measurements. The spectral range of the lamp goes from 120 to 400 nm with most of the emission concentrated at wavelengths <180 nm. In the following we will focus on the range 120–180 nm (10.3–6.9 eV) and will assume that photon absorption by ices of water or CH3NCO is negligible for longer wavelengths. The VUV absorption cross section for water ice has recently been measured by Cruz-Diaz et al. (2014) and is presented in Figure 1. As far as we know, no absorption cross sections have been reported for CH3NCO ice, but the gas-phase measurements of Tokue et al. (1987) show that UV absorption for wavelengths >180 nm is very small compared with that in the range 120–180 nm.
Figure 1. Upper panel: irradiance spectrum, S0(λ), of the UV lamp at the location of the samples (see text). Lower panel: absorption cross section of amorphous water ice at 8 K, taken from Cruz-Diaz et al. (2014).
Download figure:
Standard image High-resolution imageThe irradiance profile of the lamp was provided by the manufacturer and is presented in Figure 1 at the location of the substrate (3 cm from the lamp). The integrated irradiance reaching the sample, S0, is given by
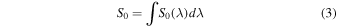
where So(λ) is the wavelength-dependent irradiance. Integrating Equation (3) over the range 120–180 nm yields S0 = 100 μW cm−2. The corresponding photon flux, ϕph, is derived as
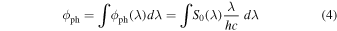
where h is Planck's constant and c the speed of light. Integration of Equation (4) over the interval 120–180 nm leads to ϕph = 7.5 × 1013 photons cm−2 s−1.
For the electron bombardment experiments, we used 5 keV electrons from an electron gun built in our laboratory (Maté et al. 2014). The gun provides a homogeneous electron flux covering the whole area of the exposed Si substrate (a circle of diameter 1 cm) available for deposition and IR spectroscopy. As in previous works (Maté et al. 2015, 2016), care was taken to ensure that the electron penetration depth was larger than the thickness of the ice samples. The flux at the substrate was measured to be ϕe = 4 × 1012 electrons cm−2 s−1. The relevant sample properties and processing conditions are summarized in Table 1.
Table 1. Column Densities in the Ice Samples Together with Energies and Fluxes of the Photons and Electrons used for Processing
| Sample | N(CH3NCO) | N(H2O) | Processing Agent | φ |
|---|---|---|---|---|
| (molecules cm−2) | (molecules cm−2) | (cm−2 s−1) | ||
| S1: CH3NCO | (4.5 ± 0.9) × 1016 | ⋯ | UV (10.3–6.9 eV) | 7.5 × 1013 |
| S2: CH3NCO(4%)/H2O | (2.5 ± 0.5) × 1016 | (6.4 ± 0.6) × 1017 | UV (10.3–6.9 eV) | 7.5 × 1013 |
| S3: CH3NCO | (4.3 ± 0.9) × 1016 | ⋯ | Electrons, 5 keV | 4 × 1012 |
| S4: CH3NCO(5%)/H2O | (3.5 ± 0.7) × 1016 | (6.5 ± 0.6) × 1017 | Electrons, 5 keV | 4 × 1012 |
Note. The column densities correspond to the deposits formed on one side of the Si substrate.
Download table as: ASCIITypeset image
IR spectra of the ices were recorded as a function of processing time, and special attention was paid to the decay of the asymmetric stretching band, νa–NCO, of CH3NCO. In our experiments, vapor deposition from the background gas takes place simultaneously on both sides of the substrate, whereas UV irradiation and electron bombardment are performed on just one side. In order to correct for the effect of the unprocessed face of the substrate, the IR spectrum of the freshly deposited samples was divided by two and subtracted from the spectra taken in the course of energetic processing. With a base pressure of ∼(0.7–1) × 10−8 mbar, water vapor from the chamber deposited on our samples at an approximate rate of ∼(3–6) × 1015 molecules cm−2 hr−1. The effect was small in the electron bombardment experiments, which lasted for less than an hour. In the longer (about 5 hr), UV irradiation experiments the deposited water was well within the estimated uncertainty of the H2O column density of the mixed CH3NCO(4%)/H2O ice, but it had some influence on the spectrum of pure CH3NCO ice, especially in the OH stretching region around ∼3200 cm−1. The effect was corrected by subtracting the appropriate H2O spectrum from the spectra of the irradiated samples. Finally, to check the effect of temperature on methyl isocyanate diluted in water, ice samples of CH3NCO(4%)/H2O mixtures were heated from deposition temperature (20 K) until sublimation at a rate of 5 K minute–1. To record the IR spectra, heating was halted at the selected temperatures.
3. Results and Discussion
3.1. Main Processing Products
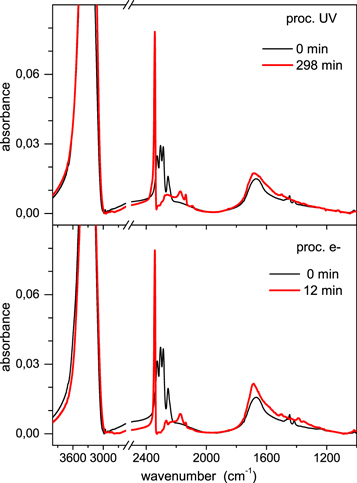
The effects of UV irradiation and electron bombardment on the samples of pure CH3NCO ice and of CH3NCO diluted in water ice are reflected in the IR spectra of Figures 2–5. In the unprocessed samples the νa–NCO band, with its characteristic quadruplet structure, stands out at 2400–2200 cm−1. There are some differences in the peak positions and in the band profiles between the spectra of pure methyl isocyanate and those of methyl isocyanate diluted in water ice, but the characteristic quadruplet structure is present in both cases (see Maté et al. 2017 for a discussion on the IR spectroscopy). The νa–NCO band is the most intense IR absorption of methyl isocyanate. After sufficiently long processing, this band virtually disappears and is replaced by four new bands, which are assigned to stretching vibrations of CO2, OCN−, CO, and HCN or CN− (see upper panels of Figures 3 and 5). The locations of the new band maxima for the different samples are listed in Table 2. Note that the same products are obtained in all cases although their relative abundances are very different. In the ices of pure methyl isocyanate (Figures 2 and 3), the main product bands are those of CO2 and CO, which appear with comparable intensities. In contrast, in the ices of CH3NCO diluted in H2O (Figures 4 and 5), the spectra of the products are largely dominated by the CO2 band. In the mixture processed with electrons (S4) the HCN/CN− feature is hardly visible. The peak frequencies of the neutral molecules CO2 and CO are stable within 2 cm−1 for all the samples, whereas those involving the OCN− and CN− anions appear redshifted (between 6 and 15 cm−1) in the mixtures with water ice as compared with those without water, which suggests that the CN bond is somewhat weakened in the solvated anions.
Figure 2. Effects of energetic processing on samples of pure CH3NCO (see Table 1). Upper panel: UV irradiation of sample S1. Lower panel: electron bombardment of sample S3.
Download figure:
Standard image High-resolution imageFigure 3. Detail of IR spectra of Figure 2. Am.I, Am.II, and Am.III refer to amide bands (see text and Table 2).
Download figure:
Standard image High-resolution imageFigure 4. Effects of energetic processing on samples of CH3NCO diluted in water ice (see Table 1). Upper panel: UV irradiation of sample S2 (4% CH3NCO in H2O). Lower panel: electron bombardment of sample S4 (5% CH3NCO in H2O).
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageTable 2. IR Bands of the Products of CH3NCO Ice Processing
| IR Frequencies of the Products of Ice Processing (cm−1) | Assignment | |||
|---|---|---|---|---|
| S1 | S2 | S3 | S4 | |
| CH3NCO, | CH3NCO(4%)/H2O, | CH3NCO, | CH3NCO(5%)/H2O, | |
| UV | UV | electrons | electrons | |
| 3572 | 3524 | ν–NH | ||
| (3700–3300) | (3701–3302) | |||
| 2951 | 3006 | ν–CHx | ||
| (3285–2466) | (3247–2466) | |||
| 2920–2800 | 2920–2800 | ν–CHx | ||
| 2342 | 2342 | 2342 | 2342 | CO2, νa |
| 2165 | 2172 | 2165 | 2171 | OCN−, ν–CN |
| 2138 | 2137 | 2139 | 2137 | CO, ν |
| 2075 | 2091 | 2078 | 2092 | HCN/CN−, ν–CN |
| 1814–1055 | 1823–1054 | Amide bands | ||
| 1728 | 1716 | ν–C=O | ||
| 1651 | 1667 | Amide I | ||
| (mostly ν–C=O) | ||||
| 1570 | 1570 | Amide II | ||
| (mostly in-phase δ–NH + ν–CN) | ||||
| 1499 | 1498 | H2CO, δ–CH2 | ||
| 1460 | 1455 | δ–CHx | ||
| 1363 | 1380 | 1378 | 1378 | δs–CHx |
| 1352 | 1351 |
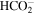 , νs–CO , νs–CO |
||
| 1303 | 1301 | Amide III | ||
| (mostly out-of-phase δ–NH + ν–CN) | ||||
Note. For the band assignment see Boogert et al. (2015), Hudson et al. (2001), Moore & Hudson (2003), Hudson & Moore (2004), Gálvez et al. (2010), Maté et al. (2012a, 2016), Cannon (1960), Barth & Zscherp (2002). Symbols ν and δ stand for stretch and deformation vibrations respectively.
Download table as: ASCIITypeset image
Besides the features just commented on, the spectra of processed ices of pure CH3NCO (Figures 2 and 3) show broad absorptions, attributable to polyamides. These broad bands appear at ∼3700–3300 cm−1 (NH stretch vibrations), ∼3250–2460 cm−1 (stretch vibrations of CHx alkyl groups), and 1820–1050 cm−1 (amide bands). The maxima of these absorptions and the peak substructure observed in the amide band region are listed and tentatively assigned in Table 2. Similar IR spectra, with peaks of CO2, OCN−, CO, HCN/CN−, and amide bands, were found after the electron bombardment of pure glycine ices (Maté et al. 2015). In the processed ices of CH3NCO diluted in H2O, minor peaks, consistent with vibrations of CHx groups, appear at ∼2950, 1500, and 1380 cm−1 and a peak possibly due to a stretching of the formate anion,  , is found at ∼1350 cm−1 (see Table 2). The intense water absorptions at ∼4000–3000 cm−1 and ∼1660 cm−1 can mask the presence of small peaks of other functional groups.
, is found at ∼1350 cm−1 (see Table 2). The intense water absorptions at ∼4000–3000 cm−1 and ∼1660 cm−1 can mask the presence of small peaks of other functional groups.
The mechanisms underlying the observed chemical transformations are not known in detail, but the similarity between the products of electron and photon processing is worth noting. In the following, we elaborate on likely reaction pathways induced by the energetic processing of the ices. Our experiments do not provide detailed mechanistic information, and the discussion, based on the observed final spectra and on literature sources, remains to a large extent speculative.
Two primary dissociation channels were identified in the gas-phase photolysis of CH3NCO with UV photons of 210 nm (Bamford & Bamford 1941):
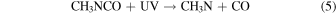
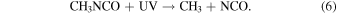
The same dissociation pathways can be assumed to hold for solid-state CH3NCO. The radicals produced in the photolysis are highly reactive and can start a chain of reactions in the ice leading to stable molecular species or even polymers. With increasing photon energy, additional channels become open. The radical CH2NCO, for instance, which corresponds to the breaking of a CH bond, was identified in the radiolysis of frozen CH3NCO at 77 K with 60Co γ-rays (Symons & Trousson 1984). The formation of OCN− was observed by Hudson et al. (2001) after bombarding mixtures of C2H5NCO and H2O at 16 K with 0.8 MeV protons. The protons induce a cascade of secondary electrons in the ice and the authors of that work suggested that OCN− is formed through dissociative electron attachment to C2H5NCO. The OCN− anion, a linear molecule with 16 valence electrons like CO2, is a stable species, especially at cryogenic temperatures. A similar mechanism can be assumed for the electron bombardment of methyl isocyanate ice in the present work:
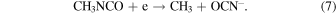
The location of the negative charge on the OCN fragment is the obvious outcome of the dissociative attachment because OCN has a high electron affinity (3.61 eV, as measured by Bradforth et al. 1993) and the  anion is less stable than the methyl radical (Marynick & Dixon 1977). The fact that a similar OCN− proportion is found in the UV photolysis and electron bombardment experiments (Figures 2 and 3) may be puzzling at first sight, since the photon energy of the present experiments (<10.3 eV) is small compared with the ionization potentials of the molecules present in the ices. However, the threshold for photoionization of molecules in polar ices might be appreciably lower than that of the same molecules in the gas phase, as discussed in Gerakines et al. (2000) and Woon (2004). This would make electrons and ions available for chemistry in the UV-irradiated ices. Chemical processes involving the dissociation products of reactions (5) and (6) can also contribute to the formation of the secondary products CO2 and HCN, and possibly also the anions OCN− and CN−. Low-temperature acid–base chemistry is often invoked to explain the appearance of charged species in ices (Hudson et al. 2001; Raunier et al. 2004; Gálvez et al. 2010; Theulé et al. 2013) and it might also be relevant for the formation of the anions in our experiments, but in the case of the pure CH3NCO samples the reaction pathways are not obvious. The formation of aggregates and polymers in the processing of pure CH3NCO ices is not entirely surprising. The polymerization of isocyanates in the liquid phase at low temperatures to yield polyamides was described in the sixties by Shashoua et al. (1960). These authors suggest a polymerization mechanism based on anionic catalysis. A similar mechanism could be at play in the present ice processing experiments.
anion is less stable than the methyl radical (Marynick & Dixon 1977). The fact that a similar OCN− proportion is found in the UV photolysis and electron bombardment experiments (Figures 2 and 3) may be puzzling at first sight, since the photon energy of the present experiments (<10.3 eV) is small compared with the ionization potentials of the molecules present in the ices. However, the threshold for photoionization of molecules in polar ices might be appreciably lower than that of the same molecules in the gas phase, as discussed in Gerakines et al. (2000) and Woon (2004). This would make electrons and ions available for chemistry in the UV-irradiated ices. Chemical processes involving the dissociation products of reactions (5) and (6) can also contribute to the formation of the secondary products CO2 and HCN, and possibly also the anions OCN− and CN−. Low-temperature acid–base chemistry is often invoked to explain the appearance of charged species in ices (Hudson et al. 2001; Raunier et al. 2004; Gálvez et al. 2010; Theulé et al. 2013) and it might also be relevant for the formation of the anions in our experiments, but in the case of the pure CH3NCO samples the reaction pathways are not obvious. The formation of aggregates and polymers in the processing of pure CH3NCO ices is not entirely surprising. The polymerization of isocyanates in the liquid phase at low temperatures to yield polyamides was described in the sixties by Shashoua et al. (1960). These authors suggest a polymerization mechanism based on anionic catalysis. A similar mechanism could be at play in the present ice processing experiments.
In the irradiation of diluted mixtures of CH3NCO in water ice (Figures 4 and 5), additional destruction mechanisms are possible. Hydrolysis of methyl isocyanate is known to produce CO2 in a two-step process (Castro et al. 1985):
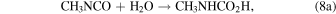
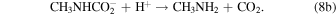
This reaction does not take place at the low temperatures of the experiments (see Section 3.3) but it could be activated if either H2O or CH3NCO is internally excited during irradiation. In the mixed ices, the dissociation of water plays a key role in the chemistry. The photolysis and radiolysis of water ice have been extensively studied by many groups (see Johnson & Quickenden 1997 and references therein). Photolysis takes place for UV photons with λ < 280 nm and the main dissociation channel is
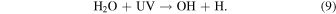
Subsequent photolysis of OH can lead to the production of O and H. Charged species can also be formed especially in the bombardment with highly energetic ions or electrons. The primary dissociation products, H and OH, recombine easily to H2O, but some of the atoms and ions react further, and in pure water ice this lead to the formation of small amounts of HO2, H2O2, O2, and H2. In the mixtures, H atoms and OH radicals can also react with CH3NCO. In the recent work of Belloche et al. (2017) hydrogenation of CH3NCO with H atoms has been suggested as a possible mechanism for formation of N-methylformamide (CH3NHCOH) in dense clouds. We are not aware of published spectra of CH3NHCOH ices but recent measurements in cold Ar matrices (Sałdyka et al. 2014) reveal the presence of two peaks at 1725 cm−1 (ν–CO) and 1518 cm−1 (δ–NH + ν–CN) that are not seen in our experiments; nevertheless the presence of N-methylformamide cannot be totally ruled out since the most intense of these peaks (ν–CO at 1725 cm−1) might be blended with the intense bending mode of water at ∼1660 cm−1. In fact, the shape of the 1660 cm−1 band changes upon processing and its maximum shifts towards 1700 cm−1.
OH radicals destroy CH3NCO molecules in the gas phase (Lu et al. 2014) and presumably in the solid phase too. In the ices, the hydroxyl radical is also known to react swiftly with CO (Oba et al. 2010):
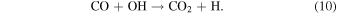
Reaction (10) is thought to be the main mechanism leading to the transformation of CO into CO2 in the photolysis (Allamandola et al. 1988; Watanabe & Kouchi 2002) and radiolysis (Hudson & Moore 1999) of diluted CO/H2O mixtures at low T. In the present work, most of the CO appearing as a primary dissociation product of CH3NCO in reaction (5) would be immediately transformed to CO2 through reaction (10), and that would contribute to the preponderance of the CO2 band in the IR spectra of the products (see Figure 4 and upper panel of Figure 5). The reaction of H atoms with CO could lead to the formation of formaldehyde and methanol (see Fuchs et al. 2009; Hama & Watanabe 2013 and references therein), and in fact a small peak at ∼1500 cm−1, attributable to H2CO, appeared in the spectra of processed CH3NCO/H2O samples. The ν–CO vibration of the molecule at ∼1720 cm−1 was not seen, but as indicated above it could be blended with large bending absorption of water at 1660 cm−1. Other chemical reactions, and particularly low-temperature acid–base chemistry, may be favored in water ice where species like H+ and OH− can be formed; but, in any case, under our experimental conditions, the breakdown of CH3NCO into smaller fragments prevails over any chemistry leading to complex molecules.
3.2. Destruction Cross Sections and Half-life Doses
The destruction rate of CH3NCO during irradiation with photons or electrons can be followed by monitoring the decay of the νa–NCO band. Assuming a first-order irreversible process, the decrease of the band intensity can be expressed as
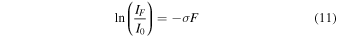
where F is the fluence (photons or electrons cm−2), IF the band intensity (absorbance) for a given fluence, I0 the band intensity before irradiation, and σ an effective destruction cross section. In the case of UV photolysis, both the cross section and the fluence depend on the photon wavelength, but our experiment yields only a cross section corresponding to the fluence integrated over the relevant wavelength range (120–180 nm). The experimental values of ln(IF/I0) are presented in Figure 6 as a function of F, and destruction cross sections can be obtained from a fit of these data to Equation (11). The experimental details and cross-section values for the four samples studied are given in Table 3. The integrated half-life fluence, i.e., the fluence needed to decrease the initial CH3NCO concentration by one half, is readily derived from Equation (11) as
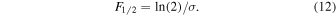
Figure 6. Decay of the intensity of the νa–NCO band (2372–2194 cm−1) during UV irradiation (upper panel) and electron bombardment (lower panel) at 20 K. The labels S1–S4 correspond to the samples of Table 1. In the UV irradiation experiments the fluence corresponds to the photon flux integrated over the interval 120–180 nm (see text). Note the different scale ranges of the horizontal axis. Black circles: samples of pure CH3NCO ice. Blue squares: samples of CH3NCO diluted in water ice. The lines correspond to a fit of the data to Equation (11). The experimental details and fitted cross-section values are listed in Tables 1 and 3.
Download figure:
Standard image High-resolution imageTable 3. Photolysis and Radiolysis of CH3NCO in the Ices of this Study
| Sample | Processing Agent | Destruction Cross Section, σ (cm−2) | Fraction of Energy Absorbed | Half-life Doses, D1/2 (eV molecule−1) |
|---|---|---|---|---|
| S1: CH3NCO | UV (10.3–6.9 eV) | (3.7 ± 0.1) × 10−18 | 0.15 ± 0.03 | 5 ± 2 |
| S2: CH3NCO(4%)/H2O | UV (10.3–6.9 eV) | (2.4 ± 0.05) × 10−18 | 0.68 ± 0.14 | 64 ± 19 |
| S3: CH3NCO | Electrons, 5 keV | (4.5 ± 0.1) × 10−16 | 0.05 ± 0.007 | 9 ± 2 |
| S4: CH3NCO(5%)/H2O | Electrons, 5 keV | (1.6 ± 0.04) × 10−15 | 0.30 ± 0.04 | 18 ± 5 |
Download table as: ASCIITypeset image
A useful measure of the destruction efficiency is the half-life dose. In the UV processing experiments it can be calculated as
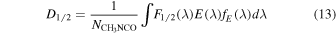
where  is the column density of methyl isocyanate, and F1/2(λ), E(λ), and fE(λ) are, respectively, the half-life fluence, the photon energy, hc/λ, and the fraction of the incident energy absorbed by the sample for a given wavelength. The product F1/2(λ)E(λ) can be expressed in terms of the incident irradiance as
is the column density of methyl isocyanate, and F1/2(λ), E(λ), and fE(λ) are, respectively, the half-life fluence, the photon energy, hc/λ, and the fraction of the incident energy absorbed by the sample for a given wavelength. The product F1/2(λ)E(λ) can be expressed in terms of the incident irradiance as
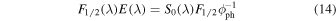
where F1/2 is the integrated half-life fluence given by Equation (12) and ϕph the integrated photon flux (note that the product  is the half-life time, t1/2). Substituting in Equation (13), we have
is the half-life time, t1/2). Substituting in Equation (13), we have
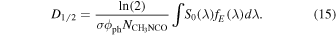
The fraction of energy absorbed can be expressed as
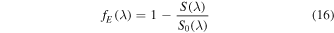
where S(λ) is the irradiance transmitted through the sample, which can be calculated using Beer's law. In the UV irradiation of CH3NCO diluted in water ice (sample S2), the energy is absorbed essentially by the much more abundant H2O molecules and we have neglected the absorption by CH3NCO. With this assumption Equation (15) can be written as
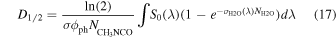
where σH2O(λ) and  are the absorption cross section and the column density of H2O. The CH3NCO destruction cross section, σ, for sample S2 is also listed in Table 3. The column densities
are the absorption cross section and the column density of H2O. The CH3NCO destruction cross section, σ, for sample S2 is also listed in Table 3. The column densities  ,
,  and the integrated photon flux, φph, are taken from Table 1. S0(λ) and σH2O(λ) are displayed in Figure 1. The half-life dose, derived by integrating Equation (17) over the range 120–180 nm, is given in Table 3.
and the integrated photon flux, φph, are taken from Table 1. S0(λ) and σH2O(λ) are displayed in Figure 1. The half-life dose, derived by integrating Equation (17) over the range 120–180 nm, is given in Table 3.
For the pure CH3NCO sample (S1) we have assumed that the irradiation of the sample leads essentially to the destruction of the molecules and we have approximated the absorption cross section to the destruction cross section. In this case the λ dependence of the cross section is unknown and we can only use the averaged value given in Table 3. Equation (17) transforms into
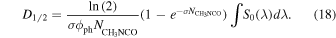
The corresponding half-life dose is also listed in Table 3.
In the electron bombardment experiments (samples S3 and S4), Equation (13) reduces to
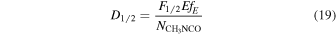
where F1/2 is obtained from Equation (12) with the cross sections of Table 3, and values of  and E are taken from Table 1. The fraction of energy, fE, deposited into the ices by the impinging electrons was calculated using the Monte Carlo Simulations of Electron Trajectories in Solids code (CASINO) (Drouin et al. 2007; Drouin 2011). We used CASINO version v3.2 in the Monsel CASINO physics mode. The simulations were run with 1000 electrons of 5 keV and trajectories were stopped for electron energies <50 eV. Input parameters also include densities and thicknesses of the processed samples. We derived the thickness values from the column densities of Table 1. A density of 1 g cm−3 was assumed for the pure CH3NCO ice (sample S3). The corresponding sample thickness was 40 nm. For the sample of CH3NCO diluted in water (sample S4) we considered just the absorption by the much more abundant water molecules. A thickness of 300 nm was obtained for this sample assuming a water density of 0.64 g cm−3, which corresponds to porous amorphous water, vapor-deposited at 20 K (Maté et al. 2012b). The half-life doses for the electron bombardment experiments are listed in Table 3.
and E are taken from Table 1. The fraction of energy, fE, deposited into the ices by the impinging electrons was calculated using the Monte Carlo Simulations of Electron Trajectories in Solids code (CASINO) (Drouin et al. 2007; Drouin 2011). We used CASINO version v3.2 in the Monsel CASINO physics mode. The simulations were run with 1000 electrons of 5 keV and trajectories were stopped for electron energies <50 eV. Input parameters also include densities and thicknesses of the processed samples. We derived the thickness values from the column densities of Table 1. A density of 1 g cm−3 was assumed for the pure CH3NCO ice (sample S3). The corresponding sample thickness was 40 nm. For the sample of CH3NCO diluted in water (sample S4) we considered just the absorption by the much more abundant water molecules. A thickness of 300 nm was obtained for this sample assuming a water density of 0.64 g cm−3, which corresponds to porous amorphous water, vapor-deposited at 20 K (Maté et al. 2012b). The half-life doses for the electron bombardment experiments are listed in Table 3.
The highest destruction efficiency is achieved, as expected, for the pure CH3NCO samples. Only a small fraction of the photons and electrons are absorbed by these thin deposits, but all the energy absorbed is invested in the dissociation of methyl isocyanate. The high half-life dose obtained for the UV photolysis of CH3NCO diluted in water ice is worth noting. The energy of the irradiating photons (<10.3 eV) is enough to dissociate the molecule, and even to produce some ionization, but the excess energy carried by the H and OH fragments is relatively small and they do not travel far within the ice. Their most likely fate is recombination to H2O, a process enhanced by the "cage effect" of the solid environment. In this case, the CH3NCO molecules are effectively shielded from the UV radiation by the water ice matrix. In contrast, the bombardment of the ice mixtures with 5 keV electrons induces a cascade of secondary electrons that propagate over comparatively long distances, provoking an extensive ionization and fragmentation of the molecules in the ice. Both the secondary electrons and the ions and radicals appearing in their wake contribute efficiently to the destruction of CH3NCO. The differences between UV and electron processing are made apparent in Figure 6. Dilution in water ice makes the decay of CH3NCO slower for UV irradiation, but faster for electron bombardment; as a result, the half-life dose for 5 keV electrons in the mixed ices is about 3.5 times lower than that for UV photons.
3.3. Thermal Processing
Thermal processing of ices can induce chemical reactions even at very low temperatures (see Theulé et al. 2013 and references therein) and could be largely responsible for the wealth of chemical species observed in hot cores and hot corinos (Boogert et al. 2015). In water-dominated astrophysical ices, hydrolysis is a likely reaction. Hydrolysis of CH3NCO (Equations (8a) and (8b) above) does not take place in ice mixtures at 20 K (Maté et al. 2017) but could happen in principle for higher ice temperatures. The effects of thermal processing on the νa–NCO band of CH3NCO diluted in water ice are shown in Figure 7. The measurements were carried out for an ice sample containing 4% CH3NCO molecules. Upon heating, the band profile is modified, but no new bands appear and the overall band intensity does not change much in the temperature interval 20–150 K. As discussed elsewhere (Reva et al. 2010; Maté et al. 2017), the multiplet structure of this band is most likely due to a coupling of NCO stretching and CH3 torsional modes. The spacing between the first torsional levels is of the order of 25–30 cm−1 and their populations should undergo significant variations with growing ice temperature, which would explain the observed modification of the band profile.
Figure 7. Evolution of the νa–NCO band of CH3NCO during the heating of a CH3NCO (4%)/H2O ice mixture.
Download figure:
Standard image High-resolution imageBetween 150 and 160 K, the process of "volcano desorption" (Smith et al. 1997) of CH3NCO associated with ice crystallization expels CH3NCO from the solid and leads to a marked decrease in the band intensity. Finally, the band disappears with ice sublimation. The absence of new peaks, and specifically of the 2345 cm−1 peak of CO2, indicates that methyl isocyanate is stable against hydrolysis over the temperature range of relevance for astronomical ices.
4. Astrophysical Implications
We can use the experimental results of this study to estimate the survival probability of methyl isocyanate in Kuiper Belt Objects (KBOs) and in the icy mantles of dust grains in cold clouds, where molecules are subjected to UV fields and CR bombardment. The Kuiper Belt, at a distance of ∼30–50 au from the Sun, is a major comet reservoir and was the original location of comet 67P/CG (Dones et al. 2015). Although ices in space are a mixture of various species (H2O, CO2, CO) we assume that the results obtained for CH3NCO molecules diluted in water ice studied in this work are representative of the behavior of hypothetical CH3NCO molecules in astronomical ices, since H2O is always the dominant component. As mentioned above, we also assume that high-energy electrons simulate the effects of CRs.
The half-lives estimated for CH3NCO molecules in KBOs or dense clouds subjected to either UV irradiation or CR bombardment are listed in Table 4. For these estimates we have used the half-life doses of Table 3 for CH3NCO molecules diluted in water ice (samples S2 and S4), and literature values for the corresponding UV and CR dose rates, which are also included in the table. These literature values, based on different measurements and models, contain many approximations and allow only order-of-magnitude estimates.
Table 4. Estimated CH3NCO Half-life in Dense Clouds and Cometary Ices
| Location of Ices in Space | Lifetime of Ices (yr) | Depth (cm) | UV Dose Rate (eV molecule−1 yr−1) | UV CH3NCO Half-life (yr) | CR Dose Rate (eV molecule−1 yr−1) | CR CH3NCO Half-life (yr) |
|---|---|---|---|---|---|---|
| Kuiper Belt Object | 4.6 × 109 | ⋯ | 2.2 × 10−2a | 1.1 × 104 | 5.6 × 10−3 b | 3.2 × 103 |
| (40 au) | 10−3 | ⋯ | ⋯ | 1.6 × 10−8 c | 1.1 × 109 | |
| Cold dense cloud | 107 | ⋯ | 4 × 10−7d | 1.6 × 108 | 3 × 10−7 d | 6 × 107 |
Notes. The half-life doses for CH3NCO diluted in H2O (samples S2 and S4) were used for these estimates. Literature dose rates refer to absorption by the dominant H2O (18 amu) ice molecules.
aMoore & Hudson (2005). UV dose rates are estimated for the top 1.5 × 10−6 cm of the ice. bCooper et al. (2003). CR dose rate for ice thickness <1 × 10−6 cm. cStrazzulla et al. (2003). dMoore et al. (2001). UV dose rates are estimated for typical grain mantles with a thickness of 2 × 10−6 cm.Download table as: ASCIITypeset image
Comet 67P/CG is a Jupiter-family comet (JFC) with origin in the Kuiper Belt (Dones et al. 2015). The most likely physical lifetime of a JFC is of the order of ∼104 yr (Levison & Duncan 1997), and hence the nuclei of present-day JFCs have resided mostly (∼4.6 × 109 yr) in the Kuiper Belt. The fluxes of UV photons and CR particles impinging on KBOs contain a large number of comparatively slow protons from the solar wind (Cooper et al. 2003). According to the estimate of the present work, at the uppermost surface of a KBO any primeval CH3NCO would have disappeared in just thousands of years. Furthermore, very efficient formation processes would be needed to counter the intense UV and CR bombardment and keep an appreciable surface concentration of the molecule. However, the water molecules in the ice provide a very efficient shield. Just 10 μm below the surface the UV field has disappeared and the CR flux decreases drastically due to the small penetration depth of the abundant low-energy solar protons. The estimated half-life of CH3NCO at this depth is ∼109 yr, of the order of the lifetime of the solar system. According to this estimate, KBOs could retain inside the ice a part of the hypothetical CH3NCO generated in the dense cloud phase. After leaving the Kuiper Belt, the fate of molecules is more difficult to estimate, but the efficient protection provided by water ice suggests that part of the primeval CH3NCO buried beneath the surface might survive the intense solar irradiation during the short JFC phase. When the comet approaches the Sun, dramatic changes can happen. In its present orbit, for instance, it is estimated that 67P/CG loses several centimeters of ice per year (Bieler et al. 2015). This should lead to the exposure and destruction of CH3NCO in the outer ice layers. However, if CH3NCO were uniformly distributed within the ice of the original comet, some of it could survive as long as there remained some ice with a thickness of the order of tens of microns.
The chemical composition of comet 67P/CG was studied by mass spectrometry in the course of the Rosetta mission. Mass spectra were acquired from three sources. The Ptolemy (Wright et al. 2015) and COSAC (Goesmann et al. 2015) instruments on board the Philae lander performed measurements after the first touchdown of the lander on the comet in 2014 November. COSAC sampled evaporated molecules from excavated material that entered the warm exhaust tubes located at the bottom of the lander, whereas Ptolemy sampled ambient coma gases. In 2016 September, the high-resolution DMFS spectrometer of the ROSINA experiment (Altwegg et al. 2017) also sampled molecules in the coma of the comet. Methyl isocyanate was only reported in the analysis of the COSAC data. A reanalysis of the first measurements in conjunction with the new high-resolution data from ROSINA (Altwegg et al. 2017) has shown that, due to limited mass resolution and low counting statistics, a unique interpretation of the Ptolemy and COSAC measurements is not possible. Specifically, the identification of methyl isocyanate could not be confirmed. Although the results of our experiments suggest that CH3NCO could persist under the surface of comet 67P/CG, the work of Altwegg et al. (2017) shows that, if present at all, its abundance should be much lower than that published in the initial COSAC report (Goesmann et al. 2015).
The interior of dense clouds is shielded from the Galactic UV field by gas molecules and dust grains, but CRs reaching these regions dissociate H2 molecules and excite H and H2, giving rise to a secondary UV field (Prasad & Tarafdar 1983). Both CRs and secondary UV photons can destroy molecules in ice mantles. Using the estimated dose rates reported by Moore et al. (2001) for ice mantles in a dense cloud, we obtain a half-life of ∼1.6 × 108 yr for CH3NCO molecules subjected to UV radiation. This time is appreciably longer than the life of a typical dense cloud (∼107 yr) and reflects the effective UV shielding provided by water ice already commented on in Section 3.2. The half-life of CH3NCO against CRs is ∼6 × 107 yr, still longer than the cloud's lifetime. Consequently, although a fraction of the hypothetical CH3NCO would be destroyed, mainly by CRs, in the dense cloud, some of it could survive the cloud collapse. As shown in Section 3.3, the molecule is stable in ice until sublimation and would desorb to the gas phase in hot cores, where it has been observed. In particular, in Orion KL, more than 400 rotational lines of CH3NCO were identified by Cernicharo et al. (2016).
5. Summary and Conclusions
The stability of methyl isocyanate ices against UV radiation and CRs has been investigated in the laboratory. Ices of pure CH3NCO and of CH3NCO(4%–5%)/H2O mixtures were deposited from the vapor phase on a substrate held at 20 K. The ices were then irradiated with a deuterium lamp, emitting in the range 120–400 nm, and bombarded with high-energy (5 keV) electrons, which are assumed to mimic CRs. The destruction of the molecule in the course of energetic processing is followed by monitoring the decay of the νa–NCO IR band (∼2372–2200 cm−1). The main results of the study are the following.
- 1.Energetic processing of CH3NCO ices for a sufficiently long time leads to the total destruction of the parent molecule and to the appearance of IR bands corresponding to CO, CO2, OCN−, and HCN/CN−. The formation of polyamides has also been observed. The results are very similar for photon and electron processing. In samples of pure CH3NCO the major products are CO2 and CO, which are present in comparable amounts. In the mixed CH3NCO(4%–5%)/H2O ices, the dominant product by far is CO2. Minor peaks in the IR spectra point to the formation of small amounts of other molecules, among them possibly H2CO, formate ions, and species containing alkyl (CHx) groups.
- 2.Destruction cross sections can be derived from the decay of the CH3NCO concentration, which is well described by a first-order irreversible reaction for both photons and electrons. The faster decay observed in the electron bombardment of mixed ices indicates that dissociation products from H2O contribute effectively to the destruction of methyl isocyanate.
- 3.Water ice provides an effective shield for CH3NCO against UV irradiation, as reflected by the high half-life energy dose (∼64 eV molecule−1) obtained for the CH3NCO(4%)/H2O mixture. The majority of the absorbed photons dissociate water molecules into H and OH, but most fragments recombine again to H2O before reacting with CH3NCO.
- 4.CH3NCO is stable in water ice. The molecule does not react with water over the temperature range 20–200 K.
- 5.Estimates based on the present measurements and on literature values of UV and CR fluxes indicate that methyl isocyanate should be stable against UV radiation and relatively stable against CRs in the mantles of dust grains over the lifetime of a typical dense cloud (∼107 yr). An appreciable fraction of hypothetical CH3NCO formed in the grain mantles should survive to the hot core phase. In an object in the Kuiper Belt (the original location of comet 67P/CG), CH3NCO should be readily destroyed on the surface by both UV photons and CRs, but it would persist for ∼109 yr under 10 μm of water ice.
This work has been funded by the Spanish MINECO under grants FIS2013-48087-C2-1P, FIS2016-C3-1P, and AYA2016-75066-C2-1-P. J.-C.G. and J.C. also thank the ANR-13-BS05-0008 IMOLABS and J.-C.G. thanks the Program PCMI (INSU-CNRS) and the Centre National d'Etudes Spatiales (CNES) for funding support. European Union funding under grant ERC-2013-Syg 610256 (NANOCOSMOS) is also acknowledged.