Abstract
Glycinamide, a glycine precursor, has been successfully generated in the gas phase by laser ablation of its hydrochloride salt, and its microwave spectrum, recorded from 6 to 16 GHz using a Chirped Pulse Fourier Transform Microwave (LA-CP-FTMW) spectrometer, is reported for the first time. The existence of a single structure stabilized by a Na–H ⋯ NtH2 hydrogen bond has been revealed in the supersonic expansion. The complex nuclear quadrupole coupling hyperfine structure due to the two 14N nuclei has been completely resolved and analyzed using a Molecular Beam Fourier Transform Microwave spectrometer in combination with laser ablation (LA-MB-FTMW spectrometer). The first precise data obtained on this glycine precursor could be of great importance for possible future identifications in the interstellar medium.
Export citation and abstract BibTeX RIS
1. Introduction
The detection of glycine in the interstellar medium (ISM) is one of the most pursued targets for the researchers in astrochemistry and astrophysics. Its presence would confirm that complex chemical reactions in this medium can synthesize the fundamental building blocks of life and thus, that its presence in the universe may be more widespread than accepted (Shaw 2006; Kwok 2011; Guillemin 2014). Attempts to observe glycine in the ISM have been reported (Brown et al. 1979; Hollis et al. 1980, 2003; Combes et al. 1996; Ceccarelli et al. 2000; Kuan et al. 2003; Cunningham et al. 2007; Jones et al. 2007) but its detection has never been confirmed (Snyder et al. 2005; Jones et al. 2007). Nevertheless, many amino acids have been found in meteorites, including glycine (Cronin & Pizzarello 1983; Ehrenfreund & Sephton 2006), which also was detected in comet Wild 2 (Elsila et al. 2009). Laboratory experiments have demonstrated that glycine can be naturally formed, together with other amino acids, in the analogs of interstellar icy grains by ultraviolet photolysis (Bernstein et al. 2002; Muñoz Caro et al. 2002). Many theoretical studies have been devoted to proposing potential mechanisms of glycine formation in the ISM (Basiuk 2001; Basiuk & Kobayashi 2002; Largo et al. 2004, 2010; Bossa et al. 2009; Redondo et al. 2015). Concretely, Garrod (2013) used chemical modeling to discuss the possible routes of glycine formation by grain-surface/mantle chemistry and gas-phase chemistry. Some chemical models include the participation of amino acid precursors (Rimola et al. 2010), which are also key candidates to be present in the ISM. The famous Strecker synthesis (Strecker 1850, 1854) is a set of chemical reactions that lead to the formation of amino acids in the laboratory by a mixture of ammonia, hydrogen cyanide, and aldehyde in water, that easily leads to aminoacetonitrile. Aminoacetonitrile can be then transformed to glycine by hydrolysis either in basic or acidic aqueous solution (Anslow & King 1925; Wyzlic & Soloway 1992). This last reaction is currently the most widely proposed synthesis mechanism of glycine in the ISM (Zhu & Ho 2004; Aponte et al. 2017). The hydrolysis of aminoacetonitrile occurs via the formation of the glycinamide intermediate (Commeyras et al. 1983; Rimola et al. 2012) (H2N–CH2–CONH2), which is then hydrolyzed into the amino acid glycine (see Figure 1). Therefore, the study of the precursors and their detection in the ISM is almost as important as that of the amino acid itself, to understand the chemical processes that could lead to the formation of building blocks of life.
Figure 1. Hydrolysis of aminoacetonitrile in glycine via the glycinamide.
Download figure:
Standard image High-resolution imageRotational spectra have been successfully used to identify most of the compounds found in interstellar space (Herbst & van Dishoeck 2009). In 2008, aminoacetonitrile was discovered in Sgr B2(N) (Belloche et al. 2008) by means of rotational spectroscopy data (Bogey et al. 1990). Thus, if the hydrolysis of aminoacetonitrile occurs in the gas phase or in the icy mantle on the grains surface in the ISM, glycinamide should also be present in the ISM. The importance of glycinamide for prebiotic chemistry was the primary motivation for the present microwave investigation. So far no experimental investigations of this vital molecule have been reported and its conformational landscape remains unknown.
In the pure form, solid glycinamide is chemically quite unstable, reacting when exposed to the air or when heated to be vaporized, thus preventing its easy measurement in the gas phase. It is only commercially available as a hydrochloride salt, where it appears in the protonated form. At the University of Valladolid, efficient procedures have been developed for the generation of neutral forms of proteogenic amino acids in supersonic expansion by laser ablation of their zwitterionic forms, allowing their conformational investigation using Fourier transform microwave techniques (Alonso & López 2015). Very recently, a comprehensive analysis of the millimeter and submillimeter-wave spectra of aminoacetonitrile (Kolesniková et al. 2017) and laser-ablated microwave spectra of hydantoin (Alonso et al. 2017), also a potential glycine precursor, have been reported. The microwave spectrum of glycinamide, successfully generated in the gas phase by laser ablation of its hydrochloride salt, is now first reported in the present work. The precise spectroscopic information provided here could be relevant to check the existence of glycinamide in the ISM.
2. Experimental Details
In the experimental procedure, finely powdered commercial hydrochloride glycinamide sample was mixed with a small amount of a copolymeric binder and pressed into cylindrical rods that were ablated using the third harmonic (355 nm) of a Nd-YAG picosecond laser. The vaporized products were seeded in neon at backing pressures of 10–12 bar and expanded adiabatically through a nozzle (pinhole 1 mm) into the vacuum chamber (10−6 mbar) of the spectrometer where the glycinamide molecules liberated from the salt were probed by laser ablation broadband chirped pulse Fourier transform microwave spectroscopy (LA-CP-FTMW) (Mata et al. 2012). A high-power excitation pulse of 300 W was used to polarize the molecules at frequencies from 6 to 16 GHz. Up to 40000 individual free induction decays (4 FIDs on each valve cycle at a 2 Hz repetition rate) were averaged in the time domain and Fourier transformed to obtain the broadband frequency domain spectrum shown in Figure 2.
Figure 2. (a) Broadband LA-CP-FTMW spectrum of glycinamide from 6 to 16 GHz. (b) A small section of the spectrum showing the unresolved hyperfine structure of the  transition. (c) Completely resolved nuclear hyperfine structure of the
transition. (c) Completely resolved nuclear hyperfine structure of the  transition by LA-MB-FTMW. Each hyperfine component is labeled with the corresponding values of I', F', I'', F'' quantum numbers.
transition by LA-MB-FTMW. Each hyperfine component is labeled with the corresponding values of I', F', I'', F'' quantum numbers.
Download figure:
Standard image High-resolution image3. Analysis and Results
The rotational spectrum of one rotamer was easily identified due to the very intense  ,
,  ,
,  and
and  μa-type R-branch transitions. Intense μb-type Q-branch and weaker μb-type R-branch transitions were also identified. No signals belonging to other species remained unidentified in the rotational spectra apart from known lines of common decomposition products (cyanoacetylene) and of water complexes. All rotational transitions of glycinamide were observed split into complicated hyperfine patterns, with many components spanning several MHz as depicted in Figure 2(b) for the
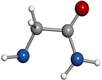
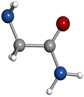
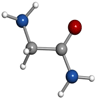
μa-type R-branch transitions. Intense μb-type Q-branch and weaker μb-type R-branch transitions were also identified. No signals belonging to other species remained unidentified in the rotational spectra apart from known lines of common decomposition products (cyanoacetylene) and of water complexes. All rotational transitions of glycinamide were observed split into complicated hyperfine patterns, with many components spanning several MHz as depicted in Figure 2(b) for the  transition. These complex hyperne patterns were accounted for in terms of nuclear quadrupole coupling interaction effects due to the presence in glycinamide of two 14N nuclei with nonzero electric quadrupole moment and spin (I = 1), which interact with the electric field gradient created by the rest of the molecule at these nuclei. The 14N nuclear quadrupole coupling splits the rotational energy levels decreasing the overall intensity of each rotational transition and giving rise to a very complex hyperfine structure (Gordy & Cook 1984). In a first step, no attempt was made to analyze the quadrupole hyperfine structure since the hyperfine components were not fully resolved. The rotational frequencies were measured as the intensity-weighted mean of the line clusters (see Table 1). A rigid rotor analysis generates a preliminary set of values for the rotational constants A = 9631.61 MHz, B = 3986.72 MHz, C = 2925.51 MHz that allows the identification of the observed rotamer of glycinamide. With this aim, a conformational search was carried out on all plausible configurations of glycinamide. Once a set of candidate structures was identified by force field and semi-empirical methods, we then employed higher-level calculations using the B3LYP density functional with the Grimme D3 dispersion interactions and Pople's 6–311++G (d,p) basis set using Gaussian (Frisch et al. 2009), in order to optimize the four different structures below 1000 cm−1. The predicted spectroscopic constants are collected in Table 2. The values of the rotational constants A, B, and C, which critically depend on the mass distribution, are usually a precise tool in the identification of conformers. The values predicted for the global minimum (conformer I) in Table 2 are very close to those experimentally determined.
transition. These complex hyperne patterns were accounted for in terms of nuclear quadrupole coupling interaction effects due to the presence in glycinamide of two 14N nuclei with nonzero electric quadrupole moment and spin (I = 1), which interact with the electric field gradient created by the rest of the molecule at these nuclei. The 14N nuclear quadrupole coupling splits the rotational energy levels decreasing the overall intensity of each rotational transition and giving rise to a very complex hyperfine structure (Gordy & Cook 1984). In a first step, no attempt was made to analyze the quadrupole hyperfine structure since the hyperfine components were not fully resolved. The rotational frequencies were measured as the intensity-weighted mean of the line clusters (see Table 1). A rigid rotor analysis generates a preliminary set of values for the rotational constants A = 9631.61 MHz, B = 3986.72 MHz, C = 2925.51 MHz that allows the identification of the observed rotamer of glycinamide. With this aim, a conformational search was carried out on all plausible configurations of glycinamide. Once a set of candidate structures was identified by force field and semi-empirical methods, we then employed higher-level calculations using the B3LYP density functional with the Grimme D3 dispersion interactions and Pople's 6–311++G (d,p) basis set using Gaussian (Frisch et al. 2009), in order to optimize the four different structures below 1000 cm−1. The predicted spectroscopic constants are collected in Table 2. The values of the rotational constants A, B, and C, which critically depend on the mass distribution, are usually a precise tool in the identification of conformers. The values predicted for the global minimum (conformer I) in Table 2 are very close to those experimentally determined.
Table 1. Observed Centers of Frequency and Residuals (in MHz) for the Rotational Transitions of Conformer I of Glycinamide
| J' |
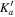
|
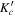
|
J'' |
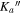
|
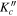
|
νobs | νobs–νcalc |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 1 | 0 | 0 | 0 | 6912.185 | −0.042 |
| 2 | 0 | 2 | 1 | 0 | 1 | 13688.501 | 0.066 |
| 1 | 1 | 0 | 1 | 0 | 1 | 6706.186 | 0.082 |
| 2 | 1 | 1 | 2 | 0 | 2 | 7903.277 | −0.051 |
| 3 | 1 | 2 | 3 | 0 | 3 | 9933.778 | −0.040 |
| 2 | 0 | 2 | 1 | 1 | 1 | 8043.501 | −0.034 |
| 4 | 1 | 3 | 4 | 1 | 4 | 10535.716 | −0.011 |
| 3 | 1 | 2 | 3 | 1 | 3 | 6356.274 | 0.055 |
| 2 | 1 | 2 | 1 | 1 | 1 | 12763.220 | −0.031 |
| 2 | 1 | 1 | 1 | 1 | 0 | 14885.629 | −0.030 |
| 3 | 0 | 3 | 2 | 1 | 2 | 15487.127 | 0.034 |
Note. νobs is the observed frequency and νobs–νcalc is the residual.
Download table as: ASCIITypeset image
Table 2. Spectroscopic Parameters and Relative Energies Calculated at the B3LYP/6-311++G(d,p) with Grimme Dispersion for the Lowest Energy Conformers of Glycinamide
| Parameter | I | II | III | IV |
|---|---|---|---|---|
| A/B/C | 9641/3976/2925 | 10066/3835/2867 | 9952/3939/2944 | 9515/3894/2855 |
| Pc | 6.7 | 5.7 | 7.4 | 5.9 |
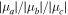
|
3.8/1.5/0.0 | 1.9/2.8/0.0 | 1.0/3.5/1.0 | 2.3/3.5/0.0 |
| 14Nt χaa/χbb/χcc | 2.40/−3.66/1.26 | −1.48/−0.52/2.00 | 2.95/2.04/−5.00 | −1.50/−0.91/2.41 |
| 14Na χaa/χbb/χcc | 1.79/2.19/−3.99 | 2.32/2.31/−4.62 | 2.13/2.18/−4.32 | 2.13/2.18/−4.32 |
| ΔE/ΔG | 0/0 | 745/257 | 1096/866 | 1162/934 |
|
|
|
|
Note. A, B, and C represent the rotational constants (in MHz); Pc is the planar inertial moment (in uÅ2), conversion factor: 505379.1 MHz-uÅ2; μa, μb and μc are the electric dipole moment components (in D); χaa, χbb and χcc are the diagonal elements of the 14N nuclear quadrupole coupling tensor (in MHz), Nt and Na correspond to the terminal and amide 14N nuclei, respectively; ΔE and ΔG are the relative and Gibbs energies (in cm−1) at 298 K with respect to the global minimum calculated at the B3LYP/6-311++G(d,p) with Grimme dispersion.
Download table as: ASCIITypeset image
The presence of 14N nuclei in our molecule is also a helpful tool to identify structures. In our predictions, we also included the values of the quadrupole coupling constants for both 14Nt (amine) and 14Na (amide) nuclei. The rotational constants give information of the mass distribution, while the nuclear quadrupole coupling interactions have a strong dependence on the electronic environment, position, and orientation of the 14N nuclei. The 14N nuclei introduce hyperfine rotational probes at defined sites of glycinamide and act as a probe of the chemical environment of the Nt and Na quadrupolar nuclei. The distinct orientation of the terminal amino group in all of the conformers causes a significant effect on the values of the quadrupole coupling constants for the 14Nt (see Table 2). To unveil, conclusively, the observed species, the treatment and interpretation of the quadrupole hyperfine structure of glycinamide is needed.
At this point, we took advantage of the sub-Doppler resolution of our LA-MB-FTMW spectrometer (Alonso et al. 2009; Bermúdez et al. 2014) to fully resolve the nuclear quadrupole hyperfine structure. The analysis began with the measurement of a total of 15 hyperfine components by interpreting the quadrupole coupling pattern for the  rotational transition. Then, new predictions were carried out to finally assign a total of 63 hyperfine components (see Table 3) corresponding to four a- and two b-type transitions. Watsons A-reduced semirigid rotor Hamiltonians in the Ir-representation (Watson 1977) complemented with a term to account for the nuclear quadrupole coupling contribution (Foley 1947; Robinson & Cornwell 1953) was used for the analysis of the transitions. The quadrupole coupling Hamiltonian was set up in the coupled basis set (I1I2IJKF), where I1 + I2 = I, I + J = F. The energy levels involved in each transition are thus labeled with the quantum numbers J, Ka, Kc, I, F. Table 4 reports accurate rotational and nuclear quadrupole constants determined from such analysis. Thus, a final comparison between the experimental and theoretical values of spectroscopic constants allows the unambiguous identification of the observed rotamer as conformer I.
rotational transition. Then, new predictions were carried out to finally assign a total of 63 hyperfine components (see Table 3) corresponding to four a- and two b-type transitions. Watsons A-reduced semirigid rotor Hamiltonians in the Ir-representation (Watson 1977) complemented with a term to account for the nuclear quadrupole coupling contribution (Foley 1947; Robinson & Cornwell 1953) was used for the analysis of the transitions. The quadrupole coupling Hamiltonian was set up in the coupled basis set (I1I2IJKF), where I1 + I2 = I, I + J = F. The energy levels involved in each transition are thus labeled with the quantum numbers J, Ka, Kc, I, F. Table 4 reports accurate rotational and nuclear quadrupole constants determined from such analysis. Thus, a final comparison between the experimental and theoretical values of spectroscopic constants allows the unambiguous identification of the observed rotamer as conformer I.
Table 3. Observed Frequencies and Residuals with or without Centrifugal Distortional Constants (in MHz) for the Rotational Transitions of Conformer I of Glycinamide
| J' |
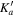
|
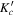
|
I' | F' | J'' |
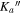
|
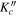
|
I' | F' | νobs | νobs–νcalc | νobs–νcalc | J' |
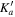
|
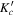
|
I' | F' | J'' |
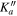
|

|
I' | F' | νobs | νobs–νcalc | νobs–νcalc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| with | without | with | without | ||||||||||||||||||||||
| Distortion | Distortion | Distortion | Distortion | ||||||||||||||||||||||
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 12555.898 | −0.003 | −0.004 | 1 | 1 | 0 | 2 | 2 | 1 | 0 | 1 | 2 | 3 | 6705.377 | −0.001 | 0.006 |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 12555.898 | −0.003 | −0.004 | 1 | 1 | 0 | 2 | 3 | 1 | 0 | 1 | 2 | 2 | 6705.539 | 0.002 | 0.003 |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 12555.898 | −0.003 | −0.004 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 6706.205 | 0.001 | 0.018 |
| 1 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 12556.447 | −0.001 | −0.004 | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 2 | 6706.339 | −0.006 | 0.008 |
| 1 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 12556.447 | −0.001 | −0.004 | 1 | 1 | 0 | 2 | 3 | 1 | 0 | 1 | 2 | 3 | 6706.374 | 0.002 | 0.004 |
| 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 12556.938 | −0.001 | −0.006 | 1 | 1 | 0 | 2 | 2 | 1 | 0 | 1 | 2 | 1 | 6706.421 | 0.002 | 0.012 |
| 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 12557.231 | 0.000 | −0.005 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 1 | 0 | 1 | 13687.508 | −0.001 | 0.021 |
| 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 12557.231 | 0.000 | −0.005 | 2 | 0 | 2 | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 13687.571 | −0.002 | 0.020 |
| 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 12557.231 | 0.000 | −0.005 | 2 | 0 | 2 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 13687.660 | 0.001 | 0.022 |
| 1 | 1 | 1 | 2 | 3 | 0 | 0 | 0 | 2 | 2 | 12557.284 | −0.001 | −0.006 | 2 | 0 | 2 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 13687.660 | 0.001 | 0.022 |
| 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 12557.774 | −0.002 | −0.009 | 2 | 0 | 2 | 1 | 2 | 1 | 0 | 1 | 1 | 2 | 13688.147 | −0.001 | 0.021 |
| 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 2 | 2 | 12557.774 | −0.002 | −0.009 | 2 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 13688.246 | −0.003 | 0.018 |
| 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 12558.891 | −0.003 | −0.012 | 2 | 0 | 2 | 2 | 4 | 1 | 0 | 1 | 2 | 3 | 13688.610 | −0.001 | 0.021 |
| 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 1 | 12558.891 | −0.003 | −0.012 | 2 | 0 | 2 | 1 | 3 | 1 | 0 | 1 | 1 | 2 | 13688.742 | 0.000 | 0.024 |
| 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | 12558.891 | −0.003 | −0.012 | 2 | 0 | 2 | 0 | 2 | 1 | 0 | 1 | 2 | 1 | 13689.303 | 0.001 | 0.027 |
| 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 6911.146 | 0.003 | 0.026 | 2 | 0 | 2 | 2 | 3 | 1 | 0 | 1 | 2 | 3 | 13689.422 | 0.005 | 0.027 |
| 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 1 | 6911.146 | 0.003 | 0.026 | 2 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 13689.609 | 0.000 | 0.020 |
| 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | 6911.146 | 0.003 | 0.026 | 2 | 1 | 1 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 14883.765 | 0.000 | 0.005 |
| 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 6911.924 | −0.001 | 0.026 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 2 | 14884.366 | −0.001 | 0.007 |
| 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 6911.924 | −0.001 | 0.026 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 2 | 14884.681 | 0.001 | −0.010 |
| 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 6911.924 | −0.001 | 0.026 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 14884.681 | 0.001 | −0.010 |
| 1 | 0 | 1 | 2 | 3 | 0 | 0 | 0 | 2 | 2 | 6912.191 | 0.006 | 0.032 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 0 | 2 | 1 | 14884.777 | 0.000 | −0.006 |
| 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 6912.453 | 0.002 | 0.027 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 1 | 14885.160 | −0.001 | −0.009 |
| 1 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 2 | 2 | 6912.453 | 0.002 | 0.027 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 14885.359 | −0.001 | −0.013 |
| 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6912.937 | 0.001 | 0.028 | 2 | 1 | 1 | 2 | 4 | 1 | 1 | 0 | 2 | 3 | 14885.634 | 0.002 | −0.014 |
| 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 6912.937 | 0.001 | 0.028 | 2 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 1 | 14885.779 | 0.002 | −0.015 |
| 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 6912.937 | 0.001 | 0.028 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 14885.810 | −0.005 | −0.028 |
| 1 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 6913.018 | −0.002 | 0.026 | 2 | 1 | 1 | 1 | 3 | 1 | 1 | 0 | 2 | 2 | 14886.036 | −0.002 | −0.021 |
| 1 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 6913.018 | −0.002 | 0.026 | 2 | 1 | 1 | 2 | 3 | 1 | 1 | 0 | 1 | 2 | 14886.348 | −0.001 | −0.014 |
| 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 6913.289 | 0.004 | 0.031 | 2 | 1 | 2 | 2 | 4 | 1 | 1 | 1 | 2 | 3 | 12763.202 | 0.008 | −0.075 |
| 1 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 1 | 6705.868 | 0.008 | 0.004 | 2 | 1 | 2 | 1 | 3 | 1 | 1 | 1 | 2 | 2 | 12763.736 | −0.002 | −0.080 |
| 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6705.879 | 0.002 | 0.002 | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... | ... |
Note. νobs is the observed frequency and νobs–νcalc is the residual.
Download table as: ASCIITypeset image
Table 4. Experimental Spectroscopic Constants of the Observed Conformer I of Glycinamide
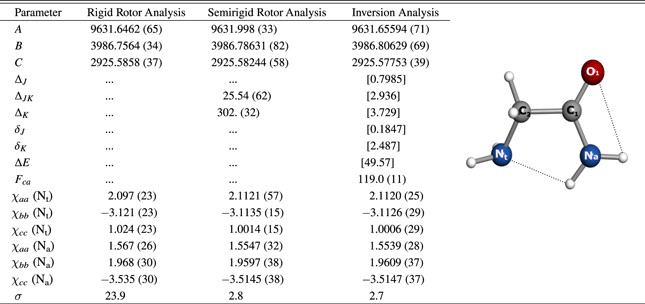 |
Note. A, B, and C are the rotational constants (in MHz); ΔJ to δK are the quartic centrifugal distortion constants (in kHz) where values in square brackets have been assumed from the quantum chemistry calculation; ΔE is the assumed ground state inversion splitting (in cm−1); Fca is the inversion coupling parameter (in MHz); χaa, χbb and χcc are the diagonal elements of the 14N nuclear quadrupole coupling tensor (in MHz); Nt and Na correspond to the amino 14N and amide 14N nuclei, respectively; σ is the root mean square deviation of the fit (in kHz).
Download table as: ASCIITypeset image
4. Discussion and Conclusion
An unexpected observation from the results of Table 4 is the abnormal nonrigid behavior. In the standard Watson Hamiltonian, this requires large values of centrifugal distortion parameters despite the low J values of the rotational energy levels involved in the measured transitions. The nonrigid contributions are most efficiently described by centrifugal distortion constants ΔJK and ΔK, as shown by comparison of the residuals. Frequencies and the rms deviations from the rigid and semirigid rotor analysis are collected in Tables 3 and 4. Nevertheless, the required values of these parameters are close to one (for ΔJK) and two (for ΔK) orders of magnitude greater than the values obtained from quantum chemistry calculations, listed in the final numerical column of Table 4. The origin of the nonrigid behavior can be attributed to the presence above the ground state of an unobserved low lying vibrational state. Decomposition of contributions to the calculated normal vibrational modes for glycinamide points to the existence of a large amplitude motion combining C2–Nt bond torsion and NtH2 inversion giving rise to a double minimum potential function with a low- energy barrier at the planar heavy atom configuration. For such potential functions, the two lowest vibrational energy levels occur at energy intervals comparable in magnitude to those of the rotational levels. Coriolis coupling interactions can, in such situation, take place between the observed ground vibrational state and the first torsional (or inversion) excited state, but the latter is not observed because it is expected to be depopulated due to vibrational cooling. When these interactions are small, they can be treated as perturbations, and their effects are reflected in abnormally high centrifugal distortion constants. In order to confirm the presence of such a double minimum potential, we varied the amine ∠CCNH dihedral angle and the calculations point to such a double minimum potential separated by a barrier of 116 cm−1 (see Figure 3). As can be seen, this torsion barrier separates two isoenergetic conformers and is sufficiently low to significantly split the ground state into two close lying states. These two states can be affected by significant mutual rotation-vibration coupling, rationalizing the need to use sizable centrifugal distortional constants at low J values. In fact, it is possible to explicitly test for such behavior by using Pickett's reduced axis Hamiltonian (Pickett 1972), as used to treat inversion in the cyanamide, H2NCN molecule (Kisiel et al. 2013). The rotational and centrifugal constants for the observed ground state (0+ state in the inversion picture) and for the excited (0−) state are set equal. The centrifugal constants are fixed and their values assumed at those from the quantum chemistry computation, while the inversion splitting between 0+ and 0− states is taken from cyanamide. The transferability of this value is not exact, as the motions in glycinamide and in cyanamide have different barriers and reduced masses but, in fact, this quantity turns out not to be critical. It is found (last numerical column of Table 4) that the nonrigid behavior can then be equally satisfactorily accounted for by fitting only the leading coupling constant, Fac, in the reduced Hamiltonian, which is equivalent to a second-order Coriolis coupling constant.
Figure 3. Potential energy surface scan of the CCNH amino torsion as a function of the energy. A barrier of 116 cm−1 interconnects the two isoenergetic conformers.
Download figure:
Standard image High-resolution imageThe first experimental information on the conformational properties of glycinamide is presented in this study. The capability provided by the laser ablation coupled with time domain Fourier transform microwave techniques to obtain very accurate spectroscopic constants, that in combination with theoretical computations, enables the unequivocal identification of the observed species. The two 14Nt and 14Na nuclei of glycinamide act as hyperfine rotational probes of molecular conformation that increase the usefulness of this spectroscopic technique even more. The obtained results indicate that glycinamide exits in the gas phase in a single conformation, stabilized by a Na–H ⋯ NtH2 hydrogen bond, which is the geometry predicted as the global minimum. The experimental approach followed in this study is a unique tool to pave the way toward more complex, unstable systems that have been discarded until now because of being considered out of reach of high-resolution spectroscopic studies. This is the case of many molecules, like glycinamide, considered to be potential candidates that are present in the ISM. The spectroscopic constants presented in this work constitute a first step to identify this glycine precursor in the ISM. Also, for observations in low-frequency regions like the ones accessible for the Green Bank Telescope, the hyperfine components of the transitions, which normally are propagated several MHz, could transpire to be a handicap for interpreting the radioastronomical observations. In this work, the experimental values of the 14N nuclear quadrupole coupling constants are also provided in order to reproduce perfectly the spectrum to enable its distinct possible identification.
The financial fundings from Ministerio de Ciencia e Innovación (Consolider-Ingenio 2010 CSD2009-00038 program "ASTROMOL," CTQ2013-40717-P and CTQ2016-76393-P), Junta de Castilla y León (VA077U16) and European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC-2013-SyG, grant Agreement No. 610256 NANOCOSMOS, are gratefully acknowledged. E.R.A. thanks Ministerio de Ciencia e Innovación for FPI grant (BES-2014-067776), and I.L.O. thanks Junta de Castilla y León for a postdoctoral contract. J.-C.G. thanks the Program PCMI (INSU-CNRS) and the Centre National d'Etudes Spatiales (CNES) for funding support.






