Abstract
High-speed atomic force microscopy (HS-AFM) is a technique that enables real-time imaging of nanoscale phenomena in solution. It was originally developed to visualize biomolecules, whose dynamics in solution significantly affect the manifestation of their functions, and has contributed to the understanding of molecular mechanisms based on the observation of single-molecule dynamics of proteins. In recent years, its application has broadened to include not only biomolecules, but also the structural dynamics of supramolecular assemblies that associate and dissociate in solution, as well as the evaluation of synthetic molecules such as polymer gels that swell in solution. In this paper, we review some of our recent studies on the application of HS-AFM to supramolecular polymers and hydrogel particles.
Export citation and abstract BibTeX RIS
1. Introduction
As the saying goes, "Seeing is believing," and nowhere is this truer than in the field of science, where direct observation is paramount to understanding the essence of natural phenomena. Since the invention of the optical microscope in the 16th century, microscopy has diversified and evolved, playing a pivotal role in viewing the world beyond the reach of the naked eye. Among the various types of microscopes, atomic force microscopy (AFM), 1) a subset of scanning probe microscopes, stands out for its wide range of applications and the diverse array of information it can provide. AFM uses a cantilever with a fine tip that interacts with the surface of a sample to produce images from the deflection of the cantilever. This allows objects to be observed in a variety of conditions, from vacuum to liquid, with nanoscale resolution, without the need to label the sample. In addition, by engaging the probe with the sample, AFM can reveal not only structural information, but also assess the mechanical properties of the surface. Since its invention in 1986, AFM has been applied to a wide range of polymers, from synthetic materials such as gels and rubbers to biological molecules such as proteins. 2–5)
However, a pervasive and significant limitation of conventional probe microscopes, including AFM, is their slow imaging speed. Compared to other types of microscopy, such as optical and electron microscopy, they suffer from slower imaging speeds, taking several seconds to minutes to acquire a single image. Poor temporal resolution is a significant problem when analyzing molecules such as proteins, where nanoscale dynamics play a critical role in their functional expression. Despite the advantage of being able to visualize nanostructures in liquids without labeling, observations have been limited to static properties. In this context, the successful development of high-speed AFM (HS-AFM), capable of imaging at rates faster than 100 ms/frame without sacrificing nanoscale spatial resolution, was a breakthrough in the observation of biomolecules. 6) HS-AFM has been instrumental in elucidating the dynamic phenomena of various proteins, providing unique insights that could not be obtained by other analytical methods. Its application has been particularly successful in the fields of protein science and biophysics. 7,8)
On the other hand, as mentioned above, AFM itself has a very wide range of applications, and thus the scope of HS-AFM is not limited to biological samples, but should be applicable to any dynamic phenomenon occurring at the solid-liquid interface. In fact, since dynamics in the solution environment is also a critical factor in understanding the properties of synthetic polymers, direct visualization techniques have been sought. In recent years, we have applied HS-AFM to the observation of synthetic polymers 9,10) and hydrogel microparticles, 11–13) aiming to elucidate their nanostructures and dynamics. In this paper, we present our recent results as a novel application of HS-AFM in the field of chemistry.
2. Application to the imaging of supramolecular dynamics
Assemblies based on non-covalent interactions, such as intermolecular hydrogen bonding and coordination bonds, are called supramolecular polymers and have shown great promise in recent years as high-function materials in fields such as electronics and medicine. 14) Supramolecular polymers, which are polymers held together by relatively weak interactions, can reversibly polymerize and depolymerize, a property that gives them exceptional self-healing properties. Structural analysis of supramolecular polymers has been performed using a variety of techniques, including X-ray crystallography, Nuclear Magnetic Resonance (NMR), circular dichroism spectroscopy, electron microscopy, and AFM, while fluorescence microscopy has been used to observe dynamics such as polymerization/depolymerization and self-healing processes. 15) The spatial resolution of fluorescence microscopy is limited to a few hundred nanometers, making it difficult to visualize nanoscale dynamics. It also requires the labeling of molecules with fluorescent dyes. On the other hand, HS-AFM can visualize various dynamics of supramolecular polymers at high resolution without the need for fluorescent labeling. Here, we present two cases where HS-AFM was employed to capture the intricate dynamics of fibrous 9) and toroidal 10) structures formed by porphyrin derivatives, demonstrating the method's ability to detail dynamic processes such as polymerization and depolymerization.
Porphyrin molecules are cyclic organic dye compounds that are widely available in nature as functional molecules. Porphyrin derivative 1 has the property of self-assembly through hydrogen bonding of the amide group and π–π interactions of the porphyrin plane, and forms nanoparticle-like aggregates (1NP) when heated and dispersed in methylcyclohexane (MCH) solvent and then cooled. 1NP is considered a metastable state and undergoes a structural transition to a fibrous aggregate, which is a stable structure, after a delay time of several hours. On the other hand, when fibers are fragmented by ultrasonication and mixed with nanoparticle aggregates, the fiber fragments act as nuclei and the nanoparticle aggregates rapidly transform into fibers. 16,17)
To observe the dynamics of supramolecular polymers with HS-AFM, the molecules must be supported on a solid substrate. In the case of proteins, hydrophilic mica surface is commonly used as the support substrate; however, hydrophobic supramolecular polymers do not adsorb to mica surface. Therefore, the nucleating fragmented nanofibers were adsorbed onto a hydrophobic highly oriented pyrolytic graphite (HOPG) surface. When nanofiber fragments were observed in MCH with HS-AFM and 1NP was added to the solvent, 1NP first adsorbed onto the HOPG substrate. When the HOPG surface was almost completely covered with 1NP, they began to bind to both ends of the fiber, initiating the growth of the fiber fragments.
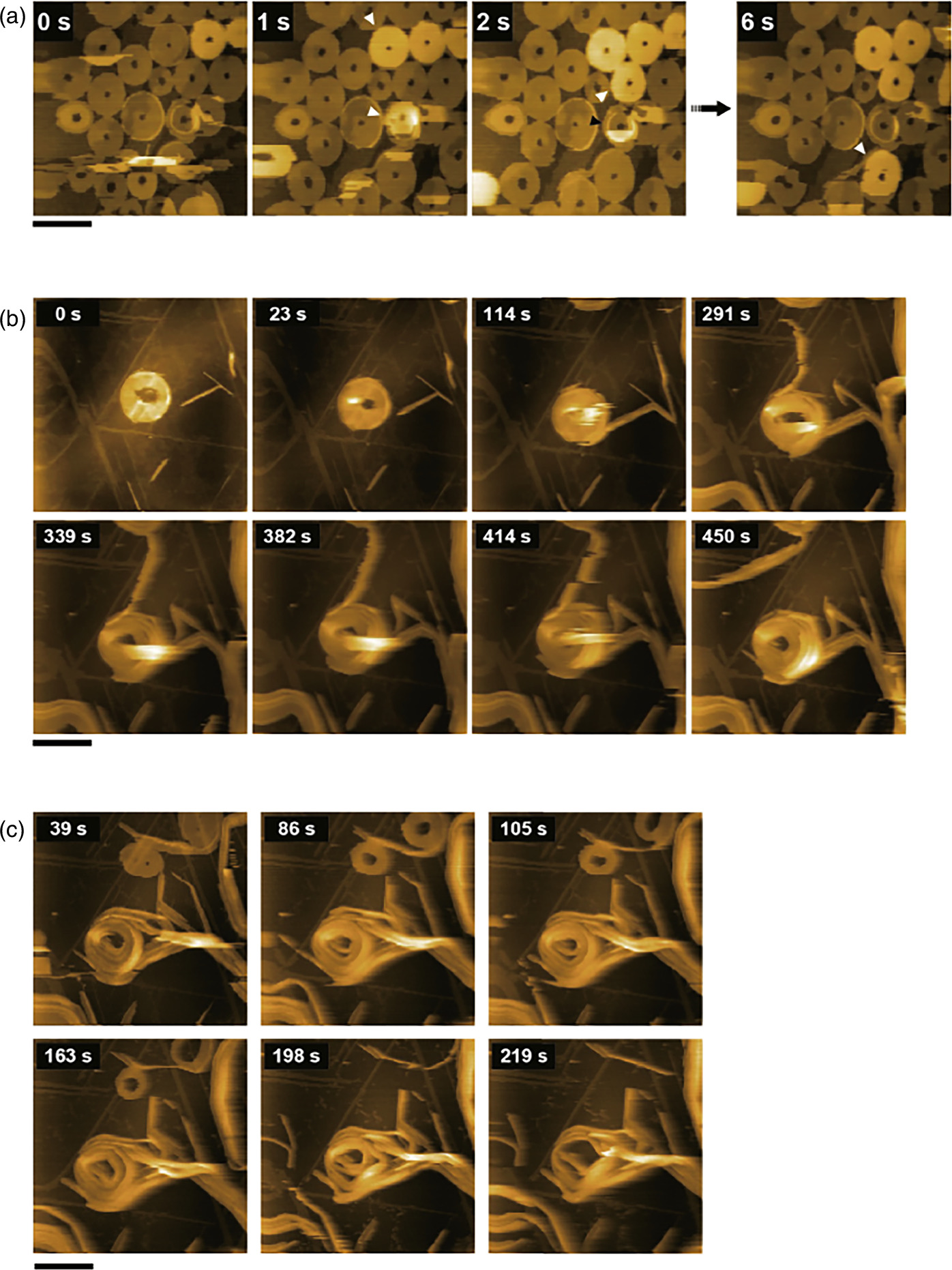
Figure 1(a) shows a series of HS-AFM images capturing the growth process of a single fiber. A single nanofiber grew continuously at an average rate of about 12 nm s−1 up to a length of about 10–20 nm, after which the fiber was often observed to shorten rapidly. This is probably due to the detachment of the fiber end from the substrate during polymerization and subsequent breakage by the lateral forces applied by the scanning AFM probe, as shown in Fig. 1(a), suggesting that single fibers are extremely unstable and their structure can be easily destroyed by small perturbations. It was also confirmed that the growth at both ends of the fiber is isotropic, with both ends growing at the same average rate. In addition, dozens of fibers bundled together were often observed, as shown in Fig. 1(b). In such bundled fibers, short fibers grow relatively stably along the longer fibers, indicating that fiber growth is stabilized by lateral interactions between fibers.
Fig. 1. Nanofiber formation process of porphyrin derivatives. (a) Clipped HS-AFM images of a single nanofiber growth process (left, scale bar: 15 nm, imaging speed: 0.1 s/frame). Time variation of the distance traveled by the fiber end (center) and the model diagram (right). (b) Growth of a bundle fiber (scale bar: 15 nm, imaging speed: 0.15 s/frame). Arrowheads indicate the ends of growing fibers. Reprinted with permission from Ref. 9. Copyright 2018 Wiley-VCH.
Download figure:
Standard image High-resolution imageIn AFM, the probe and sample are in mechanical contact during imaging, so mechanical loading can be applied to specific areas of the sample by probe contact. By temporarily lowering the feedback setpoint in predefined areas during raster scanning imaging, the amplitude of the cantilever oscillation is reduced, allowing a greater force to be applied by the AFM probe to a local area. 18) Using this function, we applied sufficient load to the fiber to cut it and form a gap. In the presence of 1NP in the solution, the self-healing process could be observed immediately after the gap formation, with polymerization progressing from the fiber ends on both sides of the gap and eventually joining, as shown in Fig. 2(a). Plotting the XY positions of the two fiber ends on either side of the gap over time [Fig. 2(b)] shows that the fiber ends repeatedly elongated and contracted. As seen at 7.9 s in Fig. 2(a), one fiber can grow in a curved fashion and pass the end of the other fiber. Although both fibers may continue to polymerize without crossing, in many cases the fiber ends will shorten again, and when the molecular orientation of the fiber ends on both sides align and come close together, the gap will eventually disappear. This demonstrates that fibers can change direction as they grow and contract, as if the molecules are "trying" to find a binding partner for self-repair. As can be seen in this example, HS-AFM enables us to directly observe the growth dynamics of supramolecular fibers and, by exploiting local structural changes due to external forces, to study in detail how locally damaged fibers self-repair.
Fig. 2. Gap formation in a nanofiber and self-healing process. (a) Clipped HS-AFM images (scale bar: 15 nm, imaging speed: 0.5 s frame−1). (b) Time variation of the XY positions of the fiber ends on both sides of the gap. The green and blue dots indicate the positions of the fiber ends after the gap formation, as shown in (a). Reprinted with permission from Ref. 9. Copyright 2018 Wiley-VCH.
Download figure:
Standard image High-resolution imageAs another example of supramolecular assembly, we briefly introduce the observation of the self-assembled concentric toroidal structures (SCTs) formed by the supramolecular assembly of the porphyrinato zinc derivative 1Zn in which each toroid consists of H aggregates (i.e. face-to-face stacking with a large overlap) of the porphyrin molecules. 10) HS-AFM imaging was performed in a 1:9 mixed solvent of toluene and dodecane, where the STCs, like the aforementioned supramolecular nanofibers, were well adsorbed on the HOPG substrate as shown in Fig. 3(a). Furthermore, successive images show that the SCTs suspended in the solvent are stacked on top of the SCTs of similar diameter adsorbed on the substrate. When monomer was added to the solvent, it was observed that thick fibers grew by wrapping around the SCT adsorbed on the substrate as shown in Fig. 3(b). On the other hand, in areas without the SCTs on the substrate, the growth of thick fibrous structures was observed, confirming that the molecules first form bundled fibers, which then wrap around the template structure to form a toroidal structure. Lewis bases such as 4-(dimethylamino)pyridine (DMAP) bind axially to the zinc atoms of 1Zn and depolymerize the H-aggregates, thereby decomposing the STC. Indeed, when DMAP was added to the observation solvent, the decomposition of the SCT could be observed and the central pore of the toroidal structure expanded as shown in Fig. 3(c).
Fig. 3. Polymerization and depolymerization of supramolecular concentric toroids (SCTs) (a) clipped HS-AFM images of SCTs on a HOPG substrate. The white arrowheads indicate the SCT stacked on top of the underlying SCT in the corresponding image. (b) Process of SCT with monomer addition. (d) Depolymerization of STC by binding of ligand (DMAP) binding. In all images, the scale bar is 200 nm and the imaging speed is 1 s/frame.
Download figure:
Standard image High-resolution imageThus, HS-AFM is capable of monitoring the elementary processes of polymerization and depolymerization of supramolecular self-assemblies in organic solvents in real time. In addition, although not presented here, it has also been demonstrated that by applying localized force exerted by the probe and exploiting the intrinsic self-repair mechanisms of the supramolecular self-assemblies, we can achieve the construction of localized block copolymers. 9)
3. Application to the observation of hydrogel particle
Polymer hydrogel microparticles, ranging in size from tens of nanometers to several micrometers, are composed of water and cross-linked polymers with hydrophilic or amphiphilic properties. As such, these hydrogels embody a unique material that integrates the inherent properties of hydrogels, such as stimulus-responsiveness, biocompatibility, and the ability to encapsulate molecules within the cross-linked network, with the properties of colloids, such as Brownian motion. 19–21) The major difference from bulk gels is their small size, which allows rapid diffusion of external stimuli such as changes in temperature or pH, resulting in instantaneous changes in physicochemical properties such as volume and deformability. These unique properties make them promising for applications in molecular separation supports, functional catalysts, and biosensors.
Hydrogel microparticles are typically synthesized by aqueous free-radical precipitation polymerization, which is driven by hydrophobic interactions of the precipitated polymer chains in water. This method allows the synthesis of gel microparticles of uniform size, which has led to their extensive use in a wide range of research, from fundamental studies to practical applications. 22) In the quest to elucidate the nanostructures that govern the properties of hydrogel microparticles, structural evaluations have mainly been performed by scattering methods 23) and microscopy, 24,25) revealing a characteristic crosslink density distribution originating from the precipitation polymerization method. However, due to their small size and rapid stimuli-responsiveness, it has been difficult to obtain information on the dynamics of individual hydrogel microparticles, including structural changes and disassembly processes in response to external stimuli. Here, we present some examples of the dynamic behavior of hydrogel particles, including thermoresponsive structural changes, chemical-induced degradation processes, and the initial polymerization process.
3.1. Structural changes of thermoresponsive hydrogel particle
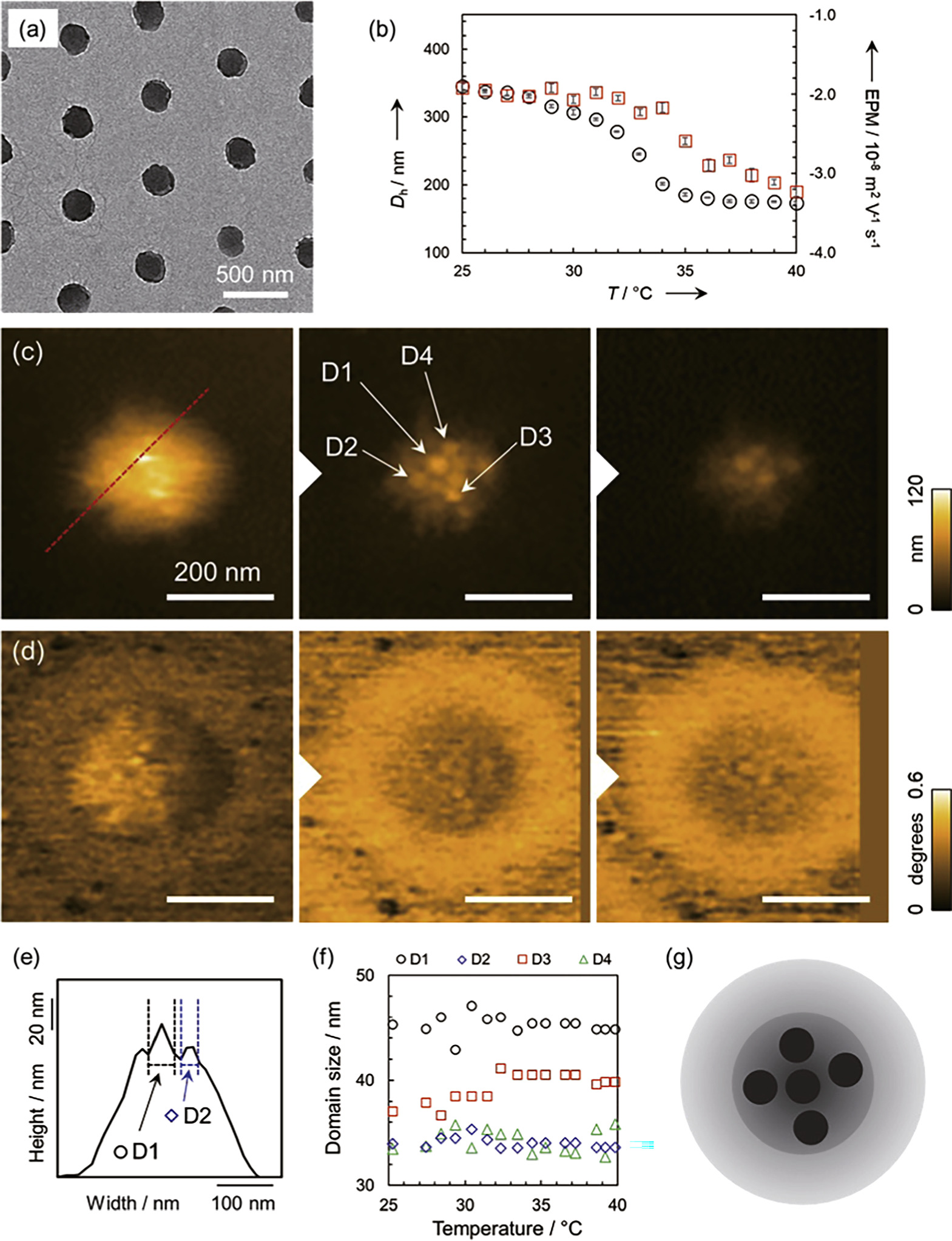
Using hydrogel microparticles composed of the thermoresponsive polymer poly(N-isopropylacrylamide) (pNIPAm) with a lower critical solution temperature (LCST) around 32 °C, and the cross-linker N,N'-methylenebis(acrylamide) (BIS) as a model [Figs. 4(a), 4(b)], nanostructural changes in response to temperature variations were evaluated using HS-AFM equipped with temperature control. 13) Figures 4(c) and 4(d) show topographic and phase images of the pNIPAm hydrogel microparticles taken at different solution temperatures. The microparticles were adsorbed on a hydrophobized mica substrate coated with a hydrophobic agent. HS-AFM observations were also performed in milli-Q water. Comparison between the topographic and phase images revealed distinct areas around the particles that are only visible in the phase images. It is known that hydrogel microparticles synthesized by precipitation polymerization form a core–shell heterogeneous structure with a high cross-linking density in the center of the gel particles because the initially formed polymer is introduced into the center of the particles and the cross-linking agent is more reactive than the monomer. Therefore, the regions observed only in the phase images are thought to be low density shell layers that have deformed on the substrate. In addition, within the core region visualized in the topographic images, spherical domain structures with a width of about tens of nanometers were found to exist in a swollen state at room temperature, as shown in Fig. 4(e). Although the overall volume of the microparticle decreases with increasing temperature, the size of the spherical domains does not change regardless of temperature, indicating the presence of non-thermoresponsive domains within the thermoresponsive hydrogel microparticles as shown in Figs. 4(f) and 4(g). Considering the mechanism of precipitation polymerization, the non-thermoresponsive domains are thought to be aggregates of polymer chains rich in cross-linking agents formed during the initial stages of polymerization process. This hypothesis was supported by the absence of heterogeneous non-thermoresponsive domains in microparticles synthesized by inverse miniemulsion polymerization, 26) in which polymer chains remain coiled state during cross-linking.
Fig. 4. Deswelling of pNIPAm-based thermoresponsive hydrogel particles synthesized by precipitation polymerization with increasing temperature. (a) TEM image of pNIPAm microgels. The amount of cross-linking agent BIS used was 3 mol%. (b) Temperature dependence of hydrodynamic diameter (Dh) and electrophoretic mobility (EPM) of microgels in the dispersed states derived by dynamic light scattering and electrophoretic light scattering, respectively. (c) Topographic and (d) phase images obtained by HS-AFM. (e) Cross section along the red line in the topographic image and the method for defining spherical domain structures. (f) Temperature dependence of spherical domain size. (g) Schematic illustration of plausible nanostructure for pNIPAm microgels. Reprinted with permission from Ref. 13. Copyright 2019 Wiley-VCH.
Download figure:
Standard image High-resolution image3.2. Decomposition process of hydrogel particle
For the application of hydrogel microparticles as carriers for drug delivery systems, the synthesis of degradable hydrogel microparticles has been developed using cross-linkers that cleave via chemical reactions. 27) However, the degradation mechanisms of hydrogel microparticles with heterogeneous structures at the microparticle scale as shown above remain to be elucidated. To address this issue, the degradation behavior of hydrogel microparticles cross-linked with N,N'-(1,2-dihydroxyethylene)bisacrylamide, which is cleaved by oxidation reactions, was monitored. 28)
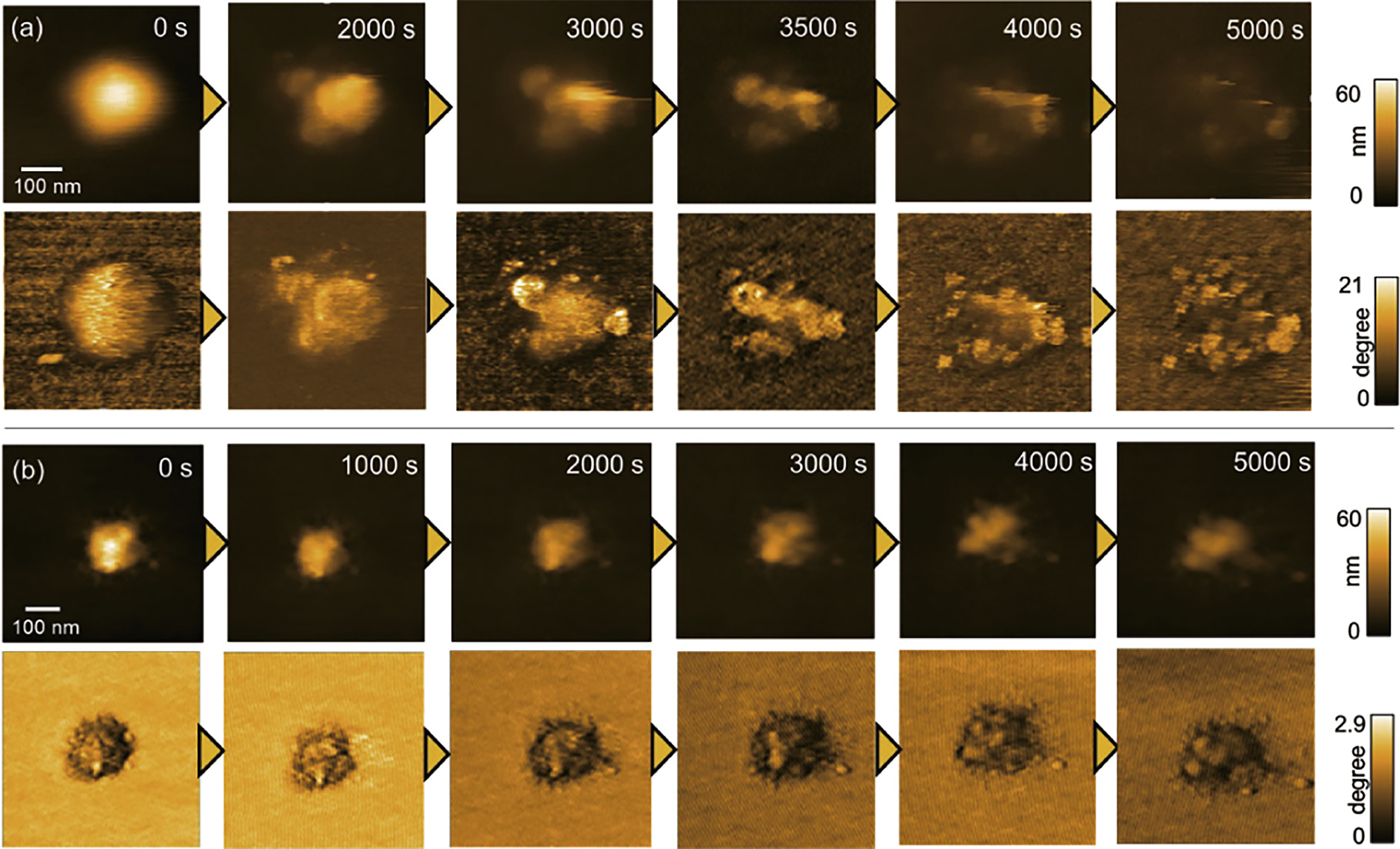
Figure 5 shows the degradation processes of the hydrogel microparticle after the addition of sodium periodate, an oxidizing agent, to the observation solution (milli-Q water) at different temperatures. The consecutive HS-AFM images shown in Fig. 5(a) indicate that the hydrogel microparticle in the swollen state at 25 °C degrades heterogeneously over one hour, accompanied by the formation of spherical degradation products. According to light scattering analysis, the degradation rate is slower in areas with higher crosslink density. 29) Therefore, the heterogeneity in degradation rates observed at the microparticle scale can be attributed to the uneven cross-linked structure resulting from precipitation polymerization. On the other hand, conducting imaging at 40 °C, which causes the hydrogel microparticles to deswell, resulted in a change in the degradation process, transitioning to a gradual degradation initiating from the particle surface Fig. 5(b). It is known that dehydrated polymers form hydrophobic networks that inhibit the diffusion of hydrophilic substrates. 30) Since the oxidizing agent used in this study was hydrophilic, it is believed that its diffusion into the deswollen hydrogel microparticles was inhibited, and degradation proceeded from the hydrogel surface that was in contact with the oxidizing agent. Taken together, it is clear that the degradation behavior of hydrogel microparticles changes depending on their swelling state, and particularly in the swollen state, the heterogeneous cross-linking structure of the microparticles leads to heterogeneity in degradation rates at the microparticle scale.
Fig. 5. Degradation of hydrogel particles induced by oxidizing agent. Clipped HS-AFM images taken at (a) 25 °C and (b) 40 °C solution temperature. The upper panel shows the topography images and the lower panel shows the phase images. Reprinted from Ref. 28 with permission from the Royal Society of Chemistry.
Download figure:
Standard image High-resolution image3.3. Initial process of precipitation polymerization
The mechanism of precipitation polymerization is a key factor in the structural formation of hydrogel microparticles. Therefore, elucidating the mechanism of precipitation polymerization is crucial for controlling the structure of hydrogel microparticles. So far, analytical methods have been limited to scattering methods, 31,32) and the understanding of the phenomena occurring during precipitation polymerization has remained largely speculative. Thus we applied HS-AFM to directly visualize the precipitation polymerization process, aiming to clarify the formation process of hydrogel microparticles. 33)
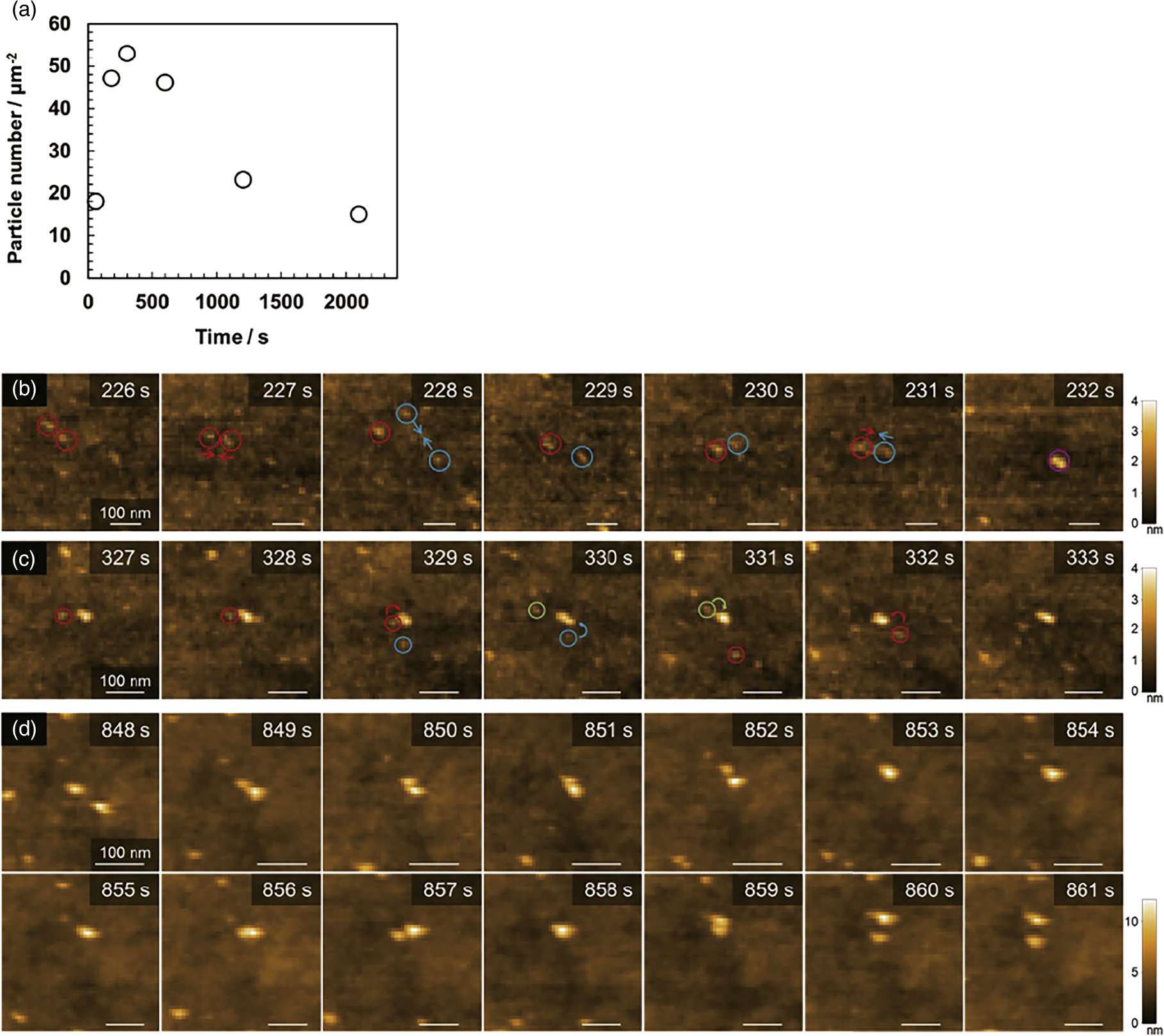
Precipitation polymerization of pNIPAM hydrogel particles was initiated by adding a redox initiator, a mixture of ammonium persulfate and N,N,N',N'-tetramethylethylenediamine, after the observation solution containing the monomer was set at 40 °C. Here, the polymerization was carried out at a low concentration of 20 mM due to the formation of microparticles that caused the solution to become turbid, which changed the transmission rate of the laser light used to detect the deflection of the AFM cantilever and resulted in an unstable amplitude. A hydrophobic mica surface was also used to ensure that the formed microparticles were adsorbed onto the substrate. As the polymerization initiated, the precipitated polymer chains, known as precursor particles, were visualized anchoring to the substrate and diffusing on the surface. The number of precursor particles continued to increase for approximately the first 5 min after polymerization was initiated, and then the number of particles gradually decreased, as shown in Fig. 6(a). This indicates that the precursor particles associate with each other by diffusion on the substrate surface.
Fig. 6. The initial process of precipitation polymerization. (a) Changes in number of precursor particles with the progress of polymerization. (b) Multi-step aggregation process of precursor particles. (c) Adsorption process of precursor particles on the nucleus. (d) Repulsion behaviors between nuclei. Reprinted with permission from Ref. 33. Copyright 2021 American Chemical Society.
Download figure:
Standard image High-resolution imageA more detailed analysis of the polymerization process revealed that the precipitation polymerization proceeds through three distinct processes. In the initial stage, the precursor particles associate to form aggregates, followed by a multi-step aggregation process in which these initial aggregates further associate with other aggregates, resulting in an increasingly complex aggregation structure as shown in Fig. 6(b). In addition, throughout the polymerization process, adsorption of precursor particles onto the formed particle nuclei was observed, as shown in Fig. 6(c). Approximately 5 min after the start of polymerization, it was confirmed that the nuclei did not aggregate when they approached each other but repelled each other as shown in Fig. 6(d). In this polymerization system, a potassium peroxide is used as the initiator. Thus, as the polymerization proceeds, sulfate groups originating from the initiator would be introduced onto the microparticles. This introduction is likely to result in electrostatic repulsion becoming the dominant force over van der Waals attractions or hydrophobic interactions, leading to the achievement of dispersion stability. Thus, direct visualization of the initial polymerization process of hydrogel microparticles by HS-AFM has revealed the existence of a previously unknown multi-step aggregation process in precipitation polymerization.
4. Summary
Using HS-AFM, we have now achieved the ability to observe the polymerization and depolymerization processes of supramolecular assemblies, as well as the dynamic behavior of polymer hydrogels undergoing solvent-induced swelling, with unprecedented spatial and temporal resolution. The potential of this advanced imaging technique extends to the visualization of diverse dynamic phenomena in various synthetic polymers, including supramolecules, colloids, and gels. AFM is generally capable of measuring not only the surface structure of samples, but also their mechanical properties. By complementing HS-AFM with the ability to simultaneously assess surface structure and mechanical property dynamics via HS-AFM, 34) insightful analyzes of how polymer hydrogel microparticles adjust their mechanical parameters such as elasticity and viscoelasticity in response to environmental stimuli would be within reach. However, AFM has the inherent limitation of only being able to study samples adsorbed on solid substrates. It is expected that the integration of HS-AFM observations with other ensemble measurement techniques, including scattering methods, and computational approaches, such as molecular simulations, will provide a more comprehensive and hierarchical understanding of the physicochemical intricacies within synthetic polymers.
Acknowledgments
The research on supramolecular assemblies presented in this paper was conducted in collaboration with Prof. Kazunori Sugiyasu of Kyoto University, and the research on hydrogel microparticles is a collaboration with Prof. Daisuke Suzuki of Shinshu University. Part of this research was supported by a CREST Grant-in-Aid (JPMJCR21L2) from the Japan Science and Technology Agency (JST), JSPS KAKENHI Grant No. JP18H04512 and JP20H04669 within the Grant-in-Aid for Scientific Research on Innovative Areas "Soft Crystals: Area No. 2903", and a Japan Society for the Promotion of Science Grant-in-Aid for JSPS Fellows (20J1272700).