Abstract
Design and optimization of electrode materials plays the pivotal role on the performance of capacitive deionization (CDI). Activated carbon (AC) has been a workhorse material for electrode fabrication in capacitive technologies. Several modification methods have been reported with enhanced activity and versatility attributes. Undeniably, tuning and tailoring AC properties have opened avenues for broadening the scope of applications, by meeting necessary features of electrodes for a given CDI cell configuration. This review traces the beneficial and also detrimental effects from various modifiers on AC electrodes with respect to CDI performance. Furthermore, a comprehensive classification of CDI cells based on different architectural aspects with a comparative performance is presented. On this basis, the tradeoff between physical, chemical, electrochemical properties in the course of electrode modification and the interdependence between electrode design and CDI cell configuration are discussed with disclosing some prospective guidelines on AC electrode design. It is important to evaluate the electrode materials and modifications in the way of practical including not only the electrode design, but also the cell architecture and operational parameters. This review aims to raise the attention on the rational electrode design by taking into account all necessary features of electrode in a given cell configuration.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: permissions@ioppublishing.org.
Desalination technology is considered to be an important means for an unlimited supply of fresh water by means of producing potable water from sea and brackish sources.1,2 Intriguingly, the supply of desalinated water does not depend on climate patterns.3 Desalination in general is a highly energy intensive technology. Water and energy shortage are the two of major concerns of 21st century.4 Nevertheless, innovation may allow us to meet freshwater production at a lower cost while at the same time improving upon energy utilization. Several thermal and membrane desalination technologies have been developed.5 To date, reverse osmosis (RO), multiple effect distillation (MED), multistage flash desalination (MSF) and electrodialysis (ED) are extensively used for seawater and brackish water desalination on a large scale. Unlike electrodialysis (ED), other technologies operate by removing the major component (water) rather than the minor (salts or minerals), hence becoming less efficient for brackish water desalination due to the low salt concentration.6
Capacitive deionization (CDI) is an emerging desalination technology extracting salts and minerals from saline water in a manner such that the consumed charges during electrode charging can be recaptured during electrode discharge,7 thereby allowing CDI to be an energy efficient and less costly alternative technology for desalinating saline water as compared to other conventional desalination technologies. Indeed, recapturing a part of the energy has been one of the key aspects of the CDI technology.8 The concept of CDI can be described as an electrosorption process that can be mainly explained based on features of the electric double layer capacitor. Similar to supercapacitors, ions are stored reversibly in electrical double layer (EDL) within carbon pores in CDI.9–11 This innovative desalination technology holds several advantages including the facile removal of minor products from major product by means of the capacitive effect and faradaic interaction under low cell voltage.9,12 In addition, it has other nice attributes such as energy conservation, environmentally friendly,13 simple in configuration and low maintenance.14 Basically, the performance of CDI relies heavily on the electrode, but as well on cell configuration and operational parameters.12,15 Thus, performance enhancement can be explored via the fabrication of ideal electrodes, optimization of operational parameters, development of robust CDI configurations and/or by integrating CDI with other desalination technologies.
Statistically, publications in the field of CDI research field are for the most part dedicated to the crucial role of electrode over other CDI performance contributors.15 In other words, the electrode holds the lion's share of contributing factors to the performance of CDI systems. Activated carbon (AC) has been extensively reported as the first and most commonly used material for capacitive technologies.11,16 The large surface area, electrical conductivity, chemical stability over a wide electrochemical window, inertness, easily tunable pore morphology and favorable adsorption isotherm, all point to AC being an ideal candidate for CDI electrodes.4,17,18 Moreover, AC is an environmentally friendly material and commercially available at relatively low cost.19 A CDI electrode with ideal capacitive behavior should possess a large specific surface area, high conductivity, good wettability, chemical stability and appropriate porosity.12–14,20–22 However, AC has been shown to have a lower specific capacitance than expected theoretical value.21 Hence, it always needs to be modified and engineered using different modifiers (conductive additives, binders, reducers, oxidizers, pseudocapacitive materials, metal oxides, surfactants, polymers, etc.) in order to improve performance. In essence, the rational design of the AC electrode is of utmost importance for optimal CDI performance.23 A design of AC electrodes has become almost the Holy-Grail of research in CDI technology. Different physical, chemical and biological modification methods of AC have been reported,24 showing various electrochemical performance enhancements. However, most of those enhancements come at the expense of impairment of some electrode properties.
Recently, several reviews concerning CDI electrode materials have been presented. Oladunni et al.,15 Liu et al.,17 Huang et al.25 and Thamilselvan et al.26 presented a comprehensive review on recently developed carbon based nanocomposites for application in capacitive deionization process. Bhatnagar et al.24 reported an overview of the modification methods of AC for water treatment application. Han et al.12 reviewed the structure and functionality of novel carbon and faradaic electrode materials for high performance capacitive deionization. In addition, Ratajczak et al. presented an overview of capacitive technologies based on carbon materials.11
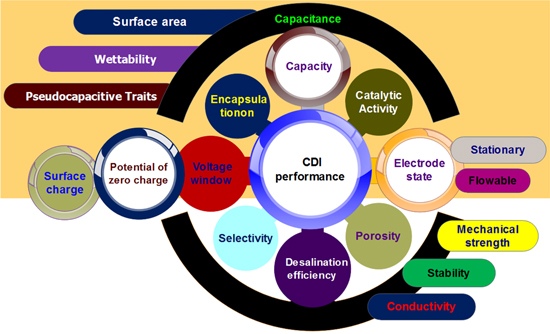
In this review, the impact of modifiers on CDI performance is delineated in the context of engineering tradeoff. The beneficial and detrimental effects of various modifiers on electrochemical activity of AC are discussed, as shown in Fig. 1. Profoundly, a comprehensive classification of CDI cells based on different architectural aspects with a comparative performance is presented. Furthermore, the interdependence between electrode aspects and CDI cell configuration has been elucidated. Finally, a brief outlook on the development of AC electrode design was summarized with some prospective research guidelines. The goal of our review here is to disclose the impact of AC electrode design on CDI performance and how to balance the tradeoff between electrode properties in the course of modification. It also introduces the interdependence between electrode aspects and CDI cell configuration. Knowing that, it provides insight into electrode modification aspects without sacrificing electrochemical performance benchmarks. In addition, electrode-CDI cell interdependence sheds the light on a rational design, in order to reap the benefits of electrode modification on appropriate CDI cell configuration.
Figure 1. Schematic diagram of electrode properties impacting capacitive deionization performance.
Download figure:
Standard image High-resolution imageThe Beneficial Effects of Various Modifications on AC Electrodes
Structural improvement
The structural properties of the electrode encompass surface area, porosity (pore structure, pore size distribution and tortuosity) and texture. The storage capacity (charge and ion storage) and kinetics strongly depend on the available surface area and the porosity of electrode.27 Thus, CDI as an interfacial process is highly sensitive to the available surface and porosity of electrode material. Capacitive ion storage and electrosorption kinetics within the matrix can be enhanced via physical and/or chemical modification of the electrode. Indeed, the surface area and pore size distribution play a crucial role in the design of carbon electrode. In order to optimize capacitive performance, much effort has been focused on extending surface area as well as control over pore size.28 Lee et al. combined AC with a limited amount of functionalized carbon nanotubes (FCNTs), wherein the homogeneous dispersion increased the specific surface area (SSA) of the electrode.29 In a sense, the addition of FCNTs prevents the occurrence of particle agglomeration and clogging of the pores.
Physical activation can broaden the pores via mild gasification and increase surface area as more pores are created.30,31 Interestingly, AC that is purposely de-functionalized via hydrogen treatment displays increased SSA, pore volume and average pore size induced by a simultaneous collapsing of micropores and creation of more mesopores, thereby improve CDI performance.32 Villar et al. subjected AC on hydrogen treatment for obtaining electrode material with a high apparent SSA. The increment of surface during AC treatment with H2 at 400 °C and enlargement of average pore diameter led to the higher charge capacity.33 In that respect, modification of the pore arrangement and size distribution can lead to a suitable pore structure for the transport of salt ions. Many researchers elucidated the importance of a strategic development of mesoporous and microporous structures that can facilitate optimal electrosorption. Indeed, electrochemical characterization unveils different capacitive behaviors due to the distinct ratio of mesopores to micropores. KOH and CO2 have been assigned to increase a tunable ratio of micropores and mesopores in AC respectively.34
Furthermore, a rational design of AC structure can improve both electrosorption and accumulation of ions.35,36 Several researchers have reported the roles of micropores, mesopores and macropores on electrode performance. Micropores increase SSA and charge storage that lead to a high specific capacitance. Mesopores enhance ionic conductivity by providing favorable and quick transport of ions in the electrode matrix, that improves electrosorption kinetics. Macropores act as ion buffering reservoirs.19,37 Therefore, the development of ordered carbon electrodes with a controlled number of mesoporous and micropores is a crucial strategy for electrode optimization. The large SSA and shortened pathways for ion diffusion were attributed to the three-dimensional (3D) mesostructures and a well-interconnected diffusion path of the electrode material. Indeed, the pore arrangement pattern (ordered or random) and pore size distribution (mesopores and micropores) have a great impact on electrode performance.38 However, some is warranted as the dominance of micropores and severe pore tortuosity may limit the CDI performance. The wide pore distribution of AC may cause a severe reduction in capacitance over a short current drain time.39,40 Several AC-based composites including: reduced graphene with AC (RG-AC), AC with metal oxides (AC-MO), AC with carbon nanotubes (AC-CNTS), AC with metal-organic frameworks (AC-MOFs), mesoporous carbon with carbon nanotubes (MC-CNTs), composite of mesoporous with microporous AC (CAs-AC) and AC with carbon nanofibers (AC-CNFs)17,39,41 have been widely developed showing an improved interconnection structure in the matrix of the electrode. For example, Wouters et al. employed metal oxides to modify carbon material for ion removal. The carbon material with SSA much lower than that of coating metal oxide xerogels, exhibited an increasing of the SSA of the entire composite. Conversely, a decreased SSA was observed when activated carbon cloth with a high SSA coated with thereof metal oxides xerogels.42 In respect to the influence of metal oxide coatings on carbon materials, the SSA of the composites relies on the nature of carbon materials. Furthermore, Ryoo and Seo reported the tetrahedral coordination of Titania dispersed on the surface of AC cloth for tuning the surface structure along with subsided physical adsorption, thereby inducing enhanced electric field adsorption. The surface chemistry modification provides more available surface with increased number of adsorption sites via the participation of titanium atoms.43
Song et al. reported a novel composite electrode by sono-assembling AC with inter/connected graphene network (ICGN) for CDI application. The fabricated electrode (AC/mPEAG) exhibited an ultra-high electrosorption capacity of 12.58 mg g−1 compared with 5.31 mg g−1 of the pristine AC electrode. Intercalation of incorporated mPEAG generates the macropores which are favorable for the buffering of ions, thereby shortening the distance of diffusion from the interface to the matrix, whereas mesopores facilitate ionic transport and electrosorption.19 The tortuosity of AC electrodes can significantly impact the rate of ionic conduction through the electrolyte in the electrode matrix. Basically, the tortuosity is a ratio that characterizes the convoluted pathways of fluid diffusion and electrical conduction in porous media. Herein, it describes the ratio of the microscopic path length within pores normalized by Cartesian distance of ion travel between the endpoints of the path.44 Thus, it impacts electrode charging dynamics by way of the effective ionic conductivity and ionic diffusivity.44–46 The addition of macroscopic and low-tortuosity pores increased ionic conductivity and improved capacitance at a high sweep rates, also macroscopic pores reduce the effective tortuosity by providing more direct paths to capacitive interfaces.45 Tang et al. developed a macropore- and micropore-dominated carbon (HPAC) material for supercapacitors and CDI electrodes.4 HPAC has shown well-distributed macropores that can provide buffering reservoirs for ions and fine particles that can provide more external surface area. The macropore size and size distribution of HPAC were smaller and denser than AC and MC. Furthermore, intrusion porosimetry unveiled the highest average pore diameter (353.2 nm) of HPAC as compares with AC (33.3 nm). The high capacitance and adsorption capacity of HPAC are generally ascribed to the adjacent pore walls (large SSA), large pore volume for distorted ions and a pore structure which induces the short ion transfer route for faster diffusion.
Additionally, the binder affects the pore size distribution of carbon materials. Using various binders result to the small or large porosity with a larger accessible porosity in the mesoporous region. Xie and coworkers applied an organic-inorganic hybrid binder to enhance CDI performance.47 The hybrid binder was inferred to improve pore structure. However, binders could block the pores in a highly porous carbon. Moreover, some hydrophilic binders with swelling behavior have been reported to change the particles contact, and consequently alter the porosity.48 Therefore, the design of binder-free electrodes provides a great opportunity to deal with the dilemma of binders' usage. Furthermore, inert gases (N2 and Ar) play a crucial role in pyrolysis, where they prevent the pores closure, breakage or shrinkage.49 Graphitization occurs readily in an atmosphere of inert gas with a simultaneous lessening of tortuosity. Also, pyrolysis under inert gas atmosphere may increase the evaporation of unusable compounds for pores formation.
Wettability
The wetter the better.50 Wettability is one of important factors that can affect electrode performance.51 CDI electrodes require a high wettability for their compatibility with aqueous media. Indeed, a better wetting of electrode material provides excellent interfacial contact and mass transfer. The surface of AC is highly hydrophobic, thus showing a poor interaction with water.52 Although AC has excellent SSA, the specific capacitance is much lower than expected.53 The low capacitance is partially attributed to the poor wettability which results in an unfavorable contact between the electrode surface and aqueous solution; thereby ions in the solution do not reach the interior part of active material. Chang et al. synthesized a hydrophilic liquid binder for AC electrode fabrication.54 Unlike other polymeric binders, this liquid binder provided improved wettability, thus exhibiting superior CDI performance. Similarly, Park and Choi fabricated carbon electrodes with a water-soluble binder instead of hydrophobic binders.51 By introducing hydrophilic functional groups onto the carbon surface via chemical means, one greatly improves wettability. Different nitrogen and oxygen functional groups including amines, sulfones, carboxylates, and carbonyls can significantly increase hydrophilicity accompanied by improved wettability of the carbon material.55 Several oxidants such as HNO3, H2O2 and KMnO4 have also been employed in order to introduce oxygen containing groups (hydroxyl, carboxyl, carbonyl, lactone and quinine) on the surface of carbon electrode, thereby improving wettability.39,56
Fic et al. studied the effect of surfactants on capacitance properties of carbon electrode.57 A significant improvement on the capacitance was ascribed to the reduction of surface tension and enhanced charge propagation. Electrode with increased wettability provides a high usable surface and reduces internal resistance which are both beneficial on electrochemical performance in aqueous electrolytes. Moreover, the penetration of electrolyte into electrode pores is not only controlled by pore structure but also surface tension.58 AC was modified with a surfactant sodium oleate for the sake of improved specific capacitance and energy storage in the electrochemical double-layer (EDL). The enhancement of capacitance was mainly attributed to wettability improvement of the carbon material thereby providing a highly usable surface area and low internal resistance. Undeniably, surfactants can profoundly improve electrode capacitance via their ability to reduce surface tension.59 Furthermore, Aslan and coworkers60 introduced a new strategy to exploit an improved porosity without sacrificing CDI salt removal capacity and efficiency in view of a limited AC wetting in aqueous media. Mixing hydrophobic and hydrophilic carbons has also been shown to improve wetting. These mixed carbons electrodes displayed a high removals and good charge efficiency. Metal oxide coatings can also increase the wettability of hydrophobic materials due to their hydrophilic nature.42 SiO2 and γ-Al2O3/γ-AlOOH were used to modify carbon material for CDI application. The coated carbon electrode exhibited an increased ion removal over the uncoated carbon. Moreover, the hybridization of carbonaceous material with the anchoring of inorganic materials such as ZnO, SnO2, ZrO2 and TiO2 has received recent attention in order to overcome some shortcomings of AC in CDI process.61 A composite of AC with nitrogen-TiO2/ZrO2 was synthesized in order to improve the low capacitance and wettability of AC.23 While the TiO2 coated electrode and pristine carbon electrode showed similar specific capacitance, the TiO2 system likely improved desalination efficiency due to increased transport of ions and water into the porous structure of the more hydrophilic surface.62
Electrical and ionic conductivity
The conductivity dictates kinetics of electrochemical process into electrode. By improving kinetics, one improves salt removal rate and can reduce CDI size and/or improve performance.63 Although the textural features directly impact electrochemical performance of the carbon electrode, there is a complementary role of conductivity to be considered. Increasing conductivity improves kinetics, thus enhancing electrosorption capacity, efficient salt removal and decreased internal resistance. AC is not solid throughout and is filled with millions of micro-pockets (microscopic holes and pores) that make it one of the most porous materials known. These micro-pockets packed with air significantly reduce the ability of AC to conduct electricity. Also, the porous nature of AC can directly affect transport of ions as well as electrons causing a slow removal of ions and a loss in conductivity.37
To transform amorphous structure of carbon into a graphitic structure, AC is subjected to pyrolysis for generating carbon with more ordered matrix associated with a high electrical conductivity. Basically, thermal treatment affects the oxygen and hydrogen containing terminations that constitutes the skeleton of the materials. However, the consistent enlargement of graphitic domains is only possible for temperature value above 2000 °C in inert atmosphere.64 The nature of electron transport is always proportional to the degree of crystallinity in carbon materials.65 Shi and coworkers improved AC conductivity and created a graphite-like AC via catalytic graphitization using N2 plasma and iron loading. N2 doping enhanced the surface accessibility of AC, while iron (III) loading facilitate the ordered arrangement of grains, thus enlarging the crystalline volume fraction of treated AC.64 Sánchez et al. reported improved electrochemical performance of carbon-based electrode after heat treatments up to 900 °C, which is mainly attributed to the simultaneous increase in conductivity.66 In addition, graphitization is a thermodynamic process, which can transform amorphous carbon into a well-ordered and three-dimensional graphitic structure.67
Furthermore, different additives such as Carbon black, FCNTs, Graphene and metals are widely employed to enhance AC electrode conductivity.63,68 Alencherry et al. investigated the effect of increasing electrical conductivity of carbon composites on CDI performance, by incorporating silver (Ag) and FCNTs into powdered AC. Ag impregnation resulted in increased electrical conductivity of electrode originating from a suitable interparticle charge transfer among AC particles. Moreover, silver impregnation reduces bulk resistivity, leading to increased charge accumulation, thereby delivering higher electrode potentials at the electrode-electrolyte interface.63 In addition, Wang et al. designed a three-dimensional composite by loading AC into highly conductive graphite felt framework to enhance the electron conductivity.69
The use of slurry electrodes has been a recent addition to the world of CDI. However, the loose connectivity of carbon particles in a flow electrode can lead to poor conductivity that may lower CDI performance. On the other hand, improvement of connectivity via a high carbon mass loading in the slurry can lead to increased viscosity which restricts flowability. Cho et al. introduced FCNTs into AC flow electrodes which generate conducting bridges among AC particles, hence inducing an increase in salt removal without the need of highly loaded active materials.70 Similarly, Lee et al. employed FCNTs as conductive agents of the CDI electrode. The decline of resistivity in functionalized AC was attributed to the extraordinary electrical conductivity of FCNTs with their sp2 carbon structure.29
Ismagilov et al.71 and Hulicova-Jurcakova et al.72 reported an increase in electrical conductivity generated from electron-rich nitrogen introduced into the carbon network that could move more electrons into the delocalized π-system. Furthermore, nitrogen dopants along with induced voids or defects provide excellent conductivity and transport paths, thus facilitating efficient electron and ions propagation into the porous electrode.73 Moreover, some nonionic compounds have been ascribed to create ion channels which may facilitate the transport of ions at electrode-electrolyte interface and charge propagation as well.74 The molecules can self-organize into ionic pathway structures, thereby offering a better charge propagation under a self-organization of the electrode with the surface agent. The impact of these ionic channels is significant for ion transport between the interface and the mesopores. In the study of the influence of surfactants on capacitance, Fic et al. reported a contribution of surfactants (Triton® X-100) on diffusion in the charge storage process, through faster and stable charge propagation. In addition, interaction between the hydrophobic structure of surfactants and π electrons of the carbon matrix can result in improving system conductivity.57
Surface charge and potential of zero charge shifting
Surface charge and the potential of zero charge are very important properties of carbon electrodes for CDI application.75 Recent findings have shown that a high salt removal in CDI cell needs a proper management of surface charge on carbon electrodes. Basically, AC has an inert surface and is highly favorable for nonionic interactions with organic compounds. Nevertheless, charge development on the AC surface can facilitate adsorption of ionic compounds via ionic interaction. By chemical modification a net positive or negative surface charge can be imparted on the AC electrode, that may be a promising solution for enhancing performance stability. Gao et al. employed carbon electrodes with different surface charge to develop a novel CDI cell configuration, that named as inverted capacitive deionization (i-CDI). In this cell configuration, the chemical charges on electrode surface offers adsorption when the cell is shorted.76 Commercial carbon electrodes treated with ethylenediamine amine and nitric acid solutions imparts both positive and negative chemical charges on the electrode surface. An improved salt removal in i-CDI cell was partially attributed to the chemical surface charge enhancement.75 AC was functionalized with quaternary amines surfactant (CTAB) which generated a positively charged surface to drive nitrate removal, with no external potential applied.77 Moreover, Wang et al. investigated the influence of surface potential on capacitive performance by charging with protons and specifically adsorbed ions.78,79 The fact that surface potential can be changed by the crystalline phase of an oxide material was further proved.80
The CDI process under alternating polarization has also displayed an interesting effect of surface charge on salt removal. In the equal formation of positive and negative charged surface sites during alternating polarization, electronic charges contribute more efficiently to ion adsorption, thus resulting in a high value of adsorption. In case of imbalance surface charges on the electrode, a portion of electronic charge is parasitically spent on reconciling the surface charge imbalance. Therefore, the management of surface charge on carbon electrodes has been a promising avenue for mitigating the loss of electronic charge due to charge imbalance.81 Also, introducing a surface charge on the electrode can minimize the co-ion repulsion effect. Nafion-AC composite exhibited an induced ionic repulsion, thereby attenuating the co-ion effect.8 Furthermore, the coincidence of an external applied potential with an electrode surface charge favors electrosorption.42 In other words, the surface charge on CDI electrode offers enhanced adsorption and quick regeneration of oppositely charged ions. In essence, cations and anions would normally cross over to the electrode with the negative and positive potentials respectively. During regeneration this tendency might cause an incomplete regeneration of CDI electrode. Hence, the opposite surface charge can be utilized to solve this issue by preventing ion crossover from one electrode to another.42,82
The location of the potential of zero charge (EPZC) over the working voltage window plays an important role on electrosorption.83,84 This potential can be defined as the transition stage of surface charge. In other words, at the EPZC, a simultaneous adsorption of cations and desorption of anions begins as the applied potential negatively passes the EPZC and vice versa.85 The EPZC of an electrode can greatly influence salt removal, charge efficiency and cyclic stability in CDI.86 The working voltage window is regulated by the potential difference between the EPZC of anode and the EPZC of cathode. This distribution of EPZC plays an important role on CDI performance. Moreover, the adsorption status of CDI electrode can be predicted based on the value of EPZC and the potential of electrode (E).87 The same value of EPZC and E implicates a minimized net ionic charge on the electrode. When E is greater than EPZC anions adsorption is favored whereas a high value of EPZC than E cations are adsorbed.88 In other words, the least adsorption of ions falls in the region of EPZC. Due to surface modification, positively or negatively charged functional groups can relocate EPZC in AC electrode. Acid treatment,89 metal oxide,90 and sulfonation91 have been employed for positively shifting the EPZC as a result of introducing negatively charged species. Quaternized poly (4-vinylpyridine),86 amines92 can introduce positive charged groups, and negatively shift the EPZC of the cathode. An imbalanced distribution of applied potential due the gradual oxidation of anode can lead to an inversion phenomenon in which co-ion desorption becomes dominant during charging and re-adsorption upon discharging.85
There is a dependence of the CDI performance on EPZC shifting of carbon electrodes during long-term operation. After extended cycling, the relocation of the EPZC occurs and the positive shifting of EPZC is due to the slow oxidation of the anode.83,85 The development of positive electrode oxidation can protect the working potential domain. For a symmetric CDI cell operating at constant voltage, ion adsorption becomes efficient when the pair of electrodes possess a net surface charge equal to zero. Cohen and coworkers employed controlled oxidation of AC electrodes in HNO3 solution to positively shift the EPZC for the sake of developing a wider potential domain.93 An anode with positive surface charge paired with a cathode with negative charge can enhance and extend the CDI voltage effect. Moreover, commercial carbon electrodes were repetitively oxidized in order to enhance the negative surface charge, thereby creating an electrode pair with different EPZC values at short circuit (Eo).76,92 Gao and coworkers reported a more favorable adsorption of Cl− at the anode as compared with the adsorption of Na+ at the cathode that can limit CDI performance. In order to induce negative charge, a carbon electrode was modified by coating with SiO2 and by the surface –COOH groups from oxidation. These modifications were used to adjust the location of EPZC for the cathode, which resulted in enhancing Na+ adsorption and diminishing co-ion repulsion.83
Stability
The cyclic stability is the ratio of capacity at the nth cycle to the maximum ionic removal capacity,94 and it is an important factor in evaluating the durability of the electrode to maintain its maximum performance. A high stability is counted among the major properties of an ideal electrode. Naturally, AC electrodes exhibit deionization decay induced by cyclability fading after several cycles. A significant effort aimed at mitigating the chemical degradation of CDI electrodes has drawn significant attention of the CDI community. The development of CDI electrodes with a minimum chemical degradation is highly important for the sake of extended lifetime. Corrosion of the anode in CDI cells is a major problem which causes poor cycling stability during desalination process.9,85 Gradual oxidation of the anode leads to imbalanced distribution of applied potential with a simultaneous pore damage originating from the formation of redox products on the carbon surface.
Furthermore, a continuous corrosion of the positively polarized electrode under charging results in a phenomenon called the "inversion effect" which refers to desorption of ions while the cell is still polarized and charged.85 Different methods have been developed in order to retain the stability during extended cycling. Surface modification (coating, oxidation/reduction and doping) is regarded to be an effective strategy for alleviating electrode corrosion. Srimuk et al. modified AC with Titania in order to prevent oxygen from participating in carbon oxidation. Thus, AC-Titania hybrid exhibited increased salt adsorption capacity (SAC) and prolonged cycling stability in oxygen-saturated saline media.9 Under realistic conditions, the atmospheric oxygen (21%) has a radical influence on the CDI stability fading. When oxygen diffuses into water and reacts with the carbon electrode, it results in the oxygen reduction reaction followed by the evolution of H2O2, which causes the AC electrodes to degrade.95 Pore structure and surface functionalities also affect stability. An electrode having a very small pore size and high amount of oxygen functional groups has been shown to have a highly pronounced degradation as cycle numbers increase.96 Though, oxygen functional groups improve surface wettability, AC with high oxygen functional groups content must be operated at low voltage to maintain the stability. Alternatively, employment of inverted CDI configuration can solve the challenge of redox reaction restrictions.
The development of mesostructured carbon electrodes with ordered and well-interconnected mesochannels offers stable cycling in CDI performance.10 An activated Novolac-derived carbon was de-functionalized via hydrogen treatment. This treatment was shown to enhance electrode stability related to a reduced number of surface carboxylic groups.32 AC modified with surfactants was additionally reported to exhibit excellent cycle stability over a wide potential range and it was attributed to the side reactions inhibition of the electrode surface.57,97 Furthermore, AC modified with carbon nanodots (C-dots) has shown to have a superior cyclic stability over many thousands of consecutive cycles with excellent capacity retention. The ability of C-dots to carry charge and modify the interface seems to indicate that AC/C-dots may be a useful means of greatly increasing electrode stability.98
Encapsulation of carbon materials is a novel approach for designing a more efficient electrode with enhanced stability. Through encapsulating, electrode is embedded in a chemical substrate including CNT or polymers to impart selectivity or electrochemical stability (mitigating the decomposition of electrolyte on the electrode surface). Jung et al. employed zwitterionic polymers for coating AC, in order to provide a resistant barrier for stabilizing electrode structure. The polymeric layer inhibits reactions between carbon electrode and electrolyte. Moreover, encapsulating the AC surface also can increase the number of ionic adsorption sites and surface area, thus improving the charge separation and ion removal efficiency.99 AC electrodes with excellent electrochemical stability and ultrahigh performance were synthesized by means of encapsulation with ultrathin layer of Al2O3 via atomic layer deposition. This remarkable performance was ascribed to the effect of Al2O3 layer of protecting oxygen functional groups against faradaic reactions. In other words, these AC electrodes were able to be protected against undesirable reactions with electrolyte.100 Furthermore, Zhao et al. applied a polyaniline (PANI)-based carbon encapsulation strategy to improve specific capacity of sulfur/starch-based AC sphere composites. The improved electrochemical performance was attributed to the encapsulated AC (PANI-AC) ability to act as cushion as well as a barrier to trap soluble intermediates during the charging-discharging process.101
Mechanical strength
In order to obtain excellent mechanical stability and interface adhesion, binders are a prerequisite component in the fabrication of AC electrodes.47 Binders play a critical role for binding active materials with conductive additives and achieving stable attachment to the current collector. Several binders and their derivatives specifically, polyvinylidene difluoride (PVDF), polytetrafluoroethylene (PTFE), polyvinyl alcohol (PVA), polymethacrylic acid (PMAA), sulfosuccinic-acid (SSA)102 have been widely employed to bind the AC powder with a proper mechanical strength. PVDF is the most widely used binder because of its outstanding properties including high mechanical strength and thermal stability.103 Asquith et al. prepared AC electrodes using sulfonated poly (arylene ether sulfone) copolymers as binder. The copolymer displayed an adequate binding of carbon particles with a good adhesion of carbon black to AC.104 Organic-inorganic hybrid binder was used in the fabrication of a robust AC electrode for a high-performance CDI application. The prepared AC electrode gained significant mechanical properties with a desirable flexibility for the construction of a compact parallel cylinder CDI unit.47 Inorganic-organic binders maintain good thermal stability with a substantial suppression of cracking and fragility.
Park et al. employed polyvinyl alcohol (PVA) binder cross-linked with glutaric acid as a novel hydrophilic binder, which might provide mechanical strength without sacrificing wettability.51 Polyurethane elastomer utilization as a novel binder for AC electrode resulted in an increased flexibility and the inhibition of mechanical crack formation, thus addressing the problem associated with a more rigid PVDF.105 Phenolic resin (PR) and epoxy resin (ER) binders exhibit an outstanding stability under high temperature and pressure. A liquid binder denoted as AA was synthesized with azodiisobutyronitrile and acrylic acid for the development of AC electrode in CDI process. Compared with other binders (PTFE, PR and ER), AA displayed the highest flexibility and durability.54 Furthermore, polyvinylpyrrolidone (PVP) blended with polyvinyl butyral (PVB) resulted in a composite binder with good mechanical stability and water resistance making it more attractive for electrode fabrication.106 Cai et al. prepared AC electrode with Nafion as a binder. Improved adhesion and mechanical properties were attributed to the Nafion addition.8 Moreover, AC impregnated with nitrogen and sulfur-containing species (dicyandiamide, urea and thiourea) under a high temperature, led to an enhanced mechanical strength.107
Pseudocapacitive traits
Although AC possesses large SSA which is responsible for superior charge storage at the interface, over the course of electrochemical cycling, crude AC suffers from a relatively low specific capacitance as compared with its theoretical capacitance.108 To enhance the specific capacitance of AC electrodes, some research efforts have been focused on pseudocapacitive behavior through the creation of surface functional groups via chemical treatment,109 metal oxide (MnO2, RuO2, V2O5, MgO, ZnO, etc.) impregnation41,110 and doping.41,111,112 The pseudocapacitive process is a reversible redox reaction or intercalation process associated with charge transfer. Pseudocapacitive charge storage is achieved through reversible faradaic reactions on the surface of electrode material.113 Unlike electrostatic storage in electrical double layers (EDL), pseudocapacitors store charge through reversible redox reactions which may be slightly a slower process.114 The functionalities, mainly carboxylic, phenolic and lactone groups can provide an extra capacitance through pseudocapacitive mechanism.25,115 MnO2/AC and RuO2/AC composite electrodes with the mixed capacitive-Faradaic behavior were fabricated for CDI applications. High performance was attributed to the mixed capacitive functionality corresponding to the EDL charging of AC and the pseudocapacitive redox reaction of MnO2 or RuO2 respectively.116 Moreover, atomic layer deposition (ALD) of vanadium oxide (V2O5) on AC surface created a composite electrode with an improved charge store and increased capacitance due to the pseudocapacitance contribution.117
Heteroatoms have drawn more interest for rivaling expensive pseudocapacitive materials such as RuO2.111 Doping provides a pseudocapacitive contribution to the total capacitance of the electrode, and thereby heteroatom-doped carbon exhibits both electrical double layers capacitance (EDLC) and pseudocapacitance.118 Nitrogen, sulfur and phosphorous are efficient heteroatoms for providing pseudocapacitive functionality.119 This pseudocapacitive contribution rises from faradaic redox reaction of electroactive species of the functional groups on the surface of carbon electrodes.120 Carbonization process which allows the transfer of heteroatom functionality was employed to introduce pseudocapacitance into the carbonaceous material. The transfer of parent heteroatoms or doping during carbonization induces both EDLC and pseudocapacitance into the carbon material.118 Although heteroatoms-doping reduces SSA via leaching effect, these species endows the electron donor characteristics and provides abundant electrochemically active sites for pseudocapacitive reactions leading to an enhancement in the ion storage capability regardless of the reduced SSA. A nitrogen-doped carbon electrode was synthesized for understanding the role of nitrogen induced redox transitions. The carbonization with simultaneous nitrogen doping under ammonium treatment causes replacement of carbon atoms with nitrogen heteroatoms while maintaining a constant oxygen content.120 The redox potential over the course of redox reactions of heteroatoms via reversible attachment/detachment of ionic species induces pseudocapacitance. Hence, the capacitance of heteroatom-containing carbon exhibits a higher capacitance in comparison with the heteroatom-free carbon. In a sense, there is always a pseudocapacitive contribution to the total capacitance of carbon electrode from heteroatoms (nitrogen, oxygen, sulfur, etc.) of surface functional groups. An effective tuning of heteroatom doping via nitrogen and oxygen self-doped carbon resulted in the optimal pseudocapacitive contribution even with a moderate level of nitrogen.113
A pristine AC treated with melamine and urea exhibited pseudocapacitive behavior ascribed to the nitrogen and oxygen content of surface functional groups.72 Urine was employed as a precursor for carbon and heteroatoms to obtain a porous and heteroatom-doped carbon electrode with enhanced pseudocapacitance and EDLC.119 AC electrodes were modified by ozone treatment followed by impregnation with cobalt (II) hydroxide to obtain high capacitance. Incorporation of oxygen and transition metal oxides leads to additional pseudocapacitive faradaic reactions. During polarization, cobalt (II) hydroxide is electrochemically converted into cobaltosic oxide (Co3O4) which is responsible for a pseudocapacitive effect.121 He and coworkers122 in their study of the capacitive mechanism of oxygen functional groups on the surface of carbon electrodes reported improvement of capacitance through the pseudocapacitive behavior furnished by oxygen functional groups. The pseudocapacitance was ascribed to the electron transfer between oxygen functional groups and H3O+ in acidic medium accompanied by the separation of positive and negative charges. In alkaline medium, pseudocapacitance was attributed to the insertion/deinsertion reaction of hydrated ions in the pore.
Catalytic activity (electrocatalysis and photocatalysis)
The metal oxides such as TiO2, MnO2, NiCo2O4, Co3O4, Fe2O3 and Fe3O4 show catalytic activity with respect to the oxygen reduction reaction (ORR),9,123,124 which can indirectly affect CDI electrode. Srimuk et al. employed Titania for chemical modification of AC in order to enhance the ORR, which could be used as a mechanism to prevent oxygen from participating in carbon electrode corrosion. AC-Titania hybrids displayed superior stability in CDI systems operating in oxygen saturated saline water. Moreover, catalytic activity with respect to the oxygen reduction impedes hydrogen peroxide formation.9 A photoelectrode material was prepared for a photocatalysis-CDI system (PCS) for the sake of a synergistic conversion and removal of total chromium ions from aqueous solution. Two opposite electrodes, a positive photoelectrode, MOFs MIL-53(Fe) and a negative electrosorption electrode were utilized. Direct current (DC) voltage and visible light were applied on PCS for a simultaneous conversion and removal of Cr utilizing the synergistic effect of both photocatalysis and CDI.125
Selectivity
AC functionalized with ion selective functional groups is regarded as new avenue to compete on the selectivity which has been unique to membrane capacitive deionization (MCDI). The removal of specific ions rather than the removal of all ions from the feed solution offers an advantage of reduced energy cost.22 Selective ion removal has been attributed mainly to the ion valence, steric effects and the interaction between pore size and hydrated radius.83 Basically, electrodes rely purely on electric field based mechanism for accumulating charge in the EDL, and hence standard CDI does not offer any desired ionic selectivity. Redox-active materials hold a promise platform to control selectivity toward various ions at the interface of redox modified electrode. Su and Hatton reported that AC electrode coated with redox-active material (PVF/CNT) displayed an interesting selectivity that depended on the nature of the charged species.126 Oyarzun et al. functionalized AC electrodes with cetyltrimethylammonium bromide (CTAB) and a counter electrode with sodium dodecylbenzenesulfonate (SDBS) for the selective removal of nitrate (NO3−) over chloride (Cl−) in i-CDI.77 TiO2 nanoparticles grafted with Disodium 4, 5-dihydroxy-1, 3-benzenedisulfonate (Tiron) were coated on AC to form an ion-selective layer in CDI process. The prepared AC composite electrode displayed ion selectivity and reduced co-ion repulsion.55 An anion-exchange resin (BHP55 resin) was used in the fabrication of nitrate-selective carbon electrode from the mixture of chloride, nitrate and sulfate ions in a CDI cell.22 Wu et al. coated AC with an anion-selective quaternized poly (4-vinylpyridine) for a hybrid CDI application.127 In addition, examination of the relation between electrode properties and electrosorption behavior of ions unveiled the contribution of the mesoporosity/microporosity ratio as a means to controlling ion selectivity.20 More recently, ion selectivity was achieved by the application of ultramicroporous carbon in electrosorption process based on the differences in the hydrated size.128,129
Flowability and rheological properties of FCDI
The flowability and rheological properties are indispensable with respect to capacitive technologies based on flowable electrodes.130 The rheological property is essential for ensuring that there is no clogging while the electrode suspension flows along a narrow channel.131,132 Basically, a high carbon loading offers an improved conductivity that led to a high current, and thus an enhanced salt removal. On the other hand, a high carbon load results in an increased viscosity, thereby requiring a high amount of energy for pumping.133 AC has been modified to improve the flow behavior and lessening viscosity (parasitic) of carbon suspensions specifically in flow electrode CDI (FCDI) systems. By utilizing an oxidized active material, Hatzell et al. demonstrated the use of a high mass density without increasing energy requirements for pumping in FCDI. Moreover, the rheology of carbon slurries is noticeably altered by changes in surface heteroatoms. The functionalization of AC leads to separation of particles and keeps the slurry flowing with a greater dispersibility. Consequently, the lessening of aggregation alters flowability.6 Park et al. modified AC suspension with ionic head-groups for flow electrode applications. AC coated with anion or cation exchange polymers exhibited a decreased viscosity with a high loading of carbon. The ionic functional groups on AC surface were ascribed to reduce the intrinsic viscosity via induced electrostatic repulsion that led to a desirable dispersion of AC particles without aggregation.131
The Detrimental Effects of Various Modifications on AC Electrodes
Porosity impairment and surface area decreasing
The porosity and surface area are the main targets for EDLC improvement.134 Nevertheless, most of enhanced electrochemical performance of AC electrodes comes at the expense of reduced or blockage of the pores and decreased SSA. Functionalization of AC with HNO3, H2O2, H2SO4, impart heteroatoms which can improve capacitance and the stability of CDI electrodes. However, AC treatment with oxidizing agents has revealed a reduced pore volume due to the new functionalities inside or at the entrance of the pores. Zhang et al. reported the destruction of pore structure from heteroatom-introducing fabrication strategy.113 Furthermore, in the context of a mass balance, all various functional groups introduced into AC may cause a decrease of available surface area.115
The BET data unveiled a low SSA of AC composite (AC/MnO2) as compared with pristine AC. MnO2 was ascribed to block some pores and a rise in resistance with reduced pore diameter.135 AC electrode coated with γ-Al2O3 as anode and SiO2 as cathode in asymmetric CDI, exhibited enhanced electrosorption capacity, but a radical reduction of SSA from 1630 m2g−1 to 1290 m2g−1 with 1.7% SiO2 and from 1630 m2g−1 to 1293 m2g−1 with 0.35% of γ-Al2O3 was noticed.42 Enhanced performance stability of AC with Titania hybrid electrodes (AC/TiO2) during CDI process in oxygen saturated saline water was achieved at the expense of reduced SSA due to pores blockage from Titania loading.9 Moreover, AC was coated with vanadium oxide to improve electrochemical charge storage capacity via pseudocapacitive mechanism. However, the addition of pseudocapacitive layers diminished the accessible pores and double layer capacitance.117
Surfactants also influence AC surface area and the structure of the pore. BET measurements of modified AC show a decreasing SSA as compared with pristine AC, and as well to a reduction in pore volume for both micropores and mesopores. The aggregation of surfactants at a high concentration leads to retarded transport of electrolyte ions due to blockage of AC pores, reduced pore volume and a SSA decrease.58,59 Moreover, Alencherry et al. incorporated CNTs and silver (Ag) to increase hydrophilicity and electrical conductivity of AC electrodes for CDI, while these modified electrodes displayed a significant pore blockage associated with a decreased SSA which has been attributed to the Ag and CNTs respectively.63 AC treated with a silane coupling agent for improving the compatibility between AC, the binder and collector also resulted in decreasing SSA and reduced pore diameter.21 Indeed, binders or cohesive agents inevitably cover some surface areas or pores of carbon electrodes. Hence, the properties of binders and their amounts in electrode fabrication influence electrochemical performance.
Electrical double layer overlapping
The overlapping of EDL associated with the confinement effect of pore structure can decrease both the rate of mass transfer of ions and the number of ions inside the pores at equilibrium.136 EDL overlapping in the micropores of AC occurs when the average pore size is generally smaller than the Debye length.16 The imbalance between the EDL overlapping effect and the number of electrosorptive sites in microporous AC may significantly cause the loss in capacitive desalination.137 Yang et al. developed an EDL model to predict electrosorption of ions from aqueous solutions by carbon electrodes.138 Electrodes with a high number of micropores exhibited a significant reduction of electrosorption capacity due to the presence of pores with a width smaller than a specific value (cutoff pore width), which do not contribute to the total capacity because of the EDL overlapping. Furthermore, under an electric field, electrosorption in micropores of carbon electrodes can be affected by both SSA and EDL overlapping.
The change in pore structure during AC modification may result in decreasing in pore width to values less than the cutoff pore width; hence these pores do not contribute to the total adsorption capacity. In other words, modification might cause the loss of balance between the number of electrosorptive sites and the EDL overlapping. Under an electrical field, as SSA increases, the effect of EDL overlapping becomes larger; consequently, ion adsorption is reduced. On another hand, when the SSA is too low, the electrode provides a very little number of adsorptive sites on the surface leading to a decreased desalination capacity. The presence of mesopores in the carbon electrode could weaken the EDL overlapping effect.139 Porada and coworkers reported the role of mesopores in mitigating EDL overlapping.140 Generally, AC electrode preparation needs additives especially carbon black as a conductive additive in order to fill the voids between the particles of the active material. Therefore, an unsuitable change in the mesoporous/microporous fraction could impede the sorption kinetics via distortion of the EDL.20 Pi et al. reported advantages of a partly graphitized AC with improved conductivity and an intact hierarchical porous structure in the absence of conductive agents.141
Electronic and ionic resistance
Electronic and ionic resistances in CDI electrodes dictate the transport of electrons and ions respectively, in the electrode matrix. These resistances contribute to the voltage drops associated with high energy consumption and impeded adsorption kinetics.63,142 The hierarchical structure of pores in AC plays an important role in the electrosorption process. Macropores act as the transport pathways of ions while micropores host EDL formation and ion storage.142 Modification of AC and addition of additives can affect AC structure possibly leading to the corruption of itinerary and impeded propagation of electrons and ions in the electrode matrix. While the use of binders is imperative for binding AC powder to the back contact, these binders can hinder ion access to the pores51,143 and yield electrodes with a poor electrical conductivity.102,104 Moreover, the copolymer binders may exhibit swelling associated with reduced charge transfer pathways among carbon particles, thereby reducing EDL formation in micropores. Furthermore, heteroatom functionalities can enhance wettability and impart pseudocapacitive behavior. However, there are some disadvantages of these surface functional groups such as, diminishing electrode conductivity, preventing ions from entering pores due to reduced volumes.122 In addition, Min et al. reported resistance to charging and reduced ionic diffusion originating from the interfacial resistance of carbon electrode with Tiron-grafted TiO2 nanoparticle layer.55
Hydrophobicity
The surface functionality is the parameter which determines the wettability of carbon electrode.144 De-functionalization has been reported as detriment to AC wettability in aqueous media. Ding et al. investigated the impact of functional groups on electrochemical performance of AC electrode. Although de-functionalization of AC under Argon and hydrogen atmosphere at a high temperature led to a relatively high SSA in comparison with pristine AC, the surface became more hydrophobic accompanied by a poor wettability.115 AC treatment with CO2 increases SSA and improves porosity at the expense of decarboxylation. Hence, carbon materials become more hydrophobic making the wetting process more difficult.60 Villar et al. subjected AC to various treatments (carbon dioxide treatment, hydrogen treatment and thermal treatment), in order to modify porosity and surface chemistry. Intriguingly, the sample with more SSA and larger average pore diameter exhibited less adsorption capacity than others. A high hydrophobicity induced by the surface functional group removal was ascribed to hindered diffusion of ions into the carbon matrix.33 Moreover, AC electrode fabrication requires polymeric binders and the commonly used binders are hydrophobic such as polyvinylidene fluoride (PVDF) and polytetrafluoroethylene (PTFE). Hence, these binders reduce the wettability of the electrode104 and generate extra hydrophobicity on carbon electrode, accompanied by impeding electrolyte diffusion rates into the carbon structure.
Rigidness and fragility
The use of binders affects the mechanical behavior of carbon electrodes and impact electrochemical performance.145 Rigid binders can provide a high mechanical strength, whereas flexible binders yield low mechanical strength. However, a high mechanical strength can lead to a decrease in capacitance,102 whereas a lower mechanical strength electrode might cause the loss of active material which is loosely bound and a failure of electrode interface. PVDF has an outstanding mechanical, thermal and chemical stability102,105 making it the most widely used binder in AC electrode fabrication. However, its rigidness can generate cracks on electrode surface and thus a subsequent loss in electrode performance.105,145 Asquith and coworkers substituted PVDF with hydrophilic copolymers (Polyarylene ether sulfone) with reduced mechanical strength. An enhanced wettability but reduced strength and brittleness were attained resulting in the loss of carbon particles.104 Fang et al. employed polyurethane elastomer as a flexible binder, to solve the problem of rigidness in AC electrode. Despite this achievement, the conductivity and stability were found to be unsatisfactory as compared with PVDF.105
Corrosion and stability decay
While incorporating pseudocapacitive material into a carbon electrode can lead to an increase in charge storage, the faradaic reactions can impede the rate of charge transfer accompanied by decay in electrode stability.146 Similar to the battery electrodes, composite electrodes of carbon with metal oxides often suffer from poor stability.41,117,147 Furthermore, chemical treatment is regarded as the preferred avenue for increasing the concentration of surface functional groups.76 However, these surface functionalities can involve into faradaic reactions16 and instigate the accumulation of irreversible redox products deposited into pores leading to capacitance fading. On the other hand, the removal of surface functional groups through reduction in Ar/H2 confers to the AC electrode a reduced electrochemical stability window (ESW), thus hindering a wider voltage window of operation.115
In Table I, the beneficial and detrimental effects of various modifiers on AC electrode were summarized, as well as the possible future advances.
Table I. The effects of various AC modifiers and future advances reviewed herein.
| AC Electrode Modifiers | Advantages and Benefits | Challenges and Detriments | Future Advances |
|---|---|---|---|
| Without modifier | High SSA | Low capacitance | Integrate AC with novel materials with superior electrochemical properties |
| Highly porosity | High tortuosity | Combination of different dimensionalities | |
| Oxidation | |||
| No selectivity | |||
| Carbon black | Conductivity | Reduced SSA | New and superior conductive additives |
| Impaired porosity | Electron mediators in flow electrodes | ||
| EDL overlapping | |||
| Binders | Mechanical strength | Hydrophobicity | Fabrication of binder-less electrodes |
| Adhesion | Resistance | Synthesis of hydrophilic binders with suitable mechanical strength | |
| Enhanced porosity | Rigidness | ||
| Swelling | |||
| Oxidizers (HNO3, O3, O2, H2O2, KMnO4, H2SO4, H3PO4, etc.) | Functional groups | Reduce pore volume | |
| Wettability | Reduced SSA | ||
| Pseudocapacitance | Corrosion | ||
| Surface charge | |||
| Flowability | |||
| Reducers (H2, Amine, NH3, NaOH, etc.) | Enhanced porosity | Hydrophobicity | |
| Increased SSA | Reduced ESW | ||
| Surface charge | Reduced pore volume | ||
| De-functionalization | |||
| Metal oxides (TiO2, SiO2, Al2O3, etc.) | Surface charge | Pore blockage | Multi-metal oxide coatings with controlled pore size distribution |
| Wettability | Reduced SSA | ||
| Catalytic activity | |||
| Encapsulation | |||
| Nanocarbons (FCNTs, Graphene, etc.) | Conductivity | Reduced SSA | Hybrids and hydrophilic nanostructures |
| Hydrophilicity | Pore blockage | ||
| Pseudocapacitive materials (RuO2, MnO2, Conducting polymers, etc.) | Pseudocapacitance | Poor stability | Introducing synergetic properties of different pseudocapacitive materials |
| Conductivity | Pore blockage | ||
| Reduced SSA | |||
| Heteroatom dopants (N2, S, P, F, B, etc.) | Pseudocapacitance | Reduced pore volume | multiple heteroatom co-doping |
| Conductivity | |||
| Wettability | |||
| Stability | |||
| Surfactants (Cationic, ionic and nanoionic) | Surface charge | Reduced pore volume | Employing mixed surfactants for the sake of synergism |
| Wettability | Electronic resistance | ||
| Selectivity | |||
| Active sites | |||
| Ions channels | |||
| Prevent corrosion | |||
| Ion-exchange resins (BHP55, etc.) and zwiterionic polymers | Surface charge | Resistance | Novel ion-exchange resins with superior properties |
| Selectivity | Reduced volume | ||
| Mechanical strength | |||
| Encapsulation | |||
| Inert gases (Ar, He, N2, etc.) | Enhanced porosity |
Classification and Comparison of CDI Cells
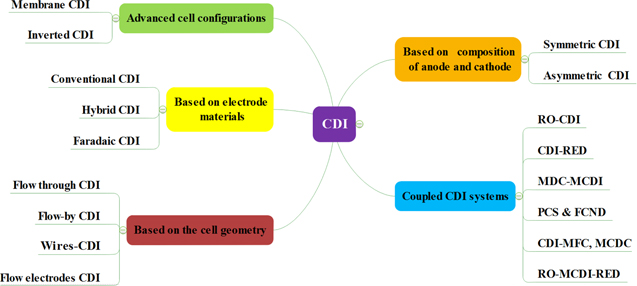
Several carbon-based electrodes have been fabricated with improved performance; albeit some of them have not been yet applied in CDI cells. Importantly, there is interdependence between electrode design and CDI cell architecture. Indeed, one of the main goals of AC modification is to function synergistically with CDI cell architectures. For example, plate and frame reactors require different electrodes than flow cell CDI systems. Therefore, it is imperative to understand the architectural aspects for each CDI cell configuration, in order to reap the benefits of that interdependence. The general classification (Fig. 2) of CDI cells based on different architectural aspects illustrates the known CDI configurations which are currently employed in CDI technology.
Figure 2. Classification of CDI cell configurations based on different architectural aspects.
Download figure:
Standard image High-resolution imageClassification according to electrode materials
Conventional CDI
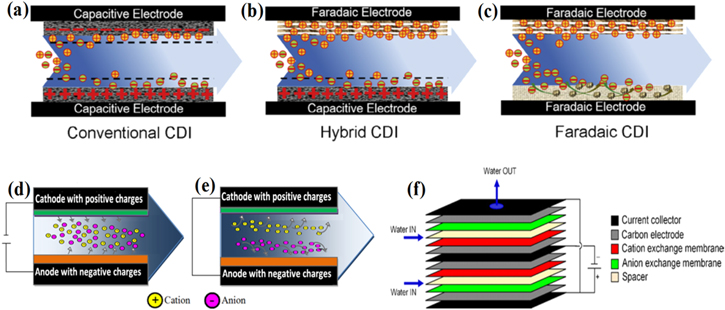
A conventional CDI cell (Fig. 3a) is the fundamental CDI architecture with two capacitive electrodes separated by a spacer wherein the feed water passes through these electrodes either in a perpendicular or parallel fashion. Both positive and negative electrodes are made from carbon material and store ions in EDLs via capacitive effects. Conventional CDI is the simplest design with excellent scalability. Moreover, ions in this system are removed in a non-selective manner.148,149
Figure 3. Desalination mechanism: (a) conventional CDI, (b) hybrid CDI and (c) faradaic CDI.12 Salt separation in i-CDI cell: (d) Regeneration, (e) Adsorption, (f) Schematic representation of two MCDI cells.150
Download figure:
Standard image High-resolution imageHybrid CDI
The hybrid CDI (HCDI) cell (Fig. 3b) consists of a capacitive electrode and a faradaic electrode.151,152 The mode of ion adsorption occurs through capacitive and faradaic mechanisms. In other words, under polarization, ions are stored capacitively in the EDL of the capacitive electrode, while in faradaic electrode, adsorption occurs by means of intercalation or surface redox reactions. HCDI combines the best performance attributes of both conventional CDI and faradaic CDI cells. The combination of capacitive and faradaic effects in the same system have achieved a high ion removal capacity (31.2 mg g−1) as compared with conventional CDI (13.5 mg g−1) and favorable ion kinetics.151
Faradaic CDI
A faradaic CDI cell (Fig. 3c) or desalination battery employs two faradaic electrodes for both the cathode and anode. Unlike capacitive electrodes, faradaic electrodes store ions through the mechanism of intercalation/conversion or by specific ion-redox activity at the interface.12,153 Faradaic electrodes have gained attractive attention due to their superior salt removal capacity, remarkable ion selectivity and a long-term cycling stability. The excellent ion storage capacity makes it a good candidate for the desalination of high-salinity water, specifically sea water. Additionally, this system is capable of reduced energy consumption. Basically, ions are stored in crystallographic sites (interstitial lattice sites) of electrode active materials.154 Intercalating materials as a constituent of faradaic electrodes have been able to achieve a high charge efficiency as compared to capacitive electrodes without the need for ion exchange membranes (IEMs) or any surface modification. This reduces the complexity of faradaic CDI systems as compared with CDI equipped with IEMs.155
Classification according to the electrode composition or structure
Symmetric CDI
A symmetric CDI cell consists of a pair of identical electrodes.156 The term "symmetric" in this context refers to the similar composition in mass, functionalities and structure (porosity, dimensionality, etc.) of the anode and cathode.73 Both electrodes can be used as pristine or after modification. However, this configuration might gradually lose electrochemical symmetry, under electrochemical cycling due to oxidation of the anode. Eventually, the applied potential cannot be distributed equally. In other words, most of the potential difference falls on positive electrode which accelerates its oxidation.84 The main challenges of a symmetric CDI cell performance are the weaker counter-ion adsorption and reduced charge efficiency.89,156
Asymmetric CDI
Asymmetric CDI cell consists of electrodes pair with different composition, such as different surface chemistry, structure, mass or thickness, etc.25,86 Basically, the size of cation in solution is different than the anion resulting in an unbalance of electrosorption of these ions. It is very rare for a carbon electron to achieve an equal electrosorption for both cation and anion. Therefore, the advantages of asymmetrical configuration in CDI rise from non-identical potential changes occurring with the anode and cathode. Asymmetric CDI systems exhibit a relatively high removal capacity, enhanced charge efficiency and better cyclability as compared with symmetric CDI. In addition, asymmetric system may induce ion-selectivity which is an attribute of CDI systems equipped with IEMs.89 However, asymmetric systems may show an inversion effect, a phenomenon discussed previously in which desorption of co-ions become dominant during charging and re-adsorption during discharging.85
Advanced CDI cell configurations
Inverted CDI (i-CDI)
The i-CDI (Figs. 3d, 3e) is a CDI system operated in an opposite manner to the conventional CDI.76 Unlike conventional CDI, in i-CDI cells, the formation of EDL takes place at a chemically modified surface (with net surface charges) of carbon electrodes without an external power source. The adsorption of ions occurs passively, whereas the external voltage is used for ion desorption.75,76,92 The noteworthy advantages of i-CDI are the excellent longevity and exclusion of co-ion expulsion as compared with a conventional CDI operated under the same conditions. The use of an oxidized anode avoids the possible decay in performance stability. However, i-CDI systems have a small working voltage window between the anodic and cathodic EPZC, thereby adsorption capacity remains less than that when compared with conventional CDI and MCDI.76
Membrane CDI (MCDI)
The MCDI (Fig. 3f) is a complex upgraded conventional CDI cell wherein ion-exchange membranes (IEMs) are positioned in front of electrodes for boosting energy and salt removal efficiencies.150 A cations exchange membrane (CEM) and an anions exchange membrane (AEM) are placed on cathode and anode respectively. Inclusion of the membranes holds the benefit of preventing co-ions from leaving electrodes, thus inhibiting the co-ion repulsion effect thereby resulting in better salt removal efficiency. Moreover, when the reversed voltage is applied for ion desorption, the counter-ions are efficiently flushed from the electrodes, thus maintaining the driving force and removal efficiency in subsequent cycles.150,157
Classification according to cell geometry
Flow-By CDI (FBCDI)
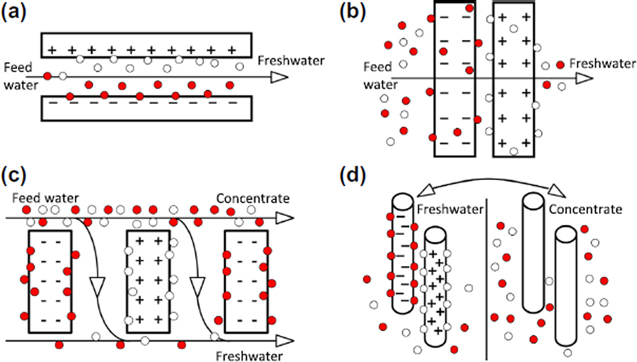
The FBCDI geometry (Fig. 4a) employs static electrode therein the feed stream flows between electrodes, through a thick porous separator element parallel to the electrodes.16 FBCDI is the oldest and commonly used CDI geometry due to simplicity of design, low fouling and limited faradaic reactions. However, the flow of feed water between electrodes imposes some restrictions on the transport of ions from reaching the inner part of electrode. Moreover, the ions in adsorbed state on the surface of electrode might prevent further ionic diffusion towards inner sites. Hence, the flow-by mode exhibits several shortcomings such as the low concentration reduction per charge, long desalination time requirements due to the slow and limited diffusion of salt separation. Furthermore, the spacer thickness which provides channels for influent flow may cause cell internal resistance.15,158 As a consequence, due to the insufficient utilization of adsorption, FBCDI offers a relatively low salt removal and charge efficiency compared with other geometries.159
Figure 4. The CDI system geometries: (a) flow-by CDI, (b) flow-through CDI, (c) flow electrode CDI and (d) CDI using wires.16
Download figure:
Standard image High-resolution imageFlow-Through CDI (FTECDI)
In the FTECDI geometry (Fig. 4b), the feed water flows through static electrodes separated by a very thin spacer vertically positioned to the electrode. The feed water first passes through the first electrode, followed by separator and finally to the next electrode. The flow through mode provides pores space to be more accessible to the ions with a full usage of active sites on the electrodes.148 Unlike FBCDI, the FTECDI exhibits a fast desalination rate with a high reduction in salt concentration of the feed per charge. Furthermore, FTECDI provides better adsorption capacity and charge efficiency than FBCDI. The rate of salt separation is controlled by resistive-capacitive timescale instead of a diffusive timescale.159,160 While FTECDI can desalinate a high salinity feed per charge, it is highly susceptible to electrode degradation after repeated cycles with poor cell regeneration in the case of inappropriate choice of morphology of the carbon electrodes. In addition, FTECDI may exhibit a higher pH fluctuation compared with FBCDI, which likely is indicative of faradaic reactions.161
Flow electrodes CDI (FCDI)
The FCDI geometry (Fig. 4c) employs a dynamic slurry-type electrode which flows between ion exchange membranes and current collectors. Desorption is performed separately downstream of the flow by mixing the effluent of electrode particles from both anode and cathode. Both ion adsorption and desorption inside FCDI occurs continuously whereas in FBCDI and FTCDI, the adsorption process always needs two sequential steps for one cycle.160,162 The use of flow electrodes allows this type of cell to be applied in high-salinity desalination. In addition, flow electrodes exhibit enhanced capacitance as compared with static electrodes. However, the poor electronic conductivity due to discontinuous network of carbon particles is a major limitation of FCDI.132
CDI using wires
Desalination with wire-based electrodes (Fig. 4d) is a capacitive technology based on alternative dipping an array of electrode pairs into the streams of freshwater and brine with and without an external voltage respectively.163 Both anode and cathode are made of thin conductive rods or wires of graphite coated with a porous carbon layer. Before desalination, the feed stream is split into two streams: the salt is taken out from one stream before the water is released into another stream. After a given time of contact in the freshwater stream, the wire pair is lifted and brought in contact with brine stream for ion desorption under a shorted-circuiting cell. Subsequently, the wires are returned to the freshwater compartment. This system using carbon coated wires offers some advantages over FBCDI and FTECDI including, a continuous desalination process instead of an intermittent flow. Furthermore, the configuration does not require a separator (spacer) between wire-based electrodes, associated with reduced resistance and less fouling compared with other geometries.16
Coupled CDI systems
Flow electrodes capacitive neutralization deionization system (FCND)
FCND (Fig. 5a) is a desalination system which combines FCDI with neutralization dialysis (ND). The key property of FCND is a simultaneous capacitive adsorption of ions and a neutralization dialysis process. The FCND design is based on FCDI cell though salt solutions are replaced by the acid and alkaline solutions in the flow electrode channels. ND desalination process is driven by the Donnan potential emerging from the salt concentration difference across the membrane with no need of an external electrostatic force. A simultaneous penetration of H+ and OH− into the salt water compartment leads to a neutralization reaction. Compared with FCDI and ND, FCND exhibits one of the highest average salt adsorption rates (ASAR) and salt removal efficiencies (SRE).164
Figure 5. (a) Schematic view of FCND164 and (b) PCS,125 (c) Diagram of MCDC reactor configuration and operation.165
Download figure:
Standard image High-resolution imagePhotocatalytic-CDI system (PCS)
PCS (Fig. 5b) interbreeds photocatalysis and CDI. The system has been proposed for effective and complete removal of high-valence heavy metal ions. PCS was first employed for enhanced chromium (Cr) removal with a relatively high removal ratio (72.2%) as compare with photocatalysis (35.2%) and CDI (42.5%). The use of metal-organic frameworks (MOFs), specifically MIL-53 (Fe) as a positive photoelectrode induces a synergistic effect between CDI and photocatalysis under visible light and a low direct current (DC) voltage. The conversion of the metal ion on the photoelectrode followed by adsorption on the AC electrode enhances the removal. A simultaneous photoreduction and enhanced removal of metal ions can be ascribed to the ability of PCS to suppress the recombination of photo-generated carriers, thereby promoting the migration of charged ions.123
Microbial capacitive desalination system (MCDC)
MCDC (Fig. 5c) is a desalination system that combines the functions of a microbial desalination cell (MDC) with CDI. Basically, MDC utilizes a bio-electrochemical approach for a simultaneous wastewater treatment and bioenergy production. However, MDC suffers from problems associated with salt migration and pH fluctuations. The MCDC development outstrips MDC on the fact that the ions were adsorbed in the EDL without migrating the salt to the anolyte or catholyte. Moreover, the free transfer of protons across the MCDC system prevents a significant pH change. Noteworthy is that this type of system permits CDI to operate with a high desalination efficiency (7–25 times over CDI).165
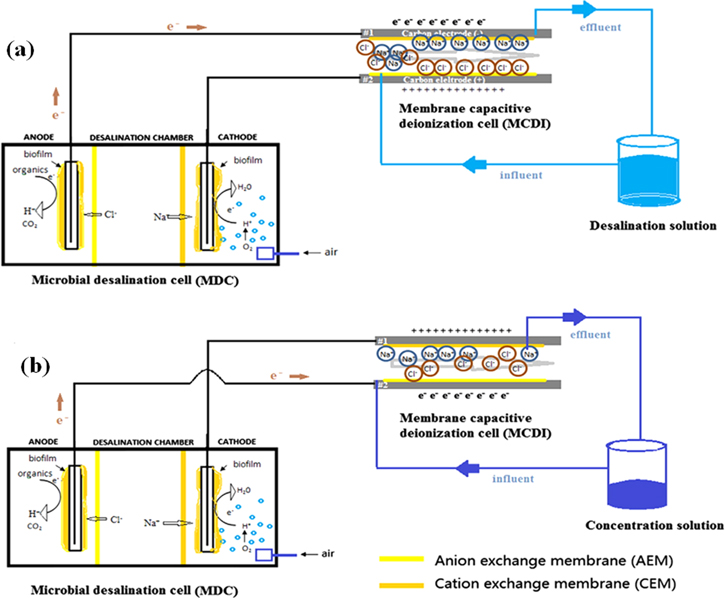
Microbial desalination cell-MCDI system (MDC-MCDI)
MDC-MCDI system (Figs. 6a, 6b) has been developed in order to reap the mutual benefits between MCDI and MDC. Based on the salt concentration required for both MDC and MCDI technology, MDC-MCDI system shows an effective desalination of high salinity water. Primarily, MDC acts as the power source for MCDI. The MDC desalinated high salinity water is obtained before the downstream desalination in MCDI for complete salt removal. The main advantages of MDC-MCDI system are its high desalination rate and superior electrosorption capacity (ECA) with a further desalination of the effluent of MDC under low energy flows from MDC to MCDI.166
Figure 6. Schematic diagram of MDC-MCDI system: (a) adsorption process, (b) desorption process.166
Download figure:
Standard image High-resolution imageReverse Osmosis-CDI system (RO-CDI)
In RO-CDI system (Fig. 7a), reverse osmosis (RO) equipped with an energy recovery device (ERD) is integrated with CDI for seawater desalination into ultrapure water and fresh water production in the same hybrid system.167,168 Furthermore, RO coupling with CDI offers a pretreatment of CDI influent, thus reducing fouling in the CDI process.169
Figure 7. (a) Schematic of integrated RO-CDI system,167 (b) The hybrid CDI-RED system,170 (c) The MFCs driven CDI system,171 (d) RO-MCDI-RED hybrid system.172
Download figure:
Standard image High-resolution imageCDI-Reverse electrodialysis system (CDI-RED)
The CDI-RED (Fig. 7b) is a hybrid system combining CDI with reverse electrodialysis (RED). The system was developed for simultaneous desalination and energy production. The saline water initially passes through the CDI cell. The effluent of the CDI cell is divided into four streams: A fresh water stream, a low salinity stream (LSS), a high salinity stream (HSS) and a rejected water stream. The LSS and HSS pass through the RED cell for the generation of electricity. Since RED employs both high and low salinity streams, the effluent of CDI (brine) contributes to the generation of a Donnan potential which drives the CDI in return. The dual advantages of the CDI-RED system have shown it to be a promising and environmentally friendly desalination system due to the pretreatment of brine.170
Microbial fuel Cell-CDI system (MFC-CDI)
MFC-CDI (Fig. 7c) consist of a microbial fuel cell (MFC) coupled with CDI. The MFC utilizes wastewater in order to generate energy, originating from electron transport from exo-electrogenic bacteria. The brine flows circularly through CDI with a simultaneous deionization under an electrical field, while MFC acts as the power source for CDI operation. Generally, CDI-MFC system provides a concomitant treatment and desalination of wastewater with a low salt concentration. In addition, it is highly energy efficient due to the recovery and reuse of electrostatic energy stored by CDI cell for enhancing electron production in MFC.171,173
RO-MCDI- RED system (RO-MCDI-RED)
The RO-MCDI-RED hybrid system (Fig.7d) emerged from a combination of both RO and RED with MCDI for desalinating seawater with a high total dissolved solid (TDS) concentration. MCDI was adopted for brackish water desalination while RED employment produces energy by utilizing the brine from RO and the effluent of MCDI. The system displays a remarkable energy efficiency that can be ascribed to the synergism between MCDI and RED.172
Interdependence Between the AC Electrode and the Configuration of CDI Cell
During electrochemical operation, the nature and design of the electrode directly affects the performance of CDI cell in a given configuration (arrangement of cathode, anode, separator and saline water together) and vice versa. Optimization of AC properties towards ideality cannot be divorced from a given CDI cell configuration. While electrodes contribute substantially to CDI performance, they should be configured in the manner that yields high capacity, robust cycle stability, high removal rates, etc. Moreover, the selection of a CDI cell configuration and with a proper marriage of matching electrodes is essential for the success of this technology.
There is interdependence (Fig. 8) between the electrodes, CDI cell configuration and operational parameters.25 Therefore, it is important to make a choice of a CDI cell configuration that considers the design and properties of electrodes. Alternatively, characteristics of electrodes can determine a given cell design. Furthermore, a function of a particular CDI cell configuration can be imparted in a conventional CDI cell via electrode modification. As an example, Min et al.55 employed an inorganic porous film of tiron-grafted TiO2 on AC electrode to acquire the ion exchange and salt removal efficiency properties of MCDI (Figs. 9a–9c). The electrode displayed good ion-exchange capacity and reduced co-ion repulsion effect without the use of ion-exchange membrane.
Figure 8. Simplified schematic illustration of interdependence in CDI performance and benchmarking. Electrode, Configuration of CDI cell and Operational parameters undergo interdependence with the key impact on the whole performance of CDI system. At the same time, the electrode activity is dictated by the tradeoff between physical, chemical and electrochemical properties appertained to the modifiers in the course of design.
Download figure:
Standard image High-resolution imageFigure 9. (a) Comparison of the conductivity change (b) Adsorption rates, (c) Salt removal efficiency,55 (d) Average salt adsorption rates (ASAR),86 (e) In FBCDI the feed water flows through separator between electrodes consist of nanoscale pores which maximize the surface area but prevent flow through the electrodes, (f) The flow in FTECDI, where the feed water flows directly through electrode with hierarchical porosity associated with low hydraulic resistance.161
Download figure:
Standard image High-resolution imageThe efficiency of salt removal in a CDI cell configured with symmetric electrodes relies on the balance between negative and positive chemical charges on the electrode surface.156 In other words, the optimal performance was obtained when the net surface charge was equal to zero for both the anode and cathode. Nevertheless, a symmetric configuration is more susceptible to electrochemical degradation as compared with asymmetric configuration, especially under a high operating voltage. Thus, encapsulation of electrodes and the adoption of IEMs play a significant role for mitigating cyclability fading in symmetric CDI. On the other side, the asymmetric CDI is based on differentiating the two electrodes for establishing an extended voltage window which can operate beyond the thermodynamic decomposition voltage.174 The necessary features of an asymmetric configuration can be obtained by integrating AC with other materials. By selecting electrode materials or modifying these materials, one influences overall performance of a CDI system for a given application scenario and also improves the stability of the system against degradation with increased cycling.175 However, due to the many choices of electrode materials with which to match a given voltage window, selected pairings of anode and cathode in asymmetric or hybrid configurations become more complicated with respect to capacity as well as rates of performance and efficiencies.
In order to circumvent the capacitance mismatch between oxide-based positive electrodes and carbon-based negative electrodes, AC-doped with nitrogen and oxygen heteroatoms was assigned to be used as the ideal negative electrode in an asymmetric configuration.113 On another hand, a symmetric configuration with nitrogen-rich porous carbon materials exhibits a competitive edge over asymmetric configuration by avoiding the power imbalance at the two ends (anode and cathode).73 Furthermore, by incorporating pseudocapacitive materials into the carbon electrodes provides one with a high capacitance that might attenuate the mass imbalance in an asymmetric configuration. In essence, the electrodes utilize the pseudocapacitive property to alleviate the mismatch of capacity and kinetics between the capacitive and faradaic electrode. The carbon materials modified by ozone are eligible candidates for negative electrode fabrication; hence a positive electrode with a high overpotential for oxygen evolution is required for an efficient asymmetric configuration. Additionally, AC impregnated with transition metal oxides or pseudocapacitive based electrodes have been assigned as a positive electrode in order to extend the operating voltage window and avoid oxidative degradation.121,125 Generally, the hybrid or asymmetric configurations provides enhanced electrochemical performance through a strategic design. Specifically, hybrid configurations allow the combination of capacitive and faradaic electrodes or a pseudocapacitive electrode in the same CDI cell for enhancing both capacitance and rate capability.176 Moreover, the capacitance of a hybrid configuration is partially determined by the faradaic electrode; hence the design of an electrode with a high rate capability is crucial as faradaic reactions are slow.
The performance of i-CDI configuration solely relies on chemical surface charge. The formation of chemical charge on AC surface, positions it to be an adequate electrode for i-CDI as compared with MCDI or conventional CDI (Fig. 9d). Through modification, the AC electrode can drive the performance of a new CDI design with a different operational mode from that of a conventional CDI cell. Furthermore, AC electrodes with shifted EPZC exhibit a better performance in i-CDI than conventional CDI. The maximum driving force for adsorption prevents any positive co-ion expulsion in i-CDI by shifting of the EPZC of the anode due to anodic oxidation.86 Similar to conventional CDI, i-CDI is also limited by low cell voltage. Nevertheless, relocating the EPZC of electrode can enlarge the working voltage window of i-CDI.
The flow-regime in the FTECDI geometry is largely governed by electrode porosity. Also, the geometry has an impact on the high electro-oxidation rate on positively polarized electrodes.7 Basically, addition of macropores can mitigate the tortuosity of an AC electrode thereby increasing capacitance and enhancing kinetics. However, the integral efficiency of that electrode depends on the specific cell architecture.45 The feed stream in FBCDI flows through the separator (Fig. 9e) between electrodes that often consists of sub-50nm of nanopores (high SSA) that suffer from the poor flow due to the high hydraulic resistance.161 There is enhancement tradeoff between SSA and diffusion in FTECDI. Thus, throughout electrode design, the flow mode in a particular geometry should be taken into consideration. The FTECDI requires a highly porous electrode with hierarchical porosity (Fig. 9f), thereby enabling both low fluidic resistance and high specific capacitance. Suss et al. reported the worthiness of carbon electrodes with a bimodal pore structure associated with low hydraulic resistance in FTECDI geometries. The use of a macroscopic porous electrode in FBCDI requires much longer time of cell charging and desalination.158,161 Contrarily, macroscopic porous electrodes in FTECDI enable a significant reduction in desalination time and desalinate at higher salinity feeds per charge,161 due to the advantages of low tortuosity associated with efficient flow. For a highly microporous AC, a further activation may create more mesopores associated with reduced SSA. On the other hand, AC with a high SSA may not have a high capacitance when majority of the surface area is not involved in electrosorption.
Furthermore, the FCDI geometry emerged with employment of flowable carbon electrodes,177 therein the whole surface might be involved in the desalination process thereby generating high capacitance. However, flow electrodes suffer from a relatively low conductivity due to the poor connectivity of AC electrode particles which can impede the efficiency of FCDI. In essence, a high mass loading of AC in the slurry results in a simultaneous increasing of connectivity and viscosity.70 Nevertheless, there is a great enhancement of performance through novel cell design and the optimization of the characteristics of the suspension.160
Conclusion and Perspectives
CDI performance relies on electrodes, cell architecture and operational parameters. Ideally optimized electrodes can gain the lion's share of performance in CDI systems. AC has been the workhorse material for CDI electrode fabrication and the generation of new opportunities for electrode optimization has been obtained using different modifiers. By tuning physical and chemical properties, AC that exhibits enhanced activity and versatility on electrochemical performance can be further improved to enhance the performance of CDI systems. However, it must be cautioned, as we have suggested above, some attempts to modify electrodes with respect to one favorable characteristic may affect another in a deleterious fashion. Indeed, in this era of intensive employing electrode modifiers, there is a need of a rational design without sacrificing some of the electrochemical performance benchmarks. In the context of electrochemical performance, AC possesses some intrinsic deficiencies such as hydrophobicity and low conductivity, and thus one might consider modifying the AC electrode with materials of superior electrochemical properties.
Several possible future advances of AC modification have been proposed based on the discussion. Conductive additives with similar textures to AC could maintain surface integrity and alleviate EDL overlapping. Moreover, AC textural characteristics should be determined before coating with metal oxides in order to control pore size in their composites. Fabrication of binder-less electrodes or employing hydrophilic binders with suitable mechanical strength could suppress the challenges of binder use. Efficient electron mediators might enhance conductivity in flow electrodes with desirable rheological properties. Employment of multiple modifiers can boost the capacitance improvement through exploitation of their synergism and/or coexistence. For instance, multiple heteroatom co-doping might be used to increase capacitance. Also, surfactants can play a role on both interfacial behavior and electrochemical performance of carbon electrodes. Hence, we can postulate that the enhancement of AC activity in a mixed surfactant system (synergism) may be far superior to the use of a single surfactant.
However, when multiple modifiers are introduced into electrode formulation, the activity must be evaluated taking into consideration of potential health hazards. Another suggestion is that, AC electrode design must consider the dimensionalities. By interweaving AC (zero-dimension) with multi-dimensional materials could improve electrode performance. Indeed, the development of AC with one-dimension (1D), two-dimensions(2D) or three-dimension(3D) using multiple dimensionalities materials as templates, or a combination of different dimensionalities in the same electrode might be an approach to expand AC electrode design domain for enhanced performance. Also, the majority of electrode designs covered in the literature are lab-scale studies. Therefore, a full assessment of modifiers effects in the practical context is yet to be validated on a large scale. Broadly speaking, knowing the effects of modifiers in the course of electrode design, provide insight on a rational design.
In essence, tuning the entire system is a prerequisite in order to balance the tradeoff between conductivity-porosity, functional group-porosity, mechanical strength-flexibility, SSA-diffusion, conductivity-flowability, desalination efficiency-charge efficiency, etc. Thanks to the tunable behaviors of AC, that could be further engineered for the sake of superior performance. Tailoring physic-chemical properties of AC and hybridization with other materials (with superior electrochemical activity) will continue to offers large room for enhancing CDI performance. Last but not the least, the selection of a modifier should take into consideration the necessary features of electrodes for a given CDI configuration. In another word, electrode design should match the given features of a particular CDI configuration to be employed for a given application in order to reap the benefits of interdependence. With unremitting endeavor, optimization of AC electrodes through a rational design will continue to be the cradle of further breakthroughs in advancing CDI technology.
Acknowledgments
The authors would like to acknowledge the financial support of the National Natural Science Foundation of China (No. 21808160).