Abstract
Here, we report nano-mediated Cu–Co–Ni-based nitrogen-doped carbon nanotubes (N-CNTs/T-CCN) by hydrothermal and procedural calcination strategy. The nitrogen-doped carbon nanotubes (N-CNTs) show more average diameter and the N-CNTs are uniformly modified with ternary Cu–Co–Ni-based nanoparticles (T-CCN). The hybrid exhibits excellent ORR catalytic activity. The onset potential (Eonset) and half-wave potential (E1/2) are 0.96 V and 0.87 V (versus reversible hydrogen electrode, RHE) in 0.1 M KOH. Most importantly, compared to 20% Pt/C, N-CNTs/T-CCN catalyst displays better methanol tolerance and higher stability. The H2O2 yield of the N-CNTs/T-CCN is less than 7.5% and the electron-transfer number (n) is about 3.9. High ORR performance may be related to the synergistic enhancement effect. The N-CNTs supply good electrical conductivity and allow large numbers of active sites to efficiently participate; the T-CCN can improve the local work function of the N-CNTs by synergistic electronic interaction and promote O2 adsorption; the stability of embedded T-CCN can be greatly improved, mainly due to the weakness of Ostwald effect. All these advantages make the hybrid a promising ORR catalyst.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Increasing energy demand and environmental problems have promoted tremendous researches and kinetically sluggish ORR is a major limiting factor [1–5]. Pt-based precious metals have excellent ORR catalytic properties [6–8], while they suffer from low reserve, high cost and methanol poisoning [9–11].
Recent researches prove that ORR activity of transition metal-based carbon can be enhanced [12, 13]. Compared to the metal-based nanoparticles supported on carbon materials, nanoparticles coated by carbon materials can weaken the Ostwald effect in ORR progress. Meanwhile, the Fermi level of the metal is lower than that of the CNT. When the metal and the carbon material are in contact, the formed buffer layer can allow electrons to flow from the metal to the CNT to maintain the balance of the work function, which can promote the ORR process accordingly [14–17]. So, a solution to obtain effective ORR catalysts is to construct carbon-coated transition metal nanoparticles. However, how to construct the hybrids remains a challenge. As for nanomaterials, the periodic boundary conditions are destroyed, and the atomic density near the surface layer of nanoparticle is reduced, and the locality and the coherence are enhanced. That can homogenize the distribution of transition metals and enhance the surface activity of nanomaterials. Therefore, how to use the nano-mediated synthesis for encapsulated transition metal-based carbon nanomaterials is important.
Especially for ORR catalysts, it is critical to enhance the ORR efficiency by controlling the structure and maintaining large numbers of accessible specific activity area. An effective way is to fabricate encapsulated transition metal-based nanoparticles in carbon nanotubes, which can enhance the electrical conductivity and avoid the 'dead volume' [18–21].
On these bases, we report the nano-mediated Cu–Co–Ni-based nitrogen-doped carbon nanotubes (N-CNTs/T-CCN) by hydrothermal and calcination strategy. The Cu–Co-based nanosheets are firstly introduced on the surface of foamed nickel by hydrothermal reaction and then N-CNTs/T-CCN is obtained by procedural calcination of the mixture of surface-nanosized-foamed nickel and melamine in Ar. The nitrogen-doped carbon nanotubes (N-CNTs) show more average diameter. Ternary Cu–Co–Ni-based nanoparticles (T-CCN) are not only distributed on the tip, but also evenly distributed in the carbon nanotubes. Based on theoretical calculations, Cu and Co shows high activity for ORR, and its position in 'volcano plot' is close to Pt [22]. Moreover, the introduction of Cu and Co into nanostructure can promote the synergistic effect between them [23, 24] and accordingly their d orbital electron can weaken O–O bonds in ORR process [25, 26]. The remarkable features of the N-CNTs/T-CCN lead to strong durability and methanol tolerance. The onset potential (Eonset) and half-wave potential (E1/2) is 0.96 V and 0.87 V in 0.1 M KOH, respectively. The H2O2 yield of the N-CNTs/T-CCN is less than 7.5% and the electron-transfer number (n) is about 3.9. Based on previous researches and experimental results, we speculate that it is a nearly 4e− ORR pathway [27–29]. Specifically, it shows great prospects for the direct production of electrocatalysts for supercapacitors and lithium-ion batteries [30–33].
2. Experimental section
2.1. Synthesis of N-CNTs/T-CCN
Cu(NO3)2 (1 mmol), Co(NO3)2, (1 mmol) and urea (9 mmol) were added into deionized water (60 ml) and stirred for 1 h. Then, cleaned nickel (1 × 3 cm) and the mixed solution were placed in the Teflon-lined autoclave. They were kept in an electric oven at 120 °C for 12 h. Next, the obtained product was washed with water and ethanol for several times and dried at 60 °C. After the product was annealed at 400 °C for 1 h in Ar, the N-CNTs/T-CCN were obtained by one-step procedural calcination of mixture of the product and melamine (3 g) under 520 °C for 2 h, 540 °C for 2 h, and 700 °C for 2 h in Ar. The heating rate was 3 °C min−1.
2.2. Material characterizations
The morphology was studied by scanning electron micro-scope (SEM) (FEI FEG250) and transmission electron microscopy (TEM) (FEI LM1-125). X-ray diffraction (XRD) and x-ray photoelectron spectroscopy (XPS) was tested by Powder diffractometer (D8 ADVANCE, Bruker) and x-ray photoelectron spectrometer (Thermo ESCALAB 250XI), respectively. Pore size and distribution of the samples and BET (Brunauer–Emmett–Teller) were obtained by Pore Master-60.
2.3. Electrochemical measurements
Electrochemical data was carried out on CHI760E with rotating ring disk electrode (RRDE, the diameter of GC disk, Pt inner ring, and Pt outer ring is 4, 5, and 7 mm, respectively.) and rotating disk electrode (RDE, the GC disk diameter is 5 mm). The saturated calomel electrode (SCE, ESCE = 0.242 V) and Pt wire were reference electrode and counter electrode. In this paper, all potentials are converted to RHE.
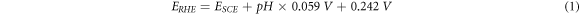
10 mg of catalyst, 2 ml of isopropanol and 10 μl of 5 wt% Nafion were dispersed in 0.5 ml of deionized water. Homogeneous ink can be obtained by ultrasonication about 30 min. Then, 20 μl of catalyst ink was coated onto the working electrode. Measurements were tested in N2 or O2 saturated 0.1 M KOH solution. The electron transfer number was calculated by Koutecky-Levich (K-L) equation [34, 35].
3. Results and discussion
Figure 1 exhibits the preparation of N-CNTs/T-CCN. Typically, Cu-Co nanosheets supported on foam nickel were firstly fabricated by hydrothermal reaction and then the N-CNTs/T-CCN was obtained by procedural calcination of mixture of surface-nanosized foam nickel and melamine in Ar. We speculate that the possible formation mechanism of N-CNTs/T-CCN is similar to 'polymer sugar-blowing process' accompanied by in situ catalytic growth of CNTs [17]. In detail, Cu-Co nanosheets have high surface tension and can react with melamine-pyrolyzed derivative. When Ni, Co, and Cu are transformed into nanoparticles, they act as catalytic centers to catalyse the in situ growth of N-CNTs.
Figure 1. Synthesis schematic of nano-mediated uniform N-CNTs/T-CCN.
Download figure:
Standard image High-resolution imageSEM images (figures 2(a)–(c)) illustrate the characteristics of the N-CNTs/T-CCN. The length of Cu–Co nanosheets is about 1 μm and they are uniformly covered on foam nickel skeleton (insets of figure 2(a)). After the procedural calcination, the hybrids are obtained (figures 2(d)–(f)). The lattice distance in figure 2(f) is approximately 0.235 nm, corresponding to the C (100) plane. The average diameter of these carbon nanotubes is about 800 nm and they are more uniform than carbon nanotubes prepared by procedural calcination of mixture of nickel foam and melamine (figure S1 is available online at stacks.iop.org/NANOX/2/010026/mmedia). According to the high-resolution TEM image (figure 2(f)) and element mapping images (figures 2(g)–(m)), the T-CCN are coated by the carbon layer, and they are not only distributed at the tip of the carbon nanotubes, but also distributed in the carbon nanotubes. As for carbon nanotubes prepared by just procedural calcination of mixture of nickel foam and melamine, only Ni-based nanoparticles are distributed at the tip of N-CNTs (figure S2), rather than uniformly dispersed on the entire N-CNTs [36].
Figure 2. (a) SEM image of the binary Cu–Co nanosheets-based foamed nickel, (b), (c) SEM images of the N-CNTs/T-CCN, (d), (e) TEM images of the N-CNTs/T-CCN, (f) High-resolution TEM image of the N-CNTs/T-CCN, (g)–(m) elemental mapping images of the N-CNTs/T-CCN.
Download figure:
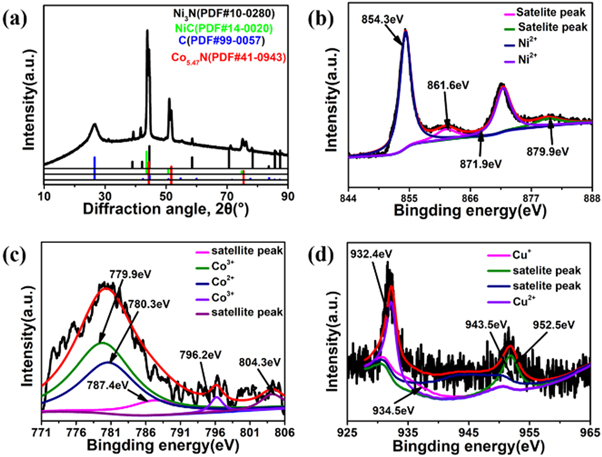
Standard image High-resolution imageThe composition and structure of the N-CNTs/T-CCN were further investigated by XRD and XPS. As shown in figure 3(a), T-CCN is mainly assigned to nitride and carbide. The high-resolution Ni 2p spectra (figure 3(b)) display the 2p1/2 (854.3 eV) and 2p3/2 (871.9 eV) peaks of Ni2+ and satellite peaks (861.6 and 879.9 eV) [37]. The curve-fitting Co 2p spectra appear two valence peaks which corresponding to Co2+ and Co3+ (figure 3(c)). Specially, the peaks at 779.9 and 796.2 eV are attributable to Co3+, while the peaks at 780.3 is ascribable to Co2+ and the peaks at 787.6 and 804.3 eV are the couple of shakeup satellites [37]. Figure 3(d) is the spectra of Cu 2p. The two peaks generated at 932.4 and 934.5 eV are mainly assigned to the Cu2+ and Cu+, respectively. The peaks at 943.5 and 952.5 eV in the hybrids are satellite peaks [38]. As for N 1s spectra (figure S3(a)), the two peaks at 398.1 and 400.2 eV are assigned to pyridinic N and pyrrolic N [39], respectively. In deconvoluted O 1s spectra (figure S3(b)), the peaks of C=O and C–O are located at 531.5 eV and 532.4 eV [40]. The spectra of C 1s in figure S3(c) can be well deconvoluted into three peaks at 284.3, 285.4, and 287.7 eV, which are assigned to sp2 carbon, C–N/C=N bands and O–C=O, respectively [41]. C–N/C=N bond indicates that N is successfully doped into carbon nanotubes [42]. Combined with previous work, the N-CNTs/T-CCN can be formed by the combination of Co2+ and the N atom in organic ligands decomposed during pyrolysis [43]. Moreover, the ID/IG of the hybrid is 0.85 (figure S4(a)), indicating that it has a high degree of graphitization and good electrical conductivity. As shown in figure S4(b), the BET surface area of N-CNTs/T-CCN is 71.7 m2/g, and the pore is about 4.2 nm, which is beneficial to enhance the ORR mass transfer.
Figure 3. (a) XRD diffraction pattern of the N-CNTs/T-CCN, (b) Ni 2p, (c) Co 2p and (d) Cu 2p high-resolution XPS spectra of the N-CNTs/T-CCN.
Download figure:
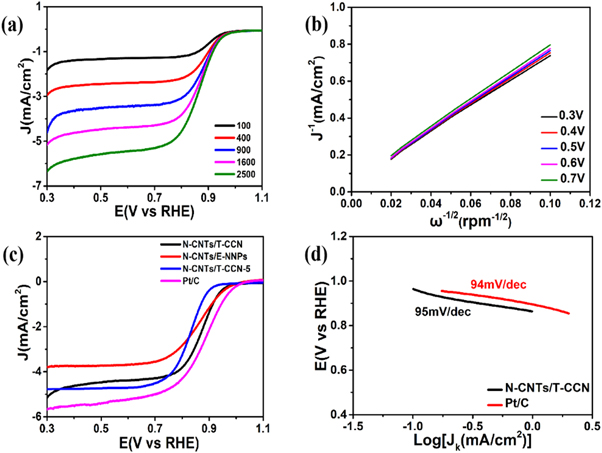
Standard image High-resolution imageThen we performed electrochemical performance on a three-electrode electrochemical workstation. Compared to the cyclic voltammetry (CV) in N2-saturated KOH solution, a clearly cathode peak can be observed in O2-saturated KOH solution (figure S5), indicating that the hybrid has ORR activity. According to LSV curves at different speeds, the hybrid shows high ORR activity (figure 4(a)) with onset potential of 0.96 V and half-wave potential of 0.87 V at 1600 rpm. It is worth noting that its ORR catalytic activity is higher than that of the transition metals and nitrogen-doped carbon materials previously reported (table S1). The K-L curve indicates an approximately parallel linear relationship (figure 4(b)), and the value of the n is calculated in the range of 3.77–4.00, suggesting a nearly 4e− ORR pathway. The ORR activity of N-CNTs/T-CCN is higher than that of N-CNTs/E-NNPs and N-CNTs/T-CCN-5 (the amount of melamine is 5 g) (figure 4(c)). Though the ORR activity of N-CNTs/T-CCN is lower than that of 20% Pt/C, the tafel slope of the N-CNTs/T-CCN (95 mV/dec) is almost the same as that of 20% Pt/C (94 mV/dec) (figure 4(d)).
Figure 4. (a) ORR polarization curves of N-CNTs/T-CCN in O2-saturated 0.1 M KOH solution with different rotation speeds at a scan rate of 10 mV s−1. (b) K–L plots at different electrode potentials derived from RDE measurements. (c) LSV curves of N-CNTs/T-CCN and other catalysts in O2-saturated 0.1 M KOH solution at a scan rate of 10 mV s−1. (d) Tafel slopes of N-CNTs/T-CCN and 20% Pt/C catalysts.
Download figure:
Standard image High-resolution imageBy comparison of figures 5(a) and (b), we found that the CV curves of the N-CNTs/T-CCN did not appreciable variation after the addition of methanol, while the current density of 20% Pt/C changed drastically. The result shows that the N-CNTs/T-CCN catalyst has excellent methanol resistance in alkaline solutions. In addition, the electrochemical durability of the N-CNTs/T-CCN catalyst for the ORR was assessed by continuous CV scanning for 5000 cycles (figure 5(c)); the result shows that there is no significant displacement. We also tested the long-term stability of N-CNTs/T-CCN and 20% Pt/C under identical conditions (figure 5(d)). The relative ORR current of N-CNTs/T-CCN remained at a level of 92.3% after 8 h, while the 20% Pt/C decreased to 53.6% under the same conditions. It is proved that the stability of the N-CNTs/T-CCN is much better than that of 20% Pt/C.
Figure 5. CV curves of (a) N-CNTs/T-CCN and (b) 20% Pt/C in O2-saturated 0.1 M KOH solution with and without 1.0 M CH3OH at a scan rate of 50 mV s−1. (c) N-CNTs/T-CCN for ORR in O2-saturated 0.1 M KOH before and after 5000 cycles. (d) chronoamperometric re-sponse at 0.6 V in O2-saturated 0.1 M KOH at a rotation rate of 1600 rpm.
Download figure:
Standard image High-resolution imageFigure 6(a) shows that the ring current (attributed to H2O2 oxidation) is evidently lower than the disk current (attributed to oxygen reduction), which is manifested as the markedly product of OH−. The H2O2 yield of the N-CNTs/T-CCN is less than 7.5% and the value of the n is around 3.9 (figure 6(b)), further confirming a nearly 4e− ORR pathway. Based on previous researches and experimental results, we speculate that it is a nearly 4e− ORR pathway, as shown in catalytic free energy diagram (figure S6):
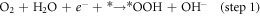
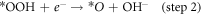
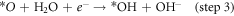
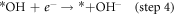
where *O, *OH, and *OOH are adsorbed intermediates. The decisive step in ORR reaction is step 2, and the lower free energy determined that the N-CNTs/T-CCN has high activity for the 4e− ORR pathway [27, 28]. The remarkable characteristics of high activity, good kinetics and strong durability are mainly attributed to the structure and compositional relationship of the N-CNTs/T-CCN: (i) N-CNTs supply good electrical conductivity and can allow large numbers of active site to efficiently participate; (ii) T-CCN are not only distributed on the tip, but also evenly distributed in the carbon nanotubes, indicating the catalyst has more ORR active sites; (iii) Synergistic enhancement promotes high ORR performance: the T-CCN can improve the local work function of the N-CNTs by synergistic electronic interaction and promote O2 adsorption; [17] (iv) the catalytic activity and stability of embedded T-CCN can also be greatly improved, mainly due to the weakness of Ostwald effect [44–46]. In general, all the advantages enhance the ORR performance of the sample, making it a promising low-cost, efficient ORR catalyst.
Figure 6. (a) RRDE curves at a scan rate of 10 mV s−1 (rotation rate: 1600 rpm min−1), (b) H2O2 yield and electron transfer number of the N-CNTs/T-CCN in O2-saturated 0.1 M KOH solution.
Download figure:
Standard image High-resolution image4. Conclusion
The nano-mediated N-CNTs/T-CCN, as the high activity and stable ORR electrocatalyst, were synthesized by hydrothermal and procedural calcination strategy. The hybrids display high active sites, good electron and mass transfer, and synergistic effects, so they have excellent electrocatalytic activity and stability. Nano-mediated collaborative strategies provide a new method that facilitates many practical applications.
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation (Grant No. 21605057, No. 21705056), Natural Science Foundation of Shandong Province (No. ZR2016BQ07), Open Founds of State Key Laboratory of Electroanaytical Chemistry (SKLEAC201907), and Study Abroad Fund.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).