Abstract
In this article we review the microfabrication approaches, with a focus on bioprinting and organ-on-chip technologies, used to engineer cardiac tissue. First, we give a brief introduction to heart anatomy and physiology, and the developmental stages of the heart from fetal stages to adulthood. We also give information on the cardiac tissue microenvironment, including the cells residing in the heart, the biochemical composition and structural organization of the heart extracellular matrix, the signaling factors playing roles in heart development and maturation, and their interactions with one another. We then give a brief summary of both cardiovascular diseases and the current treatment methods used in the clinic to treat these diseases. Second, we explain how tissue engineering recapitulates the development and maturation of the normal or diseased heart microenvironment by spatially and temporally incorporating cultured cells, biomaterials, and growth factors (GF). We briefly expand on the cells, biomaterials, and GFs used to engineer the heart, and the limitations of their use. Next, we review the state-of-the-art tissue engineering approaches, with a special focus on bioprinting and heart-on-chip technologies, intended to (i) treat or replace the injured cardiac tissue, and (ii) create cardiac disease models to study the basic biology of heart diseases, develop drugs against these diseases, and create diagnostic tools to detect heart diseases. Third, we discuss the recent trends in cardiac tissue engineering, including the use of machine learning, CRISPR/Cas editing, exosomes and microRNAs, and immune modeling in engineering the heart. Finally, we conclude our article with a brief discussion on the limitations of cardiac tissue engineering and our suggestions to engineer more reliable and clinically relevant cardiac tissues.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Cardiovascular diseases (CVDs) have been the leading cause of death in the United States and worldwide for several decades. In 2020 alone, CVDs accounted for more than 650 000 deaths in the United States with most recently estimated total healthcare cost greater than $378 billion [1]. While deaths due to CVD have steadily decreased each year since the mid 1960s, over the past decade this trend has stagnated or, in some parts of the U.S., reversed [2–4]. With the rising age of the U.S. population and associated increases in healthcare costs and CVD risk [4], as well as the increasing cardiac problems associated with the recent COVID-19 pandemic, it is imperative that we develop a more complete understanding of the progression and pathology of CVDs in order to design effective clinical interventions or otherwise preventative measures.
Study of CVDs has been stunted by the difficulty in obtaining human hearts for scientific research, in part due to the high demand of hearts for organ transplant [5], requiring the use of animal models to study both the progression and treatment of CVDs. While much of our knowledge of CVDs comes from animal models, recent studies have shown key discrepancies between animal models and human cardiac pathology, which limits the translatability of these results [6–8]. Furthermore, due to the difficulty in recreating 'true' CVDs in animal models, studies often necessitate obtaining a CVD phenotype through a physiologically irregular manner, which further confounds these results [7, 8]. Studies of the fundamental physiology and biological processes of the heart have been similarly limited by these factors, and in fact have been less addressed than studies of CVDs.
A proposed solution to these limitations is the generation of lab-made biomimetic cardiac tissue, a task which has become a cornerstone of the field of tissue engineering [9]. Engineered cardiac tissues (ECTs) have taken several forms, from the attempted growth or reseeding of whole organs to breaking down hearts into models with as few biological components as possible [9, 10]. Two approaches that have shown promising success in recapitulating in vivo heart behavior are the development of small, highly controlled tissues in the form of structured printed models, and microfluidic organ-on-a-chip models.
Printed cardiac tissue models have been widely used to mimic the structural aspects and organization of the heart, typically including defined vasculature [11]. These models have been instrumental in developing our understanding of the structural and multicellular (i.e. paracrine signaling) effects on tissue homeostasis and disease progression [10–12]. However, they have been limited by difficulties in promoting effective vascularization of larger scale tissues, as well as limitations concerning the variety of materials and cells that can be used [12]. Alternatively, small-scale approaches to mimic a portion of the cardiac tissue ex vivo have also been informative in ascertaining the importance of local signaling in cardiac tissue maintenance and disease response [9, 10]. In particular, the inclusion of extracellular matrix (ECM) components and recent interest in the role of intercellular signaling and miRNA activity in maintaining tissue homeostasis and disease response have been driving factors in this development [13]. Further advancement of models that can recapitulate both whole tissue function and microenvironment signaling are necessary for the development of truly biomimetic cardiac tissue models. This field is rapidly growing due to the convergence of several disciplines, including such diverse fields as molecular biology, mechanical engineering, and computational bioinformatics, in our understanding of cardiac tissue and how to model it. Therefore, it is necessary to address both the fundamentals of cardiac anatomy and physiology as well as modern engineering techniques to model the heart in order to better understand the applications and potential limitations of existing ECT models.
In this review, we will discuss recent trends in cardiac tissue engineering, specifically focusing on the utility of ECT models in both basic science and translational applications. While ECT models are a popular topic for review, many discussions aim to focus on a single aspect of these devices, such as the utilized cells [14–16], constructed materials [17–19], or novel fabrication methods [12, 20], and often convalesce to investigate the zeitgeist of models used to engineer the heart, the majority of which are bioprinting and heart-on-a-chip models [21, 22]. Despite this, few articles have been published on the target applications of cardiac tissue engineering [10, 23, 24]. To address this, we focus on the use of cardiac tissue engineering in (i) the treatment of heart diseases, along with potential applications in the development of strategies for cardiac tissue regeneration and replacement, and (ii) creating both healthy and diseased cardiac tissue models to both enhance our understanding of the fundamental biology of heart diseases, as well as develop more biomimetic and easily accessible platforms for drug testing and discovery, and diagnosis. Finally, we highlight cutting-edge techniques that are being explored with respect to their applications in cardiac tissue engineering, including machine learning (ML), CRISPR/Cas gene editing, exosomes and local signaling, and the role of the immune system. We bring a unique perspective by covering both the applications and the recent trends in cardiac tissue engineering, and define the roadmap towards engineering a truly biomimetic heart model.
1.1. Heart anatomy and physiology
The primary physiological role of the heart is to mobilize blood to provide oxygen to the various organs in the body, including itself [25, 26]. To do this efficiently, the human heart has developed four major parts: the left and right atria, and the left and right ventricles. The atria serve as receptacles for blood in the heart, with the left atrium collecting deoxygenated blood from the vena cava and the right atrium collecting oxygenated blood from the pulmonary veins. From the atria, blood will be pushed into the ventricles, with the right ventricle further pushing deoxygenated blood into the pulmonary artery and the left ventricle pushing blood into the aorta and the rest of the body. Because of the resistance and distance that must be overcome to mobilize blood flow in the body, the left ventricle is the largest and most muscular part of the heart [27]. Additionally, the left ventricle is the region most susceptible to CVDs, particularly myocardial infarction (MI), cardiac fibrosis, and hypertrophy [26–28].
While all four parts are major components of an individual heart, it has been shown that the organization and cell phenotype for each part is unique [26, 27]. This highly controlled spatial organization is a major feature of the heart, and the onset of most CVDs is defined by the disruption of this balance, often through cell death or adverse cell proliferation [27, 28]. Additionally, the cardiac microenvironment, particularly the ECM and intratissue signaling, has recently been investigated as actively developing and maintaining this balance [26, 28]. The signaling between these distinct parts of the heart and cell types in the heart create a complex paracrine signaling structure that we have yet to fully understand, but is certainly essential for developing effective strategies for engineering biomimetic cardiac tissue models.
1.2. Cellular and biochemical organization of the heart
While many models of the heart focus primarily on the use of native cardiac cells and application of some limited physiological factors, such as top-down organizational techniques or the application of biomimetic stimulation, recent advances have shown us that the heart is a highly organized organ consisting of several factors beyond just cells [10, 15, 16, 18, 20]. A simple way to characterize this complex organizational apparatus is to consider the heart as consisting of cells, microenvironment, and structure. For the purposes of this review, we will be focusing on the cells and surrounding microenvironment to better recapitulate a snapshot of the cardiac tissue, rather than recreation of the structure of the entire heart.
1.2.1. Cells in the heart
The main functional cell type in the heart is the cardiomyocyte (CM). Along with the CMs, there are many other cells in the heart that are crucial for heart homeostasis, including the stromal (cardiac fibroblasts (CFs) and adipocytes (CAs)), vascular (endothelial cells (ECs), smooth muscle cells (SMCs), and pericytes), mesothelial cells, hematopoietic cells (HCs) (red and white blood cells, and platelets), and cardiac stem cells (CSCs) [29, 30] (table 1). Together, these cells help maintain the structure and function of the heart via direct cell-cell interactions and secreted signaling factors.
Table 1. A table outlining the discussed major cell types in the heart, as well as the broad lineage, cardiac location, and cardiac function of these cells.
| Cell | Lineage | Location | Function | References |
|---|---|---|---|---|
| Cardiomyocyte (CM) | Muscle cell | Cardiac muscle | Heartbeat generation | [31, 32] |
| Smooth muscle cell (SMC) | Muscle cell | Cardiac vasculature | Vasodilation and constriction | [33, 34] |
| Cardiac fibroblast (CF) | Stromal cell | Cardiac muscle | Heart remodeling and paracrine signaling | [35–37] |
| Cardiac adipocyte (CA) | Stromal cell | Adipose tissue | Energy supply and paracrine signaling | [29, 30] |
| Cardiac stem cell (CSC) | Stromal cell | Cardiac muscle | Organ homeostasis and paracrine signaling | [29, 30] |
| Endothelial cell (EC) | Vascular cell | Cardiac vasculature | Nutrient transport to the heart | [38] |
| Pericyte | Vascular cell | Cardiac vasculature | Angiogensis and vascular homeostasis | [39–41] |
| Mesothelial cell (MESO) | Epithelial cell | Pericardium | Organ homeostasis and immunoregulation | [29, 30] |
| Red blood cell | Hematopoietic cell | Blood | Oxygen transport | [29, 30] |
| White blood cell | Hematopoietic cell | Blood | Immune response | [29, 30] |
| Platelet | Hematopoietic cell | Blood | Blood clotting | [29, 30] |
Although there are contradictory reports on the ratio of cells within the heart cell population, recent evidence suggests that 25%–35% of the heart cells are CMs, 40%–55% are ECs, and 10%–30% are CFs, while the remaining small proportion of cells (3%–7%) are HCs. As CMs are much larger than other cells in the heart, they occupy 70%–80% of the heart cell volume, while ECs, FCs, and HCs occupy 3%–5%, 5%–15%, and 1.5%–2% of the cell volume in the mammalian heart, respectively [42–47].
1.2.1.1. Cardiomyocytes
CMs are the cells that have the ability to beat, and generate the contractile force needed to pump the blood in and out of the heart. They are physically connected and communicate with each other through gap junctions, adherens junctions, and desmosomes, and with other cells in the heart either physically or through paracrine signaling [31, 32].
Contractility of the CMs is regulated by controlling Ca2+ exchange into and out of the cells and the sarcoplasmic reticulum (SR) through ion channels and exchangers. Free Ca2+ ions that accumulate in the myoplasm interact with troponin C and initiate cell contraction by enhancing the binding of actin and myosin [48, 49]. Ca2+ is removed from the cytoplasm through Na+/Ca2+ exchangers on the sarcolemma or through the sarco-endoplasmic reticulum Ca2+ transporters on the SR [50, 51]. Cyclic uptake and release of Ca2+ results in beating of individual CMs, and coordination between individual CMs is achieved through the cardiac conduction system which conveys electrical signals throughout the heart [49].
In mammals, CMs continue to change not only during fetal development, but also after birth. They lose their ability to divide during or just after birth; while prenatal CMs have the ability to fully divide, postnatal CMs can only replicate their DNA without dividing the cytoplasm [52]. Hence, a considerable percentage of adult CMs are binucleated: 25%–57% for humans, 31% for pigs [53], and 85% for mice [54]. Human CMs also continue to grow in size until the age of 20 years, with adult CMs being 30–40-fold larger than embryonic CMs [55]. Additionally, the sarcomeres of adult CMs tend to be longer and more organized compared to the sarcomeres of fetal CMs [20]. Finally, the resting membrane potential of fetal CMs is around −50 mV to −60 mV, and decreases to approximately −85 mV to −90 mV in adult CMs [56]. This leads to stronger beating forces for more efficient pumping of the blood.
Apart from the morphological and functional changes, CMs go through metabolic and biochemical changes as they mature. Postnatal CMs switch their energy source from glucose to the more energy-efficient fatty acids to produce ATP [57]. At the biomolecular level, CMs also switch their myofibrillar protein isoforms as they mature. For example, while fetal CMs express myosin heavy chain 6 (MYH6), myosin light chain 2 atrial isoform, and troponin I1, postnatal CMs express myosin heavy chain 7 (MYH7), myosin light chain 2 ventricular isoform, and cardiac troponin I3 [58].
1.2.1.2. Cardiac fibroblasts
CFs are stromal cells that produce the ECM and signaling molecules (growth factors (GFs) and cytokines) in the heart in response to physical, electrical, biochemical, and mechanical stimuli. Other important roles attributed to CFs are CM electrical coupling, conduction system insulation, ECM degradation, vascular maintenance, and stress sensing and response [35–37]. As both sources and targets of stimuli, CFs coordinate the chemical, mechanical, and electrical signals between the cells and the ECM in the heart [35]. Unlike mature CMs, CFs can proliferate, migrate, and differentiate into myofibroblasts (MFs) through activation and subsequent transdifferentiation.
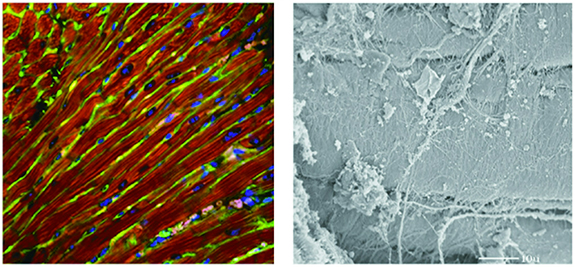
The CFs are embedded within the collagen network in the heart, which is linked to the CMs via integrins [59], and are co-aligned with the longitudinally organized CMs [60–62]. CFs fill the space between the longitudinal axis of the aligned CMs, blocking the lateral crosstalk between two adjacent CMs and inducing communication in the longitudinal direction (figure 1) [35]. This organization within the heart ECM network allows the fibroblasts to maintain the structural integrity of the heart through proliferation and ECM remodeling, convey the signals through cell-cell and cell-ECM interactions, and mechanically stimulate the CMs through collagen contraction [63–67].
Figure 1. Cell and ECM organization in the rat heart. Left: Confocal micrograph showing the co-alignment of CFs (green) and CMs (red). Nuclei: blue. CFs lie between the longitudinally aligned CMs (red) and within the collagen network in the heart. Right: Transmission electron micrograph showing the interaction between CFs, CMs and the ECM. Reproduced with permission from [51]. Copyright © 2004 the American Physiological Society.
Download figure:
Standard image High-resolution imageIn adult mammals, CMs have limited ability to proliferate and regenerate. Hence, upon injury or insult, CFs are activated and transdifferentiate to MFs to close the wound, which leads to deposition of fibrotic scar tissue and eventually to cardiac fibrosis [68, 69]. The fibrotic scar tissue has no ability to beat, affecting the biomechanics of the heart. MFs express alpha smooth muscle actin stress fibers, which gives them the ability to contract and pull the injured tissue together, producing tension and increasing the stiffness of the myocardium [70, 71]. Cardiac fibrosis increases the risk and mortality of a future MI, and heart failure in the long term [72, 73].
The crosstalk between the CMs and CFs is important for the proper functioning of the heart [44]. Crosstalk between these cells occurs via gap junctions (mainly through connexin (Cx) 40, 43 and 45) [74], membrane nanotubes (through Ca2+ exchange) [75], and paracrine signaling (through TGF-β, angiotensin II, and interleukin-6) [44, 76].
1.2.1.3. Endothelial cells
The heart is a highly vascularized organ due to its high energy and oxygen requirements, with almost every muscle fiber in the heart surrounded by at least one capillary [77]. Fittingly, ECs are the most abundant cell types in the heart. Along with their structural role in the blood vessels, ECs are metabolically active, control contraction and relaxation of the vessels, and regulate angiogenesis [38]. The endothelium, the monolayer ECs lining the inner wall of the blood vessels, is a protective barrier between the blood and tissues, and is involved in the production, release, uptake, storage, and degradation of a number of vascular substances that can regulate cardiac function and ECM remodeling [78–80]. While the vascular endothelium controls coronary artery function and blood supply to the myocardium and plays an indirect role in heart function, ECs in the myocardial capillaries and endocardial endothelium are in close contact with the CMs, allowing for direct cellular communication and signaling between both cell types. In fact, the distance between a CM and a capillary EC ranges between 1 μm–6 μm in small mammals and 10 μm and 30 μm in larger mammals, including humans [81, 82].
The crosstalk between the ECs and CMs is crucial for the healthy development of the heart. This usually takes place through signaling molecules. While CMs secrete factors that induce angiogenesis, ECs also secrete factors that promote CM maturity as well as organ growth and regeneration [83–87]. ECs play a role in not only healthy heart development and function, but also heart diseases, including hypertrophy, MI, and heart failure [82, 88]. Problems associated with EC signaling pathways may result in cardiomyopathies [89, 90].
In addition to the aforementioned cells, vascular SMCs and pericytes, as well as immune cells (mainly macrophages) play roles in heart homeostasis and function, as well as heart-related diseases [33, 34, 39–41, 91]. Finally, it should be noted that each cell type has its own subtypes. The cell subtypes may vary depending on the location and function of the tissue [29, 30]. Hence, the correct cell subpopulation must be used to engineer a specific tissue within the heart.
1.2.2. Heart extracellular matrix
Heart ECM is a complex three dimensional (3D) network of fibrillary (collagens and fibrillins) and non-fibrillary (basement membrane proteins, proteoglycans, and glycosaminoglycans) components, which have both signaling and structural functions [92]. Besides providing mechanical support and structural integrity to the cells and tissue, the ECM interacts with cells and directs them to proliferate, survive, attach, migrate, differentiate, and communicate with other cells [20, 93].
Collagen constitutes 2%–5% of the total heart weight. The main collagen types in the heart are types I (89%) and III (11%), although there are also type IV (in the basement membranes), V (in the pericellular space), and VI (in the interstitial matrix) collagens [94, 95]. Collagen contributes not only to the 3D structure but also to the mechanical properties of the heart [96]. Other components of the heart ECM are laminin, fibronectin, elastin, fibrillin 1, and heparan sulfate proteoglycans [95, 97, 98] (table 2). Moreover, ECM is a reservoir for anchored signaling molecules including the GFs, cytokines, proteases, and noncoding RNAs such as miRNAs and long noncoding (ln) RNAs [97, 99–101].
Table 2. A brief overview of several major components of the cardiac extracellular matrix.
| Protein | Class | Function | References |
|---|---|---|---|
| Collagen I | Collagen | Major structural protein | [94, 95] |
| Collagen III | Collagen | Major structural protein | [94, 95] |
| Collagen IV | Collagen | Major structural protein | [94, 95] |
| Collagen V | Collagen | Major structural protein | [94, 95] |
| Laminin | Glycoprotein | Basement membrane protein | [95, 97, 98] |
| Fibronectin | Glycoprotein | Integrin and ECM protein binding | [95, 97, 98] |
| Fibrillin 1 | Glycoprotein | Microfibril structure and load bearing | [95, 97, 98] |
| Elastin | Connective Tissue Protein | Load bearing and elasticity | [95, 97, 98] |
| Agrin | Heparin Sulfate Proteoglycan | Basement membrane protein and microvasculature nutrient exchange | [95, 97, 98] |
| Hyaluronic acid | Glycosaminoglycan | Lubrication and resilience of soft tissue | [102] |
| Interleukins | Cytokine | Immunoregulation | [95, 103, 104] |
| Matrix metalloproteinase II | Proteinase | Matrix remodeling | [95, 103, 104] |
| Matrix metalloproteinase IX | Proteinase | Matrix remodeling | [95, 103, 104] |
After birth, the heart ECM undergoes structural changes. The elastic modulus of the ventricular ECM increases after birth (from 12 kPa in prenatal to 39 kPa postnatal in mouse hearts) [105, 106], and continues to rise with age (as estimated with a ultrasound scanner from 2.6 kPa for native hearts of healthy humans below 40 years of age to 6.1 kPa for native hearts of healthy humans above 60 years of age [107]) indicating a high deposition and crosslinking of the ECM. In fact, the levels of collagen, laminin and periostin, as well as lysyl oxidase, which crosslinks collagen and elastin, increase in the heart after birth [102]. In contrast, the levels of hyaluronic acid, fibronectin, agrin, and other proteoglycans in the heart decrease after birth and with age [102]. Finally, inflammatory cytokines, proteases like matrix metalloproteinases, and fibrosis associated factors, as well as glycosylation degree increase with age [95, 103, 104].
1.2.3. Signaling molecules in heart development and maturation
Heart development and homeostasis is mediated through a plethora of autocrine, paracrine, and endocrine pathways. Developmentally, the heart is the first functional organ in an embryo and its formation is mediated by several rapid, sequential triggers controlled by secreted signaling agents [25]. The formation of the heart is initiated by Wnt GFs, mostly operating around the multifunctional protein β-catenin, which both promote and later inhibit canonical Wnt signaling in a time-dependent manner to achieve initial cardiogenesis [108, 109]. After early cardiac progenitor cells (CPCs) are formed by canonical Wnt signaling, bone morphogenic proteins (BMPs), in part through synergy with non-canonical Wnt activity, promote the differentiation of these early CPCs to differentiated cardiac cells. At this point both Wnt and BMP signaling are inhibited, with Dkk1, a major Wnt inhibitor that is released at this time, simultaneously activating 'non-canonical' pathways for later cardiac development and mature cardiac function, and development of the heart proceeds quickly. Further contributions from fibroblast GFs (FGFs), TGF-β, and other GFs help develop the final morphology and construction of the heart [108, 109]. Over the past decade, Notch signaling, particularly from NOTCH1 and NOTCH4, has also been identified as being key regulators of this process. Notch is a highly conserved intercellular signaling pathway that is required for tissue development and homeostasis. Additionally, Notch is directly involved in cardiogenesis. This effect is mediated through both BMP and non-canonical Wnt signaling pathways, which activate Notch by pathway activation and suppression, respectively, mediating downstream cardiogenesis and cardiac homeostasis [109, 110].
Many of these same pathways are also involved in maintenance of the mature heart and the onset of CVDs [109–111]. In particular, canonical Wnt signaling is mostly suppressed in mature cardiac tissue with a few notable exceptions. One of these exceptions is the maintenance of resident CSCs and resident immune cells or immune cell progenitors, although the behaviors and maintenance of these cells and their relationship with typical signaling in the myocardium are not well understood [112–114]. Wnt signaling is also involved in both the onset and resolution of diseases and injuries in the heart. Similar to heart development, Wnt signaling has been shown to be both beneficial and detrimental to cardiac health depending on the timing and degree of expression. Aging-related senescence in cardiac cells has been traced to increased levels of Wnt inhibition with increased age [115], which actively stymies the maintenance of CSCs and proliferation of CFs and ECs upon injury. On the other hand, overstimulation of Wnt, which can be triggered by MI or other cardiac insult, has been shown to contribute to TGF-β overstimulation, which can drive cardiac fibrosis and chronic inflammation [116, 117]. Wnt has been identified as an ideal target for therapeutic intervention due to the wide breadth of maintenance, aging, and disease-related pathways it may influence, but due to the complexity of these pathways and our limited understanding of their mechanisms, it has gained infamy as an 'undruggable pathway' [118, 119].
Beyond just the Wnt pathway, the complexity and multifunctionality of many cardiac signaling pathways is not well understood in current literature [28, 109, 119]. This is compounded by the difficulty in mimicking many of these multi-faceted signaling systems ex vivo, which is paradoxically difficult to resolve without greater understanding of the underlying mechanisms. Recent advances in cell biology, however, have provided an additional avenue to understand the means by which intratissue communication can mediate such complex signaling pathways. Both miRNAs and the extracellular vesicles (EVs) that have been identified as commonly transporting these miRNAs have been identified as potential key mediators of the progression and resolution of many CVD-related pathways [120].
1.2.3.1. Extracellular vesicles in heart development and maintenance
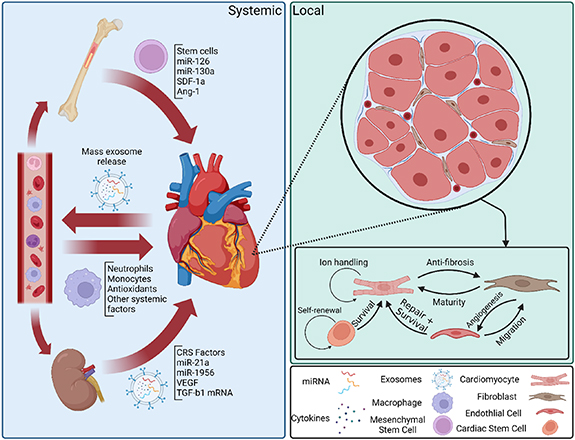
Recent studies have revealed that much of the signaling involved in heart homeostasis and disease response is mediated by EVs [120, 121] (figure 2). Specifically, EVs are often 'programmed' to seek out specific target cells and deliver miRNAs and proteins to modulate signaling activity in a highly controlled manner [122, 123]. This specificity allows for deliberate crosstalk between the varied cells in the heart, as well as between the heart and other organs. This system of communication has several advantages for the creation of ECT models and has been a useful trove of information on physiological signaling in the heart [124, 125].
Figure 2. Systemic and local exosome signaling in the heart after cardiac injury. Systemic, or endocrine, signaling after cardiac injury is primarily mediated by mass release of exosomes from the heart into the blood. These exosomes specifically target blood cells and other organs, such as the bone marrow or the kidneys, to recruit cardioprotective cells or factors to control inflammation and promote healing. Local, or paracrine and autocrine, signaling mediates intercellular signaling for maintaining cardiac homeostasis and inhibiting adverse responses to cardiac injury, such as fibrosis and hypertrophy. Cell crosstalk via exosomes has been shown to be involved in a number of specific processes, but most interactions with CMs tend to promote survival and functional maturity in order to maintain normal heart function under all circumstances.
Download figure:
Standard image High-resolution imageOne of the most useful attributes of EVs is the ability to isolate intratissue communication and identify both key compounds and targets for intervention. Intratissue signaling through EVs has been characterized between CMs and both ECs and CFs, and in both cases has shown the capability to recapitulate the effects of co-culture in a typical cell culture model [126–128]. Additionally, interference with these EVs, particularly in the case of CM-EC co-culture, has been shown to inhibit many of the effects of co-culture on CMs. Interestingly, matrix-bound EVs have also recently been found in decellularized cardiac tissue from multiple sources, including human left ventricle [101], mouse atria [129], and other decellularized tissues [130]. These EVs have been shown to attenuate inflammatory response [130] and enhance CM functional maturity [129] in vitro primarily through miRNA-mediated signaling pathways, although these pathways are not well understood, and have also demonstrated differential effects on the progression of cardiac fibrosis depending on the age and sex of the source tissue, corresponding to beneficial or adverse clinical outcomes [101]. Furthermore, these age and sex-dependent anti-fibrotic effects could be partially recapitulated utilizing a cocktail of identified miRNA targets contained in the EVs [101]. All together, these developments suggest that the microenvironment and local signaling play an important role in maintaining healthy heart homeostasis.
EVs have also been implicated in both cardiac development and disease progression and attenuation. Recent studies have shown that Wnt-related proteins are primarily ferried either in or on EVs, which are released upon the stimulation of certain trigger pathways [114, 116]. These EVs can be released during certain developmental stages or as a systemic response to cardiac injury, and appear to be a major method of Wnt signaling regulation in the myocardium. Aging and stress are currently suspected of impacting myocardial EV production, and this interaction, though it is not well understood, has been suggested as the means by which aging and stress can promote CVD through Wnt regulation [115]. However, several key factors have been isolated from cardiac EVs related to disease progression. In particular, EVs from CFs under stress conditions have been found to be enriched in miR-21, which stimulates cardiac hypertrophy [120, 131], and EVs from clinical plasma samples have implicated numerous miRNAs as either disease biomarkers or intervention targets [132].
Additionally, many EVs have also demonstrated beneficial effects on clinical outcomes for patients experiencing CVDs, and promote novel cardiac regenerative behaviors. A prime example of this is the use of mesenchymal stem cell (MSC)-derived EVs as a therapeutic. While the use of MSCs directly as a therapy has been implemented with some success, limitations such as low immune response, cell survival, and tissue targeting have been difficult to overcome with existing methodologies [121, 133, 134]. An experimental solution is the use of MSC-derived EVs to recapitulate the effects of MSC therapy on damaged myocardium, and has been met with success in pre-clinical and early clinical trials [133]. Other efforts to isolate beneficial EVs or EV contents from ECs and CSCs to attenuate immune response, stimulate functional recovery, and promote tissue regeneration have been met with varied success.
The wide range of targets and high specificity of the effects of EVs on the myocardium make them an attractive target for study and intervention. In order to accurately understand and model the cardiac microenvironment, maintaining a physiologically relevant EV population and signaling apparatus is essential.
1.3. Cardiovascular diseases and treatment methods
Many CVDs manifest through obstructive vascular lesions that can lead to thrombosis, rupture, and cardiovascular complications [135]. Onset of CVDs can be characterized by the accumulation of low-density lipoprotein (LDL) and triglyceride-rich lipoproteins along the vessel, leading to local inflammation in the artery wall [136]. If not treated, the resulting plaques can lead to thrombosis, vessel rupture, or other cardiovascular complications [137, 138]. Advances in our understanding of cardiac physiology have allowed for the identification of biomarkers associated with CVDs, such as Niemann Pick C1-like protein 1, and related to LDL cholesterol build-up, as well as potential therapeutic targets, such as proprotein convertase subtilisin/kexin type 9 to lower the LDL cholesterol levels [139, 140].
MI can result from coronary artery disease (CAD) or other diseases which limit blood supply to the cardiac tissue [141]. While initial incidence of MI is typically non-fatal, MI can result in chronic diseases, such as cardiac fibrosis or chronic inflammation, which can lead to increased risk of future MI and death [142]. MI consists of two phases, ischemic injury (II) and reperfusion injury (RI). II results from limited delivery of oxygen to tissues due to occluded blood flow. Low oxygen levels (hypoxia) in the tissues downstream occluded vessels leads to cell death, which can quickly propagate through the myocardium [143]. RI results from the restoration of the blood flow and oxygen to the ischemic tissue and is associated with elevated levels of reactive oxygen species (ROS), which can result in an up to 50% increase in cell death and can trigger chronic inflammation [143].
Current strategies to treat CVDs include treating existing plaques with statins to reduce the risk of thrombosis, and reducing the blood pressure by the use of pharmaceuticals such as angiotensin-converting enzyme inhibitors, angiotensin blockers, beta blockers, ACE-inhibitors and calcium channel blockers [144, 145]. Current treatments targeting atherosclerosis are mainly based on medications to lower cholesterol levels (statins) or decrease clotting (aspirin). However, the effectiveness of these approaches is limited by rapid drug clearance and inefficient accumulation at the site of inflammation [145]. Different nanomaterial-based strategies have been proposed to clear atherosclerotic plaques from arteries. One recent study showed the use of phospholipid nanoparticles to reduce cholesterol crystals on arterial walls and inhibit inflammation [146]. Their high-density lipoprotein-like nanoparticle design improved plaque stability in mice and reduced inflammation by inhibiting TLR4-NF-κB pathway in macrophages. Yet another recent study assessed the utility of macrophage-biomimetic nanoparticles to deliver drugs for treatment of atherosclerosis [135]. These ROS-responsive nanoparticles were coated with a macrophage-like membrane to avoid clearance through reticuloendothelial system and specifically target the inflammation zone for drug delivery.
If the initial treatment is insufficient, the lipid build-up can be removed surgically [145]. Treatment of CVDs frequently results in long-term complications, limiting the utility of existing solutions. These complications can present as additional CVDs, such as instant restenosis, or grafting failure [135, 146]. Current treatments for MI consist of administration of aspirin, antiplatelet agents, and surgical revascularization [147]. There are several approaches for acute MI treatment, such as balloon angioplasty, administration of aspirin, antiplatelet agents, and surgical revascularization [147, 148]. However, these strategies will not be able to recover dead CMs, which may eventually lead to heart failure. Because of this, heart transplantation remains the most effective means of treating MI. However, due to donor scarcity and the difficulty in obtaining hearts suitable for transplantation, over 2000 patients die yearly in the US while waiting for an available heart [149]. Even if a suitable donor is found within reasonable transport distance to allow timely transplantation, there remains several major factors that decrease the success rate post-procedure. For example, organ rejection caused by immune incompatibility is a major concern that leads to significant morbidity and mortality [150]. Even with the use of immune-suppressing medications, 40% of heart transplant patients experience complications within the first year of the procedure [151].
2. Cardiac tissue engineering
Cardiac tissue engineering is a viable alternative to the current treatment options; it offers opportunities to find novel approaches to treat or replace the damaged heart tissue without suffering from tissue rejection, or to study the heart physiology and pathology, test and discover new drugs, and develop diagnostic tools to detect and/or treat CVDs. Here, we will review the relevant cells and biomaterials used to engineer the heart, the clinical applications of these research topics, and the trends in cardiac tissue engineering.
2.1. Engineering the microenvironment
Recent advances in single cell transcriptomics and proteomics technologies have enabled researchers to fully characterize the cellular [29, 30] and biochemical [95, 103] compositions of the heart tissue in fetal, postnatal, adult, and aged subjects. These, as well as the discovery of induced pluripotent stem cells (iPSCs) [149] and the development of protocols for successful differentiation of iPSCs to CMs (iCMs) [150] have created invaluable opportunities for cardiac tissue engineering research. Currently, there are three challenges that cardiac tissue engineering researchers face: (i) using the correct cell source, (ii) accurately recreating the cardiac microenvironment that will lead to mature and functional cardiac tissue, and (iii) creating large, vascularized tissues.
2.1.1. Cells in cardiac tissue engineering
Due to the limited proliferative [150] ability of CMs, and the challenge of obtaining primary human CMs, CMs derived from stem cells have become the primary cell source for tissue engineering research [152]. While differentiating these iCMs from iPSCs offers unique advantages, such as the high proliferative capability of iPSCs and the ability to derive patient-specific cell lines, most existing differentiation protocols result in heterogeneous iCM populations, leading to tissues with nonsynchronous beating and immature electrophysiological and functional phenotypes. However, recent studies have proposed methods to obtain ventricular iCMs with distinct electrophysiology [152].
Obtaining mature-like, physiologically relevant iCMs for use in tissue engineered models has been at the crux of cardiac tissue engineering since the inception of the field and has driven much of the innovation in device design and non-obtrusive cell stimulation techniques [153]. While CMs alone can be matured using mechanically stretchable [154] or electrically conductible [155] platforms in order to directly 'train' immature CMs to emulate a mature phenotype, recent approaches have also begun to integrate other cell types in a tunable 3D matrix in order to obtain intra-tissue maturation signaling as well [153, 156]. This approach takes advantage of endogenous interactions between CMs, other cardiac cells, and the cardiac microenvironment to promote more physiological behavior [157, 158], and allows for the modeling of sophisticated CVD interactions in vitro [159]. Another notable advancement is in high-throughput 'heart-on-a-chip' models, which commonly utilize iCMs and sometime other iPSC-derived cardiac cells in a microfluidic device to succinctly model the neovasculature of the myocardium and promote a more biomimetic phenotype by inducing native physiological stresses [160]. Despite these advancements, however, iCMs are not yet a perfect model for cardiac tissue engineering and many other strategies are still employed to address the shortcomings of these models.
As a common alternative to stem cell-derived CMs, these cells can often be isolated from model organisms such as rats and mice. Of these, the most commonly utilized CMs are those from rat neonates, primarily due to relatively high yield after isolation from tissue [161] and resilience compared to mature CMs from the same animal, thereby enhancing the utility and usability of these cells for tissue engineering applications [162]. Although neonatal CMs are much more common from rat models, adult rat CMs can also be used to assess chronic diseases that would be difficult to effectively model in a neonate or otherwise in vitro model, as well as for use in models taking advantage of the endogenous maturation of CMs in vivo [163]. Yet another utility of animal models, particularly murine models, is the high degree of freedom with which transgenic animals can be designed. While these transgenic animals are most commonly utilized in in vivo studies, cell lines can be isolated from transgenic animals to link fundamental biology to the instigated cardiomyopathy in a transgenic animal, as was the case with lysyl-oxidase like protein-1 and the onset of hypertrophy in mouse models [164]. Furthermore, animal models can be obtained much more easily than human cardiac tissue, which often necessitates competing with hospitals through organ donor networks, allowing for greater statistical power and treatment conditions than would often be reasonable when utilizing human tissue. Although all these factors make animal models a valuable tool to study cardiac physiology, tissue engineered constructs using animal cells still have relatively low transferability for clinical applications [165].
An accurate and tunable model representing native tissue state is invaluable to understanding the physiology and pathology of the heart. CMs are the primary functional components of the heart and are the source of heart tissue contractility. As such, the ability to produce functional CMs is a key requirement for successful models of the myocardium. In preclinical studies, reintroducing CMs to damaged myocardium has been shown to induce healing [166]. Although CMs are crucial to achieve the base functional capabilities of cardiac tissue, they are not the most abundant cells in the heart, and in vivo they depend on both CFs and ECs to maintain normal cardiac function for features such as signal propagation, vascular maintenance and stress response [46]. Therefore, to engineer a functional and clinically translatable cardiac model, it is essential to include multiple cell types in model tissues.
In engineered models, CFs are essential to generate and maintain a biomimetic organizational structure and microenvironment as they are responsible for deposition and maintenance of the cardiac ECM and related GFs [35, 167]. It has also been established that interactions between the CM, ECM, and CFs can modulate CM phenotype to promote enhanced functionality and maturity [167]. Furthermore, CFs are the principal cells responsible for cardiac fibrosis and adverse remodeling after cardiac insult and contribute to the progression of several CVDs through adverse signaling pathways [35]. As with CMs, primary human CFs can be difficult to obtain, but can be differentiated from iPSCs [168]. Primary CFs must be isolated directly from heart tissue, and can be difficult to keep in culture in part due to the susceptibility of CFs to MF differentiation in vitro [169]. While this can complicate primary CF culture, in some cases it may be beneficial to deliberately culture primary cardiac MFs. MFs are minimally present in healthy tissue, and are implicated in a wide range of CVDs, including MI, hypertrophy, and cardiac fibrosis, and obtaining MFs through CF differentiation is essential to accurately model the damaged myocardium and any related endogenous response [35, 159]. Recently, interest has grown in modeling the CF and MF interplay even outside the scope of interactions with CMs [170].
While models do exist that utilize CMs or CFs alone or together, cardiac ECs are essential contributors to cardiac signaling and disease response, and development of a biomimetic cardiac microenvironment. Primary ECs can be isolated and harvested through various techniques, such as enzymatic dissociation, mechanical dissociation, or laser-assisted microdissection [171]. Recent heart-on-a-chip models have incorporated ECs in order to simulate physiological perfusion and nutrient transport [160], and cardiac spheroid bioprinting with CMs, CFs, and ECs demonstrated the ability to maintain viability for up to 30 d [172]. As with other cells, iPSC-derived ECs (iECs) are the primary ECs used in cardiac tissue engineering [166, 173]. While cultures of freshly-derived iECs alone can result in immature populations with high plasticity, which may revert to EC progenitor-like cells, co-cultures of iECs with CMs in tissue engineered constructs have been shown to improve maturity and functionality for both CMs and ECs [174].
2.1.2. Biomaterials in cardiac tissue engineering
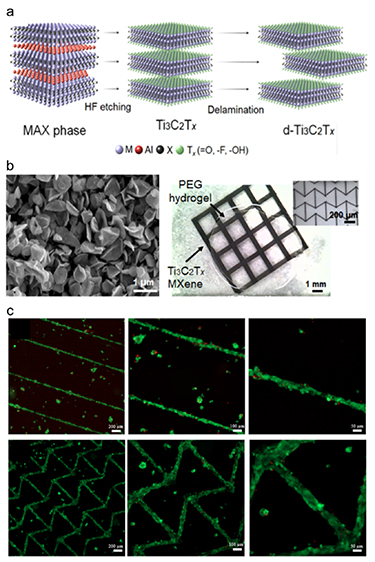
Biomaterials used in tissue engineering should (i) be biocompatible and usually biodegradable, (ii) support the delivery and attachment of cells to the injured site, while promoting cell proliferation or differentiation and ECM production, and (iii) match the ECM properties of the target tissue [175–177]. Biomaterials for cardiac tissue engineering can be in the form of hydrogels, scaffolds, substrates, and reinforcing or conductive materials. Hydrogels are crosslinked networks of polymers that can absorb high amounts of water, entrap cells, and allow diffusion of nutrients, gas, and waste products through their pores [178–180]. Scaffolds are solid materials processed into porous matrices that can replace the damaged tissue and serve as a carrier for cells and GFs [181–184]. Substrates that change hydrophilicity and cell adhesiveness in response to temperature or pH changes are used to prepare cell sheets [185–187]. Reinforcing or conductive materials can be in the form of nano/micro-sized particles or fibers, and are used to improve the mechanical or electrical properties of the constructs [188, 189] (figure 3).
Figure 3. MXenes used in cardiac tissue engineering as conductive materials. (a) Schematic showing the production of MXenes. (b) MXenes grains processed into 3D printed grids on hydrogels as conductive materials. (c) Viability of CMs seeded on the 3D printed MXenes. Reproduced from [159]. CC BY 4.0.
Download figure:
Standard image High-resolution imageEngineered cardiac constructs can be applied as patches or injectable hydrogels which are produced from natural or synthetic biomaterials [190]. Natural biomaterials are inherently biocompatible and biodegradable, and have functional groups that can induce cell attachment, proliferation, and migration, but these polymers have some drawbacks including poor mechanical properties, high variability between donors, and risk of immunogenicity [179, 191]. Collagen, the most abundant structural protein in the body, is also one of the most common natural polymers used to engineer the heart [192–194]. Other natural polymers used to produce engineered heart include gelatin [195–197], alginate [198, 199], chitosan [200, 201], fibrin [202, 203], silk [204–206], and decellularized matrices [159, 207, 208].
Synthetic biomaterials may be tailored to have high mechanical properties, with little batch-to-batch variability and immunogenicity, but they have limited functionality and resemblance to the natural cardiac ECM [179]. Synthetic biomaterials used for cardiac regeneration include polyethylene glycol [93, 209], poly(lactic acid) [210, 211], poly(lactic-co-glycolic acid) (PLGA) [212], poly (N-isopropylacrylamide) [213], and poly (glycerol sebacate) [211, 214], as well as conductive materials such as polypyrrole [204, 206], carbon nanotubes [215, 216], gold [217, 218], carbon nanofibers [219], and MXene [189] nanoparticles.
Usually, these materials are used in combination to take advantage of the natural and synthetic polymers or as composites to improve the mechanical and electrical properties of the engineered tissues (table 3).
Table 3. Details for the use, major benefits, and noted limitations of common biomaterials used in cardiac tissue engineering.
| Material | Use | Benefits | References |
|---|---|---|---|
| Collagen | Scaffolds, hydrogels | Bioactivity, biodegradability, low toxicity | [192–194] |
| Gelatin | Scaffolds, hydrogels | Bioactivity, biocompatability, biodegradability, low toxicity, low cost | [195–197] |
| Alginate | Scaffolds, hydrogels | Biocompatability, biodegradability, non-adhesive, ease of modification, low toxicity, low cost | [198, 199] |
| Chitosan | Scaffolds, hydrogels | Biocompatability, biodegradability, ease of modification, antimicrobial, low toxicity, low cost | [200, 201] |
| Fibrin | Scaffolds, hydrogels | Bioactivity, biocompatability, biodegradability, ease of fabrication, low toxicity | [202, 203] |
| Silk | Scaffolds, hydrogels | Bioactivity, biocompatibility, dynamic biodegradability, ease of modification, mechanical strength, low toxicity | [204–206] |
| ECM | Scaffolds, hydrogels | Bioactivity, biodegradability, endogenous integration, replication of complex native microenvironment | [159, 207, 208] |
| Polyethylene glycol (PEG) | Scaffolds, hydrogels | High solubility, high manipulability, non-adhesive, non-toxic, highly stable | [93, 209] |
| Poly(lactic acid) (PLA) | Scaffolds, nanofibers | Biocompatability, controllable degradation, ease of modification, non-toxic | [210, 211] |
| Poly(lactic-co-glycolic acid) (PLGA) | Scaffolds, hydrogels | Biocompatability, controllable biodegradability, ease of functionalization | [212] |
| Poly (N-isopropylacrylamide) (PIPAAm) | Hydrogels | Thermosensitivity, protein preservative, ease of conjugation to other substrates | [213] |
| Poly (glycerol sebacate) (PGS) | Scaffolds, hydrogels, nanofibers | Biocompatability, biodegradability, elasticity, tunable mechanical properties, low cost | [211, 214] |
| Polypyrrole | Scaffolds, nanofibers | Biocompatability, tunable electrical conductivity, ease of conjugation to other substrates, ease of synthesis, low toxicity | [204, 206] |
| Carbon nanotubes | Scaffolds, nanofibers | Electrical conductivity, ease of chemical modification, anisotropicity, ease of patterning | [215, 216] |
| Gold | Nanofibers, nanoparticles | Biocompatability, electrical conductivity, ease of conjugation to other substrates, low toxicity | [217, 218] |
| MXene nanoparticles | Nanoparticles | Biocompatability, electrical conductivity, ease of conjugation to other substrates, ease of patterning | [189] |
2.1.3. Growth factors and small molecules in cardiac tissue engineering
GFs are diffusible signaling molecules secreted by cells that can guide the cells to proliferate, migrate, differentiate, and produce ECM. Various GFs have been utilized extensively in cardiac tissue engineering due to their innate proangiogenic and antiapoptotic properties [220] (table 4). The utility of GFs in cardiac engineering can be broadly divided into two categories: iPSC differentiation and cardiac regeneration.
Table 4. An overview of several commonly used growth factors and related cardiac mechanisms of interest in cardiac tissue engineering.
| Growth factor | Involved cardiac mechanism | References |
|---|---|---|
| Vascular endothelial growth factor (VEGF) | Angiogenesis, antiapoptosis, cell proliferation | [233, 234] |
| Fibroblast growth factor (FGF) family | Angiogenesis, antiapoptosis, cell proliferation | [234, 235] |
| Stromal cell-derived factor 1 (SDF-1) | Antiapoptosis, systemic stem cell recruitment | [224, 236] |
| Periostin | Cell proliferation, fibroblast recruitment | [237] |
| Neurogilin1 | Improved cardiac function, cell proliferation | [238] |
| Hepatocyte growth factor (HGF) | Antiapoptosis, reparative remodeling, | [239] |
| Platelet-derived growth factor (PDGF) | Antiapoptosis, reparative remodeling | [240] |
| Insulin-like growth factor (IGF-1) | Antiapoptosis, cell proliferation | [240] |
| Granulocyte colony-stimulating factor (G-CSF) | Antiapoptosis, local stem cell recruitment, immunomodulation | [241] |
| Granulocyte-macrophage colony stimulating factor (GM-CSF) | Antiapoptosis, systemic stem cell recruitment, immunomodulation, neovascularization | [242] |
| Bone morphogenetic protein 4 (BMP4) | Cardiac development and maturation, immunomodulation | [243] |
| Angiopoietin | Angiogenesis, reparative remodeling, vascular stabilization | [233] |
| Erythropoietin | Antiapoptosis, reparative remodeling | [225] |
| Matrix metalloproteinase (MMP) family | Remodeling regulation | [244] |
| Adiponectin | Antiapoptotic, angiogenesis, immunomodulation, antioxidant | [245] |
| Growth-differentiation factor 15 (GDF-15) | Antiapoptotic, immunomodulation, cardiac development and maturation | [246] |
| Tumor necrosis factor α (TNF-α) | Antioxidant, immunomodulation | [247] |
Some GFs and small molecules are utilized to differentiate CMs from stem cells. Studies have repeatedly shown that differentiating CMs from stem cells in the presence of key GFs results in a higher differentiation success and enhanced homogeneity of the population [221]. Particular GFs of interest are BMP4 and FGF2, which contribute to achieving up to over 90% iCM differentiation yield [221].
Several key GFs have been utilized in preclinical trials to stimulate angiogenesis and promote cardiac healing after MI or other cardiac insult [222]. Vascular endothelial GF (VEGF) and FGF are the two most commonly used GFs in cardiac regenerative medicine, primarily due to well-characterized pro-angiogenic properties [222]. Their application during the early stages of ischemic damage can lead to decrease in tissue death by stimulating new vessel formation and restoring the blood flow. Cochain et al describe the importance of the newly formed microvessels in preventing subsequent heart failure in MI patients [223]. Another GF used for its angiogenic potential is stromal cell-derived factor (SDF-1). SDF-1 has also been shown to have cardioprotective effect through hematopoetic stem cell mobilization and differentiation into MFs [224].
Another use of GFs following ischemic injury is to introduce anti apoptotic signals to CMs. Recent studies have reported that periostin and neurogilin 1 can induce CM proliferation and are utilized to inhibit adverse cardiac remodeling after cardiac injury. Additionally, erythropoietin, hepatocyte GF (HGF), platelet-derived GF, Insulin-like growth factor 1 and granulocyte colony-stimulating factor are GFs with established antiapoptotic properties and are commonly utilized in ECT models to promote cardioprotective signaling [225]. Nakamura and colleagues demonstrated that antiapoptotic nature of HGF leads to reduction in infarct size and improvement of cardiac function [226].
While there have been attempts to systemically deliver GFs to stimulate in vivo differentiation of stem cells to CMs, several key shortcomings limit the progress of the approach [227, 228]. Due to the short half-life of GFs once injected into the circulation, required doses to achieve stem cell differentiation is high [229]. Additionally, GFs are shown to be cytotoxic in high systemic doses [230]. These limitations can be mitigated in part by the delivery methods used to release GFs in a controlled manner over longer period of times [231]. Formiga et al summarized the effect of different delivery techniques on angiogenic therapy using GFs [232].
2.2. Cardiac tissue engineering applications
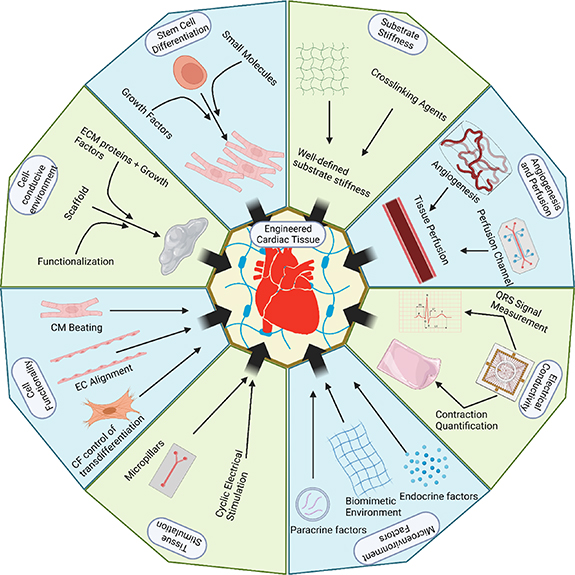
ECTs aim to recapitulate the heart microenvironment. There are two aspects that should be taken into consideration in achieving this goal: the engineering factors and the biological factors. Engineering factors are primarily dedicated to ensuring that any cells used will be in a physically similar environment to the modeled cardiac tissue, as well as allowing for easy delivery of the biological agents and useful data collection. Biological factors primarily focus on ensuring that any cells used behave as is typical in cardiac tissue, as well as providing the tissue with appropriate nutrients and signaling agents to maintain tissue health and normal function (figure 4).
Figure 4. Major engineering and biological factors for engineering cardiac tissue constructs. Schematic outlines several engineering (green) and biological (blue) factors that must be controlled when constructing biomimetic cardiac tissue to ensure that the resulting construct behaves consistently with endogenous cardiac tissue. Engineering factors primarily focus on ensuring that any cells used will be in a physically similar environment to the modeled cardiac tissue, as well as allowing for easy and useful data collection. Biological factors primarily focus on ensuring that any cells used behave as is typical in cardiac tissue, as well as providing the tissue with appropriate nutrients and signaling agents to maintain tissue health and normal function.
Download figure:
Standard image High-resolution imageCardiac tissue engineering has various applications. It can be applied to (i) regenerate, treat or replace the injured heart, (ii) construct 3D in vitro models of healthy and diseased tissues to study their biology, test drugs before they are used in animal and human clinical trials, and (iii) diagnosing heart-related problems.
2.2.1. Treatment of heart diseases
The heart has a limited capacity to regenerate. Upon injury, the fibroblasts transdifferentiate to MFs, which close the wound in the short term, but then lead to cardiac fibrosis in the long term. Cardiac fibrosis increases the risk and mortality of a future heart event [72, 73]. Cardiac tissue engineering can address this problem by inducing heart regeneration and preventing fibrosis or by replacing the damaged tissue.
2.2.1.1. Cardiac tissue regeneration
Due to inherent low regenerative capacity of the cardiac tissue, one of the main objectives of the therapies is to stimulate regeneration after heart injury. The major strategies that are utilized in aiding in heart regeneration are inducing cell proliferation and differentiation, providing biodegradable scaffolds for proper cell alignment, restoring electro conductivity, synchronization of cardiac rhythm, and promoting angiogenesis.
One approach to regenerating the heart is delivering cells to the injury site. To explore the effects of direct cell injection versus cardiac cell sheet transplantation into post MI heart on cell survival and regeneration, Sekine et al used a rat MI model. Luciferase-expressing neonatal rat cardiac cells were transplanted either in the form of cell sheets or as a direct injection of cell mass after dissociation from culture plates. To assess cardiac function and cell survival, they performed echocardiography and in vivo bioluminescence. In their study, cell-sheet transplantation resulted in better cell survival. Four weeks post-transplantation, cardiac function was observed to be significantly better using cell-sheet methods compared to direct injection of cells. In a different study, Shiba et al used fibroblast-derived iPSCs and grafted CMs to an infarcted cynomolgus monkey (Macaca fascicularis) heart and observed improved contractile function at 4 and 12 weeks after treatment [248]. Another research group developed a polymeric cardiac microneedle patch integrated with cardiac stromal cells to allow heart regeneration after MI [249]. They showed that their approach can promote healing in MI animal models by promoting angiomyogenesis and augmenting cardiac functions, such as cardiac pump function. However, their patch has to be delivered via open-heart surgery, making it invasive. In the future a less invasive delivery method could be studied [249].
Another approach to induce heart regeneration is the use of ECM proteins. In a recent paper, Ozcebe et al investigated the effect of cardiac ECM age on iPSC-derived CM function and their response to MI conditions [95]. They evaluated the effects of young, adult, and aged ECM coatings. They reported that while young ECM induced proliferation, adult ECM improved cardiac function. It was also noted that usage of the aged ECM impaired cardiac function of iPSC derived CMs. In another study related to ECM's role in cardiac regeneration, Bassat et al identified agrin, an ECM protein, to be crucial for regenerative capacity of neonatal mouse heart. They also demonstrated that agrin treatment after MI led to improved cardiac function as demonstrated by electrocardiography. They reported that agrin-treated mice had retained healthy wall thickness post-MI and had fewer occurrences of dilated cardiomyopathy [250].
Another interesting approach to regenerating cardiac tissue is the usage of secretome. In one study, nanoclay-based gels were studied to controllably deliver stem cell secretome after MI to stimulate angiogenesis. Their results showed improved heart function by secretome delivery after MI, displaying higher ejection fractions compared to untreated controls. This system provided proangiogenic and cardioprotective function and has the potential to improve the therapeutic efficacy of stem cell-derived secretome by increasing retention rate at the site of cardiac injury. They were able to prove that nanoclay-based biomaterials can direct in situ tissue regeneration [229]. MicroRNAs have recently gained attention in cardiac engineering due to their potential to regenerate the heart. Previously, functional screening has identified miR-590 and miR-199a for their ability to promote cell cycle re-entry of adult CMs when delivered in viral vectors [251]. More recently, Lesizza and colleagues showed that intracardiac injection of miRNA mimics was sufficient to induce myocardial repair [252]. They also demonstrated that the delivered miRNAs are relatively stable and their effects last at least 12 d. Furthermore, recent evidence has shown that exosomal miRNAs can synergistically inhibit fibrotic behavior and promote cell survival in CFs independent of other intervention [101].
2.2.1.2. Cardiac tissue replacement
In addition to heart regeneration, cardiac tissue engineering can also be used to produce constructs that can replace the injured heart muscle. To replace the injured tissue, cardiac patches or ECTs are used. The biochemical, mechanical, morphological and electrical properties of a patch or an ECT must match those of the specific target tissue to be replaced by the engineered construct (table 5). Additionally, it must fully integrate with the surrounding healthy tissue to recapitulate the heart function. This includes maintaining the cell-cell interactions through gap junctions, coupling of CMs, CFs, and ECs for synchronized beating, and vascularization (figure 4). Finally, the engineered construct must withstand the mechanical and electrical forces in the heart after implantation to the body, while being responsive to the stimuli at the injury site.
Table 5. The function, features, and success metrics of various cardiac tissue engineered constructs designed to provide therapeutic effects.
| Device | Function | Features | Success metric | References |
|---|---|---|---|---|
| Decellularized skeletal muscle ECM | Provide structure and nutrients to seeded CMs | Endogenous materials, complex structure | Cell survival, electrophysiology, and gene expression | [257] |
| Epicardial microtissue cardiac patch | Re-functionalization of LV scar tissue post-MI | Micro-tissue integration, electrical coupling | Cardiac pacemaking, electrophysiology, tissue integration | [265] |
| Engineered heart tissue strips | Remuscularization of injured myocardium | Large treatment area, 3D construction | LV ejection fraction, neovascularization, electrophysiology | [151] |
| Synthetic remote-reporting cardiac patch | Utilize microelectronics to report on patch engraftment without invasive observation | Integration of bio and electronic components, controlled drug release | Mechano and electrophysiology, drug release profile, patch stability | [266] |
Decellularized matrices are attractive candidates for producing ECTs. They are obtained by removing the cells in a tissue without changing the ECM characteristics, and hence retain the tissue structure. Decellularized whole hearts can be recellularized and implanted into the body. Although cells align well and start to beat regularly on the decellularized hearts [253], problems have been reported including thrombosis [254], abnormal electrical activity and beating [255], and poor integration with the host tissue [253]. Some of these problems can be addressed by choosing the appropriate decellularization technique [256], seeding method [257], and surgical procedure [253, 258]. Despite promising results, decellularized whole hearts still need some development before they can be applied clinically. Instead of whole hearts, decellularized tissue sections can be used as patches. In a recent study, decellularized matrices derived from cardiac tissue (cECM) and skeletal muscle (sECM) were recellularized with murine embryonic stem cells (mESCs) or mESC-derived CMs (mESC-CMs) to construct ECTs [257]. Researchers showed that the matrices had similar structural properties and both supported mESCs to adhere, survive, proliferate, and differentiate into functional cardiac microtissues that were responsive to electrical and adrenergic stimulation. The matrices also supported mESC-CMs adhesion, survival, and proliferation, and the cells produced ECTs with synchronized contraction.
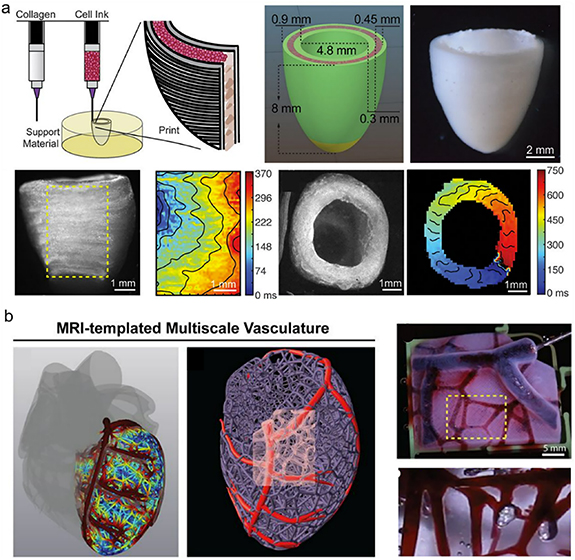
Natural and synthetic polymers have also been used to engineer cardiac patches or ECTs for replacement of the injured hearts. One example is the development clinically-relevant, heart chamber-specific, ring-shaped ECTs using ventricular or atrial iCMs differentiated from human iPSCs embedded in a collagen hydrogel [259]. The atrial ECTs demonstrated atrial-specific gene and protein expression, while the ventricular ECTs displayed more ventricular-specific expression. Functionally, the two ECT types were also distinct; the ventricular ECTs had longer action potential duration and conduction velocity than the atrial ECTs, as expected. In another study, researchers produced ECTs using patterned methacrylated tropoelastin hydrogels [260]. These highly elastic ECTs provided a mechanical environment similar to that in the native tissue, and promoted the attachment, spreading, alignment, function, and intercellular communication of the neonatal rat CMs, as well as inducing synchronous beating in response to electrical field stimulation. Lee and colleagues engineered an ellipsoidal shell-shaped construct to reproduce the left ventricle [261] (figure 5(a)). They used collagen to print the outer and inner walls of the construct and filled the space between the walls with a high concentration of ESC-CMs. They reported that the cells were beating throughout the construct with a directional wave propagation (figure 5(a), bottom). They also included blood vessels in their model, mainly printing the vasculature containing the left anterior descending (LAD) artery using collagen as the bioink (figure 5(b)). By perfusing the multiscale vasculature through the root of the LAD, they also demonstrated vessel patency. In another study, a cardiac path was developed by seeding CMs, SMCs, and ECs that had been differentiated from human iPSCs on a fibrin scaffold and maturing the construct using dynamic stimulation [262]. Cell-free and cell-laden patches were implanted into a swine model and significant improvements in left ventricular function were reported. Additionally, there were reductions in infarct size, myocardial wall stress, myocardial hypertrophy, and apoptosis in the scar border zone of the myocardium. This design was later improved by introducing helical fibers to the chambers using rotary jet spinning to mimic the native structure of the atria and ventricles [263]. When seeded with iCMs, the helically aligned constructs showed more uniform deformations, greater apical shortening, and increased ejection fractions compared to circumferentially aligned constructs.
Figure 5. Engineered left ventricle. (a) Design and bioprinting of the ellipsoidal shell-shaped left ventricular construct using collagen at the inner and outer walls of the construct and filling the space between them with cell bioink. (b) Printing of the vasculature in the left ventricle. The vessels were perfused using a red dye to show patency. From [261]. Reprinted with permission from AAAS.
Download figure:
Standard image High-resolution imageAlthough promising, ECTs need more improvement before they can be used clinically. A recent study showed only 5% increase in the remuscularization area of a cryo-injured pig heart ventricle when a cell-laden ECT patch was applied as compared to the cell-free patch, and no improvement to the function of the injured heart was recorded [264]. Integration of the engineered tissue with the injured tissue and the production of beating muscle tissue are of great importance to creating functional ECTs.
To promote the integration of the engineered tissue with the healthy tissue, cardiac microtissues or large cardiac patches were produced from hESC-CMs seeded on PDMS to produce cell sheets and implanted into rat hearts to test their integration with the healthy tissue [265]. Interestingly, the microtissues displayed better integration with the healthy tissue than the large cardiac patches. While the tissue patches were electromechanically active at 4 weeks, they were not electrically coupled to the host tissue and were separated by scar tissue. In contrast, microtissues were electrically coupled to the host heart at spontaneous rate and could follow host pacing up to 300–390 beats per minute. In another study, fibrin-based patches seeded with human iPSC-derived CMs and ECs were transplanted onto large defects (22% of the left ventricular wall defected with 35% decline in left ventricular function) of guinea pig hearts [151]. The patches supported CM proliferation and vascularization, were electrically coupled to the intact heart tissue, and remuscularized 12% of the infarct area 28 d after transplantation. The researchers reported a 31% improvement in ventricular function after implantation. A multilayered modular patch was engineered by electrospinning albumin and producing micropatterns to direct cardiac and vascular tissue formation [258]. A femtosecond laser was used to create microgrooves on one of the albumin layers to induce CM elongation and microholes to induce nutrient and gas exchange. Another layer was also produced, this time with microcages and microtunnels onto which VEGF containing PLGA microparticles were tethered to promote angiogenesis. After combining the layers and implantation into rats, the patch and the healthy heart tissue became electrically coupled and the blood vessels innervated the patch, showing good integration with the host tissue. A hybrid polyimide-based microelectronic construct with gold sensors as a cardiac patch to replace the damaged tissue in the heart was produced (figure 6(a)) [266]. A mesh of PCL and gelatin fibers were deposited onto the microelectronic sensor via electrospinning (figure 6(b)). The patch was seeded with neonatal rat CMs to produce the hybrid microelectronic tissue (figure 6(c)). The patch was shown to withstand the dynamic beating environment in vitro without any loss in electronic or mechanical functionality. The patch was also designed to sense the engineered tissue and provide stimulation for pacing and release of multiple drugs, such as dexamethasone, indomethacin, and acetylsalicylic acid.
Figure 6. A hybrid microelectrode construct with a sensor portion produced from gold electrodes and a functional tissue portion produced from PCL and CMs. (a) Design of the construct. The microelectrode after (b) electrospinning the PCL, and (c) seeding the cells [266]. John Wiley & Sons. [© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim].
Download figure:
Standard image High-resolution image2.2.2. Cardiac tissue models
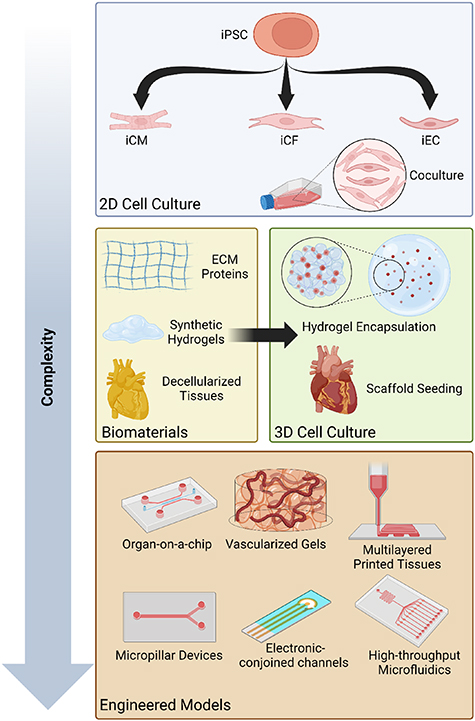
There is a lack of human representative preclinical models that can be used to study the mechanisms by which heart diseases initiate and progress, and to test drugs reliably before they are evaluated in the clinic. In vitro 2D models cannot recapitulate the clinical response due to a lack of the microenvironment and body's systemic effect. Ex vivo models preserve the complex tissue architecture; but they still lack the systemic response of the body, while in vivo models enable studying cancer at a systemic level, but they lack the human components and it is not possible to study the mechanisms of the disease and dissect the reasons for the observed outcome. Cardiac tissue engineering enables the creation of 3D in vitro heart disease models for studying the basic biology of diseases, drug testing and screening, and diagnosis of heart problems. There are various types of models with varying complexity that can be used to study the biology of heart disease, test drugs, and diagnose and treat heart diseases (figure 7).
Figure 7. Cardiac tissue engineering models for the study of heart disease and tissue manipulation. The least technically complex and, correspondingly, most widely accessible models are 2D cell culture models, consisting of monoculture or coculture with or without a patterned substrate. 3D cell culture models are more complex but are still easily accessible due in part to the ubiquity of hydrogels in tissue engineering, however, many simple 3D cell culture models can be limited by difficulties in establishing useful vasculature and manipulating cell organization. The most complex and biomimetic 3D models often utilize re-cellularized tissue scaffolds, but these models can be expensive and difficult to obtain and maintain, thereby limiting their utility. Engineered models aim to address these difficulties whilst efficiently recapitulating both the environmental and organizational properties of the original cell environment, but can rapidly become very complex and difficult to manufacture in house.
Download figure:
Standard image High-resolution image2.2.2.1. Engineered models for studying the biology of diseases
Engineered models can be particularly useful for studying the underlying mechanisms of disease progression in a highly controlled manner. In particular, many single cell-type organ-on-a-chip models are excellent platforms to ascertain a direct relationship between manipulation of key factors or pathways and changes in phenotype or overall tissue functionality [267]. One example of this is muscular thin film models, a widely utilized CM heart-on-a-chip archetype that allows for simultaneous assessment of the contractile and electrophysiological properties of the included CMs [268, 269]. This device has been used to investigate the relationship between CM functional maturity and microenvironment factors, mTOR-p53 signaling, and mechanical training post-differentiation, and enhance understanding of the pathogenesis and functional effects of metabolic syndrome in CMs in a more biomimetic environment than 2D or 3D cell culture and without the complexities of an animal model [269–272]. Similarly, a CF organ-on-a-chip, designed to allow for highly controlled study of fibrotic factors, was able to demonstrate a clear relationship between combined substrate stiffness and mechanical heterogeneity, and the onset of functional and phenotypical markers of cardiac fibrosis [273, 274]. Although simple compared to some organ-on-a-chip models and the native heart, these models permit highly controlled study of individual tissues to better ascertain mechanisms of interest for further study or intervention. This type of design has utility not only in enhancing the study of fundamental biology and sophisticated biological mechanisms, but also for providing tested and standardized platforms for pharmaceutical use, which can be for biomarker discovery, drug testing, or even drug discovery, in a system that is both easy to use and with greater mass-producibility than traditional models.
More sophisticated models consisting of multiple cell types have also been utilized for more complex analysis of known pathways of interest and to assess the interplay between specific relevant cell populations. One study used such a model, consisting of CMs and CFs in a compressible microfluidic device, to measure the effects of pro or anti-fibrotic factors on device functionality and profile paracrine miRNA signaling in the device. This model allowed for a more comprehensive assessment of the interplay between CFs and CMs during cardiac fibrosis, as well as the direct impact that CF transdifferentiation may have on CM functionality independent of cardiac insult or mass structural changes in the myocardium [275].
2.2.2.2. Engineered models for drug testing and discovery
The development of modern cardiac tissue models for drug testing are typically heart-on-a-chip systems, either as small microfluidic devices or large, machine-operated multi-organ devices [276–278]. This is largely due to the emphasis placed on high throughput and repeatability for both drug testing and drug and biomarker discovery. A high-throughput model allows for a large quantity of tests for one drug or a small quantity of tests for many drugs in a consistent model with well-defined parameters. Alternatively, a high-throughput model could also allow for either simultaneous or rapid throughput of samples for drug targets or biomarker discovery for drug development. Both of these types of models are essential for enhancing clinical intervention strategies to mitigate or prevent CVD onset and mortality (table 6).
Table 6. The function, features, and outputs of several cardiac tissue engineered constructs designed to model physiological cardiac behavior.
| Device | Function | Features | Output | References |
|---|---|---|---|---|
| Muscular thin film (MTF) | Mechano and electrical stimulation of CMs | High throughput, integrated measurements | Stress, strain, and conductivity of cells | [268] |
| Cardiac fibrosis on a chip model | Measurement of interstitial fibrosis markers in real time | Real-time, co-culture permissive, integrated measurements | Tissue stiffness, passive tension, proteomic and genomic analysis | [273] |
| Microfluidic heart on a chip MI model | Fluid flow, paracrine, and oxidative stimulation of cells | High throughput, co-culture permissive | Cell survival, paracrine signals, tissue functionality | [160] |
| Tunable myocardial ECM-integrated bioink | Spatial, mechanical, and paracrine stimulation of cells | Dynamic mechanical properties, co-culture permissive, spatial modeling of damage | Tissue beating mechanics, cell fiber alignment, gel mechanical properties | [159] |
| Microfluidic cardiac platelet aggregation and thrombosis model | Induction of shear stress in cardiovascular channels | High throughput, label-free, dynamic measurements | Platelet aggregation | [283] |
Recently, a modular microfluidic heart-on-a-chip device has been shown to be capable of replicating both miRNA and exosomal characteristics of plasma taken from patients pre- or post-MI [160] (figure 8). In this case, the model was utilized for biomarker discovery to predict the onset of II or RI, and demonstrate the feasibility of doing this rapidly and non-invasively in a clinical setting. Additionally, this model can be utilized to model individual drug responses for personalized medicine approaches [87]. The personalized modeling of disease progression and drug response allows these personalized microfluidics models to provide a more controlled and human-relevant model than cell culture and animal models. In addition, the high-throughput and modular design allows for these devices to test a large quantity of biological replicates with considerably less effort and expense than other models used for translational studies. Alternatively, some models aim to assess the effect of drug treatment on designated areas-of-interest in the heart, rather than miniaturizing the entire heart. In particular, the infarct area and infarct border zone post-MI are desirable targets for drug activity. Recently, a proof-of-concept infarct border-zone model was constructed by bioprinting a hydrogel with controlled stiffness and CM/CF ratio [159]. This type of model can benefit both drug testing for anti-fibrotic pharmaceuticals, and discovery of new target pathways that promote the propagation of fibrotic signaling from the border zone. The ability to model the heart both as a whole unit and as individual areas-of-interest is essential for accelerating the development of well-studied, safe, and efficacious pharmaceuticals.
Figure 8. The human heart anoxia and reperfusion tissue (HEART) model. (a) Schematic of the HEART model, where the cardiac channels are loaded with a solution containing iCMs and iECs-laden decellularized human heart ECM and collagen, and the microvessel channel is seeded with a monolayer of iECs. (b) Representative images of iCMs (top) and iECs (bottom) seeded into the HEART model [160]. John Wiley & Sons. [© 2022 Wiley-VCH GmbH].
Download figure:
Standard image High-resolution image2.2.2.3. Engineered models for diagnostics
Engineered models can also be used for diagnostics. Faster and more accurate diagnosis can be achieved using various biosensor devices that take advantage of cardiac tissue engineering techniques. For example, heart-on-a-chip models have been used to study several biomarkers: B-type natriuretic peptide (BNP), N-terminated pro-hormone BNP (NT-proBNP), creatine kinase isoenzyme MB (CK-MB), myoglobin (Myo), and cardiac Troponin I associated with cardiac injury [279]. Recent advances in biosensor technologies allow for detection of biomarkers with high specificity and sensitivity, and more rapid detection of biomarkers than conventional methods, such as troponin testing or biochemical assays [280, 281]. Recently, novel diagnostic approaches combining microfluidics and advanced sensor technology have been used to detect and quantify both nucleic acid biomarkers, including both DNA and miRNA, and protein biomarkers for diagnosis of several CVDs [132, 282]. These combined technologies require smaller sample quantities than conventional diagnostic assays, while simultaneously reducing the time from measurement to diagnosis by utilising simultaneous detection of several biomarkers in a single sample. This can be done via integration of putative cardiovascular biomarkers, mimicking autoantibodies against CRP, BNP, LDL, and cell free maternal DNA detection [282], or through the simultaneous polarization and capture against an ionic membrane of miRNAs with integrated exosome lysis, for efficient high-throughput detection of miRNA biomarkers from a single plasma sample in a combined system [132]. Another study proposed the implementation of a high-specificity diagnostic device for detection of a single factor, rather than simultaneous detection [283]. This device measured platelet aggregation at different shear rates in label-free whole blood, with the intention to be utilized as a point-of-care diagnostic device to detect thrombosis. Another recent study utilized a smartphone combined with a 12-lead electrocardiography device to detect ST-elevation characteristic of STEMI in a rapid manner, allowing patients at high risk of suffering from MI to be monitored remotely [284]. The integration of increasingly complex sensors into the pipeline of engineered models is quickly allowing for the development of high throughput ECT models which can provide rapid output without sacrificing sensitivity or specificity.
2.2.3. Commercialization of cardiac tissue engineering products
Knowing the ubiquity of CVDs and the severe limitations on hearts available for transplant, significant effort has also been put forward to define a path to commercialization and medical usage of many cardiac tissue engineered constructs [285–287]. A recent report estimated that the market for engineered constructs for valve replacement alone surpassed $225 million [285, 286, 288], with predictions that the market size will grow at double-digit rates in the next five years [285, 286].
In the United States, the major regulatory agency charged with evaluating potential products is the Food and Drug Administration (FDA). The FDA has classified cardiac regenerative products as a drug, device, biological product, or a combination product according to the Federal Food, Drug and Cosmetic Act [287]. This classification provides guidelines specific to regenerative products, as well as specific practical guidelines for industry manufacture and use, according to Part 1271 of the Act [287]. Despite this guidance, many cardiac tissue engineered devices are still reviewed by the FDA on a case-by-case basis due to the novelty and wide range of devices that this classification covers.
To obtain FDA clearance for the commercialization of cardiac tissue engineered products (cardiac TEPs), the FDA will often require sequential testing of the device in pre-clinical trials demonstrating both safety and efficacy in animal models [285, 287, 288]. After this, the device may be tested in Phase I clinical trials, and subsequently Phase II and Phase III trials [285, 287]. If the cardiac TEP demonstrates both safety and efficacy according the FDA guidelines in all of these trials, then the FDA may approve the construct for commercial sale and clinical use.
This whole process is typically done by or in partnership with a company, rather than by individual researchers [285, 286]. This combinational effort creates a smooth, multifaceted pipeline for the development of cardiac TEPs and subsequent translation to provide clinical benefits.
2.3. Trends in cardiac tissue engineering
Advances in understanding of basic physiology in both healthy and diseased cardiac tissues in vivo, the development of more sophisticated techniques for designing and implementing biomimetic devices, and the implementation of powerful analytical tools have been essential in the development of more accurate and relevant cardiac models [9, 10, 87, 159, 278, 289, 290]. The implementation of these more biomimetic models further allows for more repeatable and powerful study of both typical cardiophysiology and response to CVD. Below we discuss several areas of interest in the construction of these 'next generation' cardiac tissue models, including ML, CRISPR/Cas editing, exosomes, and the immune system, and how they can be utilized to enhance ECT models.
2.3.1. Machine learning
With the increasing pattern recognition and predictive capability of ML-based computational approaches, their application in detecting cardiac pathologies and developing new treatments is expanding. An emerging use of ML in cardiac tissue engineering is for predictive analysis of the impact of pharmaceutical compounds on CM function as well as potential cardiotoxicity [291]. Previous studies have demonstrated that algorithms can distinguish drug-treated iCMs from untreated iCMs [292]. Additionally, the effects of previously unassessed pharmaceuticals on the electrophysiological function of CMs was predicted using deep neural networks [292]. Another recent study reported 79% accuracy when predicting the cytotoxic effects of novel drugs on iCMs [293]. Yet another recent study utilized ML to generate a metric based on 17 functional parameters derived from mechanical readouts from iCM tissue strips [292]. This metric predicted the cardioactivity upon exposure to a given compound. These and other approaches suggest that ML could be effectively implemented to screen both existing and novel pharmaceuticals for effectiveness and toxicity before any preclinical testing.
Alternatively, the most common use of AI in cardiovascular engineering is uncovering new biomarkers for the early detection of CVDs such as MI and CAD [294]. Previous studies have shown the ability of neural networks to identify MI biomarkers from genome-wide miRNA signatures [295]. Levels of miRNAs such as miR-1, miR-133, miR-208 have been shown to rise in blood quickly before and after an acute cardiac event. The advantage of identifying miRNA signatures will be earlier detection of MI than the traditional troponin assay-based methods, which only become detectable after heart muscle damage and peaks 12–48 h after onset of MI [24, 132, 160]. Moreover, similar algorithms have been used to decipher the miRNA signature of pulmonary arterial hypertension [296].
2.3.2. CRISPR/Cas9 editing
The clustered regularly interspaced short palindromic repeats (CRISPR) associated protein 9 (Cas9) system provides a standard system for genome editing, allowing for the introduction of single functional mutations based on site-specific cleavage [297]. This approach has made gene editing easily accessible for many labs, and is compatible with many different cell types. This ease of access has promoted increased interest in advanced models and intervention strategies for numerous congenital diseases, including some CVDs [293]. Generation of iCMs using the CRISPR/Cas9 method is efficient and provides the means to study the morphological, functional, and biological differences in CMs [298]. The combination of iCM differentiation and CRISPR/Cas9 gene editing provides a pipeline by which cells can be obtained, either from an established stem cell line or on a patient specific basis, and subjected to a high degree of genetic control throughout a study in order to investigate the biomolecular mechanisms of disease onset and pathology in a highly controlled manner. A recent study investigated the mechanisms of Barth syndrome onset by introducing a mutation in tafazzin (TAZ) gene using Cas9-mediated gene editing in iPSCs derived from healthy donors [299]. Barth syndrome is a mitochondrial disorder caused by a missense or frameshift mutation of TAZ, resulting in congenital cardiomyopathy. When characterizing the iPSCs from patients with Barth syndrome compared to normal iPSC lines, the observed differences implicated the relationship between the TAZ gene mutation and the mitochondrial phenotype [300].
Another application of CRISPR/Cas9 technology is the establishment of genetically modified animal models to extensively study cardiac development and function, as well as congenital heart diseases. A recent study developed a mouse model of cardiomyopathy in which transgenic mice overexpressing Cas9 in the heart were generated to allow editing of the Myh6 locus [301]. This resulted in massive cardiac dilation and heart failure by knockdown of Myh6, however, no significant toxicity due to constitutive expression of Cas9 in the heart was observed, which enabled the researchers to dissect the effect of Myh6 expression. This and other studies have helped establish the relevance of gene editing and delivery of single-guide RNA using adeno-associated virus to study CVDs. Another study focused on researching the DiGeorge syndrome and its associated genes [263]. DiGeorge syndrome is caused by a microdeletion of chromosome 22 and characterized by cardiovascular malformations [302]. Cirino et al studied the gene expression and chromatin remodeling in cellular models of Tbx1 loss of function mutation in mouse P19Cl6 cells and mESCs [303]. Tbx1 is expressed in the developmental phase of cardiopharyngeal mesoderm and its deletion is a requirement for the development of the 4th pharyngeal arch artery. The utility of CRISPR/Cas9 in furthering our understanding of the underlying biological mechanisms that regulate the heart, as well as the genetic basis for predisposition and congenital onset of CVDs cannot be overstated.
2.3.3. Exosomes
Exosomes are a subpopulation of EVs with diameters typically between 30 and 200 nm and with distinct characteristic surface proteins [130]. These specific markers are a result of the highly controlled packaging and release of exosomes in cells and allows for highly specific isolation and identification. Additionally, exosomes commonly contain both cytokines and miRNAs and are typically the most numerous EVs released from cells [120]. Exosome content has also been shown to be highly responsive to cell conditions, such as stress, injury, or disease, and are currently being investigated as dynamic metrics of cell health [121, 133].
While exosomes have been known for many years at this point, it is only recently that they have been investigated as active signaling contributors from cells as opposed to miscellaneous cell waste. This, in conjunction with recent interest in miRNAs, has spurred many recent studies to investigate both the diagnostic and therapeutic potential of these small EVs.
2.3.3.1. Diagnostics
A major translational application of exosomes is the use of exosome populations, markers, or contents as diagnostic markers for CVDs. In addition to providing a wealth of responsive information about the cells of origin, exosomes can also be reliably captured from plasma or other biofluids by centrifugation or antibody capture, allowing for a highly informative, relatively non-invasive profiling tool [304].
Exosomes have recently been investigated as a diagnostic tool for the identification of distinct phases of MI, either ischemic injury or RI, and to distinguish these states from CAD in a fast, repeatable manner [132, 160]. This distinction was characterized by miRNA levels. While miRNAs have gained prominence as potential rapid biomarkers for a number of CVDs and other diseases, the results of studies attempting to quantify miRNA levels in a clinically relevant way are often confounding [305]. We have recently shown that utilizing miRNAs isolated from exosomes instead of attempting to obtain free-floating miRNA from plasma yields higher quantities of miRNAs with greater repeatability for miR-1, miR-208b, and miR-499a, which are well-defined precursory markers of MI [132]. Other studies have shown that exosomal miRNAs, such as miR-133a, miR-143, miR-145, and miR-210, can be utilized to reliably detect heart failure, aortic aneurism, and vascular calcification, rapidly and non-invasively [120, 306–308]. Although these methodologies have yet to reach clinical trials, there is a considerable effort to further the development of exosomes as diagnostic markers. Correspondingly, there has been a concerted effort to develop engineered models that can accurately recapitulate these biomarkers for use in drug screening and biomarker discovery [309–311].
2.3.3.2. Therapeutics
A less widely known but more thoroughly investigated application of exosomes is their direct use as therapeutics. Due to the difficulty in identifying every possible target and interaction of drug cocktails and addressing the innate concerns of directly using cells, utilizing cell-derived exosomes, instead, which are non-toxic and largely not immunogenic, has become an attractive avenue for the development of highly targeted therapeutics [312, 313]. In most applications, exosomes are collected from cell culture media and then directly applied to damaged tissue [134]. The scope of these studies has been largely limited to stem cell exosomes, typically MSC exosomes for cardiac applications, or human umbilical vein EC exosomes, due to the demonstrated clinical benefits of these cells in preclinical and clinical trials [121, 133, 306, 314]. Although less explored, recently matrix-bound exosomes from human cardiac tissue have also demonstrated cardioprotective effects against fibrosis and ischemic injury in vitro [101], and these effects could even be partially recapitulated through the application of several identified miRNA from these exosomes.
MSC exosomes have shown therapeutic benefits in 2D and 3D cardiac models, heart-on-a-chip models, preclinical trials, and some clinical trials [315–318]. While MSC exosomes have been largely investigated for applications post-MI to promote recovery and inhibit fibrosis [133, 316], several studies also suggest that MSC exosomes are applicable to address chronic inflammation and enhance CM functionality in aging hearts [319, 320]. Another advantage of MSC exosomes is that these exosomes can be applied directly or through a number of other means, with little difference in efficacy [315–317]. Clinical trials thus far have consisted of hydrogel-based cardiac patches loaded with MSC exosomes [321], and some preclinical trials have demonstrated comparable benefits through intravenous injection [316, 322].
2.3.4. Immune modeling
Another important aspect of cardiac tissue modeling and CVD response is the integration of immune cells. This step is essential for developing clinically relevant models of cardiac tissue for testing therapeutics and investigating CVDs, as immune response and inflammation is one of the primary reasons for failure of cardiac therapeutics in preclinical trials [68]. Furthermore, the immune system is intimately related to the pathogenesis of many CVDs, including fibrosis and adverse response to MI [323].
While many immune cells are involved in CVDs, most recent interest has been on the integration of macrophages into models [324, 325]. This is due to the well-characterized involvement of macrophages in injury and stress response in the heart, as well as the differential effects of type 1 and type 2 macrophages in the heart [323, 325]. Another interesting factor is the recent characterization of resident macrophages in the heart, and the suggestion that these cardiac macrophages are a major mediator of age-related differences to CVD and a key factor in the generally worsening prognosis of CVD after repeated insult [323–326].
Although there has been considerable interest in the integration of immune cells, this has been difficult to achieve due to the naturally transient nature of most macrophages, and the difficulty in characterizing and acquiring cardiac macrophages or the corresponding progenitors [326]. Due to these difficulties, there have been few successful attempts to integrate immune cells into cardiac tissue models. Another complication is the remaining difficulty in generating a truly biomimetic cardiac tissue model, which can prematurely trigger an immune response due to apoptosis or other stress signaling that may be released from the cells in a less than ideal microenvironment [325].
2.3.5. Major challenges in cardiac tissue engineering
Despite these exciting advances in cardiac tissue engineering, several major challenges still need to be addressed. One major limitation of many cardiac TEPs is their reliance on metabolically active biological components [153, 156, 327]. While the use of cells or other such components in tissue engineered constructs allows for highly biologically relevant modeling and clinical utility of these devices, their inclusion also necessitates constant and expensive care and a highly limited shelf life [153, 156, 327]. Furthermore, while many advances in cell biology have allowed significant manipulation of cell phenotype, the construction of mature cardiac cells, in particular mature adult CMs, has thus far remained elusive [153, 156, 327]. Yet another cell-related limitation of these devices is the current inability to easily recapitulate all relevant stimuli that the heart may be experiencing. While it is possible to stimulate models mechanically, electrically, or both, and even introduce some cross-talk with other organ models [328], the systemic factors, paracrine factors, and immune factors that have recently been shown to significantly impact CVD outcomes have yet to be meaningfully integrated. On a final note, there is also no single device which manages to incorporate all of these signals into a single, cost effective, easy to utilize device, which may be described as the ultimate goal of cardiac tissue engineered models.
3. Conclusion and future prospects
Here, we review the recent advances and trends in cardiac tissue engineering. To engineer a functional heart in the lab, human heart anatomy, physiology, structure, and function should be understood very well. Hence, we briefly explain the heart anatomy, physiology, and the cardiac tissue microenvironment, with a focus on the heart cells, the biochemical composition and structural organization of the heart ECM, and GFs playing roles in heart development and maturation. We shortly describe the CVDs and treatment methods currently used in clinic to treat these diseases. We then introduce the reader to cardiac tissue engineering, which may be a viable alternative treatment option to treat CVDs. We next review the cardiac tissue applications aimed toward cardiac regeneration and replacement, as well as cardiac tissue models used to study the cardiac tissue biology, test and develop drugs to treat CVDs and create diagnostic tools to detect them. Finally, we discuss the recent trends in cardiac tissue engineering, including the use of ML, CRISPR/Cas9 editing, exosomes and microRNAs, and immune modeling in engineering the heart.
Although promising progress has been made in the field of cardiac tissue engineering, many limitations remain that hinder the clinical translation of engineered tissues, whether in the form of therapeutic patches or diagnostic tools. One of the main challenges is generating functional and mature tissues that recapitulate the native tissue. One of the mains reason for the immature ECTs is the lack of effective differentiation protocols that yield mature human iCMs. Many methods have been proposed to improve the maturity of iCMs, including biochemical (modulation of the carbon source, hormone treatment, miRNA overexpression, secretory factor treatment, and co-culture with other cells) and biophysical (electrical stimulation, mechanical stimulation, and growing cells on various substrates or in 3D organoids) cues [329–334]. Although these methods have achieved some improvements, functionally, the differentiated iCMs are still far from optimal. Recent studies have shown that the ratio and organization of the cells within the engineered tissue yield higher maturity of the tissue. Giacomelli et al used iPSC-derived stromal cells in a specific ratio (iCM:iCF:iEC, 70:15:15) to produce scaffold-free 3D microtissues to enhance the interactions between the cells and improve the tissue maturity [157]. As such, aiming to incorporate various cell types of the heart while also recapitulating the structure and organization of the native heart is a direction worth pursuing. For example, introducing immune cells to recapitulate the tissue-resident macrophages could improve the functionality of the engineered tissue and its response to drugs. Furthermore, using cells and tissues of donors from various demographic groups, including from different sexes, age groups, races, and ethnicities, can generate fundamental knowledge on the biology of the heart, as well as account for the variability in human responses against drugs [335]. Combining such constructs with physiological mechanical and biochemical cues could lead to greater maturity. Hence, developing scaffolds that induce cell-cell interactions through physical and biochemical signals, while also including biomaterials that mimic the ECM, could be a viable option for obtaining more mature cardiac tissues. Signaling molecules play an important role in cardiac tissue development and maturity. Recent evidence shows that exosomes can be used for cardiac tissue regeneration [133, 336]. As such, using exosomes as a supplement could be used to improve the maturity and function of CMs. With ML, the best ratio of cells, biomaterials, and exosomes to achieve optimal cardiac tissue maturity and function can also be predicted.
Another challenge in cardiac tissue engineering is the limited thickness that can be achieved due to limited nutrient and gas transfer within these constructs. Introducing factors that will induce blood vessel formation within the ECTs after transplantation is essential for the success of these constructs [337, 338]. Additionally, it is crucial to design scaffolds that allow controlled ingrowth of blood vessels within the engineered tissue. Hence, designing scaffolds with pores at various sizes and locations within the scaffolds will be useful for maintaining the viability of the constructs [258]. This will also allow for treating larger defects in the heart.
Additionally, incorporating gene editing to create cells to study a specific condition or disease, and ML to improve the maturity of the engineered tissues and evaluate their response to drugs will open new paths in cardiovascular research.
With the recent advances in the biomedical engineering, we are closer to create functional tissues for cardiac regeneration and cardiac tissue models to faithfully test the human response.
Acknowledgments
This work was funded by the National Institute of Health (NIH), National Heart, Lung, and Blood
Institute (NHLBI) Award Number R01HL141909, and by the National Science Foundation (NSF) CAREER Award Number 1651385.
Figures 2, 4, and 7 were created in part or totally by using BioRender.
We thank Stanley Cheng, Zorlutuna Lab manager, for assisting in proofreading this manuscript prior to submission.
Data availability statement
No new data were created or analysed in this study.
Conflict of interest
The authors have no competing interests to disclose.