Abstract
Polyurethanes (PUs) have properties that make them promising in biomedical applications. PU is recognized as one of the main families of blood and biocompatible materials. PU plays a vital role in the design of medical devices in various medical fields. The structure of PU contains two segments: soft and hard. Its elastomeric feature is due to its soft segment, and its excellent and high mechanical property is because of its hard segment. It is possible to achieve specific desirable and targeted properties by changing the soft and hard chemical structures and the ratio between them. The many properties of PU each draw the attention of different medical fields. This work reviews PU highlighted properties, such as biodegradability, biostability, shape memory, and improved antibacterial activity. Also, because PU has a variety of applications, this review restricts its focus to PU's prominent applications in tissue engineering, cardiovascular medicine, drug delivery, and wound healing. In addition, it contains a brief review of PU's applications in biosensors and oral administration.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The polyurethane (PU) story began in the 1930s when it was synthesized by Professor Otto Bayer [1, 2]. In 1958, PU was used as a shell for the breast-prostheses by Pangman. Later, the same year, Mandarino and Salvatore used a PU called ostamer to fix the bone in situ [3, 4]. Since then, PU has been utilized as a biomaterial in medical applications, and some formulations have been designed explicitly for biomedical applications [4]. PU is a broad category of polymers [5] containing urethane groups formed in the reaction between isocyanate (NCO) and alcohol (–OH) [6]. PU has a complex chemical structure that includes a polyol, a diisocyanate, and a chain extender [7]. PU contains other groups depending on the chemical composition, such as urea, esters, ethers, carbonates, and aromatic components [6]. During the PU synthetic process, in addition to the formation of urethane linkages formation, reactions occur that cause the creation of different bonds, such as biuret, allophanate, and isocyanurate or acyl-urea. These bonds can lead to more branching, which affects the whole structure of the polymer in terms of chemical–physical, mechanical properties, and biocompatibility [8].
PU category can be divided as follow: (i) polyester-based PUs: poly(ester urethanes) was an initial generation of PU that was recognized as applicable but found unsuitable for long-term implantation because of the rapid hydrolytic degradation of the soft segment (aliphatic polyester). (ii) Polyether-based PUs: poly(ether urethanes) were then introduced due to their hydrolytic stability [9]. By contrast, observing the failure rate of medical devices that contained the grade of softer PUs revealed that poly(ether urethanes) can be subject to oxidative degradation [9]. (iii) Polycaprolactone-based PUs can be utilized advantageously as medical, solvent-activated, pressure-sensitive adhesives because of their quick crystallization. (iv) Polybutadiene-based PUs can be synthesized using polybutadiene diols as the soft segment, providing the material with high elasticity and flexibility. (v) Castor oil-based PUs. Castor oil-based PUs can be synthesized using castor oil as the soft segment, which provides the material with excellent flexibility and biocompatibility. These types of PUs can also exhibit excellent biodegradability, making them ideal for use in applications where a biodegradable material is desired. The last two types are limited to medical applications [8].
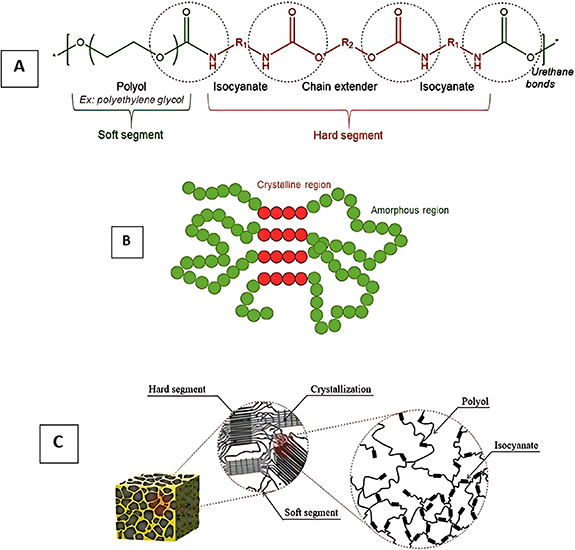
PUs can be synthesized into various classes based on their properties, such as flexibility, rigidity, thermoplasticity, binders, waterborne, adhesives, coating, elastomers, and sealants [6]. Meanwhile, PU has been recognized as versatile for applications in different fields. In recent years, PU has become necessary as a biocompatible polymer with superior mechanical properties because of the development and use of biomaterials in tissue engineering (TE). This review aimed to collect updated narrative investigations into PU's critical properties and biomedical applications. Figure 1 shows semi-crystalline polymer and PU structures in linear and foam structures.
Figure 1. (A) The schematic representation of a semi-crystalline polymer and (B) linear PU structure, and (C) structure of the inside of PU foam. (A), (B) Reprinted from [10], Copyright (2020), with permission from Elsevier. (C) Reproduced from [11]. CC BY 4.0.
Download figure:
Standard image High-resolution image2. Polyurethane properties
PU is a polymer composed of repeating units of diisocyanates and polyalcohols. It is known for its versatility and can be used in many applications due to its unique properties, such as high tear strength, good flexibility at low temperatures, low water absorption, and load-bearing capacity. PU has two critical segments in its structure: soft and hard segments. Together, these segments give rise to a versatile structure [12].
The soft and hard segments contain polyhydroxyl and polyisocyanate components [12]. The soft segments are based on long-chain diol, which provides the PU's elasticity [2]. Conversely, the hard segment depends on the low-weight diol, chain extender, and diisocyanate, producing hydrogen bonding containing urethane links and providing extra strength. Therefore, the properties of PU can be tailored by varying the ratio of soft segments to hard segments in its structure. The soft segments, typically polyether or polyester polyols, give PU flexibility and resilience. The hard segments, typically made up of diisocyanates, give PU strength and stiffness. By varying the ratio of soft segments to hard segments, PU can have a wide range of properties, from soft and flexible to stiff and rigid [2, 13]. This ability to tailor the properties of PU makes it useful in a wide range of applications, such as in flexible foam, rigid foam, elastomers, coatings, and adhesives. PUs have been used in biostable films in valves, bladders, and implants for their mechanical and flexible properties [5]. Also, because of these essential properties (mechanical and biocompatibility), PU is a good candidate for medical devices, including vascular stents, artificial organs, wound treatment, and more [4]. Poly( -caprolactone)-based PU (PCL-PU) copolymers [14, 15] and alginate-based PU composites [16] have been investigated for biomedical applications.
-caprolactone)-based PU (PCL-PU) copolymers [14, 15] and alginate-based PU composites [16] have been investigated for biomedical applications.
Different PU composites have been developed to improve and enhance their properties for additional biomedical applications. This variety can be achieved by combining PU with other polymers, ceramics, and carbon-based materials [17]. PU was applied in cellulose and collagen composition to investigate its application in manufacturing cosmetic masks. PU was selected to provide the required elasticity for this application. Also, the reason for choosing collagen and cellulose was to ensure bioadhesion and mechanical strength, respectively [18]. PUs can be used as a coating because of their properties, such as their versatile mechanical property, excellent abrasion resistance, toughness, low-temperature flexibility, and chemical resistance [19, 20].
As previously briefly introduced, PU can be made from various polyisocyanates and polyalcohols, resulting in different types of PU with different properties depending on their composition. Polyol and isocyanate are the two main chemical reagents for synthesizing PUs. The polyol contains multiple hydroxyl groups (–OH) that react with the isocyanate to form the polymer backbone. Isocyanate is a compound containing one or more isocyanate groups (–NCO) that react with the polyol's hydroxyl groups to form the polymer's urethane linkages. The specific type of polyol and isocyanate can significantly affect the properties of the resulting PU. The ratio of isocyanate to polyol also affects the properties of the resulting PU (table 1) [21].
Table 1. Various types of polyurethanes. Typically, the rate of hydrolytic degradation of polyols observes the order polycarbonate < polyether < polyester [22].
| Type of PU | General properties | Structure [6] | References |
|---|---|---|---|
| Thermoplastic polyurethane | Good hydrolytic stability, abrasion properties, suitable mechanical properties with superior chemical resistance, dimensional stability, stiffness, high impact resistance, high strength, and good process ability |
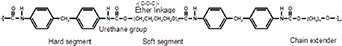
| [23] |
| Fully biodegradation rate of TPU is still a challenge as the hard segment (isocyanate) is not degradable in various environment conditions | |||
| Poly(ether urethanes) | Excellent mechanical properties, biocompatibility, flexibility, and hydrolytic resistance |
| [24, 25] |
| A disadvantage is its lower oxidative and thermal stability | |||
| Poly(ester urethanes) | They have appropriate mechanical strength and thermal stability; however, they are susceptible to hydrolysis |
| [21] |
| Poly(carbonate urethanes) | Chemical stability as compared to poly(ether urethanes). polycarbonate-based PUs provide improved mechanical properties, heat and hydrolytic stability compared to polyester and polyether-based PUs. However, long-term in vivo studies indicate susceptibility to enzymatic hydrolysis and oxidative degradation by inflammatory cells |

| [21, 24] |
2.1. Various types of PU and their properties
Thermoplastic polyurethane (TPU) is a type of PU that can be melted and reshaped multiple times. They have good abrasion resistance, high elasticity, and good chemical resistance. Polyols include di-hydroxyl terminated macroglycols of polyethers, polyesters, and polycarbonates between 1000 and 5000 Da molecular weight. The molecular weight and polyol's structure are significant in PU's mechanical, physical, and chemical properties [26, 27]. TPUs are non-crosslinked polymers typically made by reacting a polyisocyanate with a polyester or polyether polyol, chain extender, and catalyst. The resulting polymer combines hard and soft segments, with the hard segments giving TPU its strength and stiffness and the soft segments giving it flexibility and elasticity. The properties of TPU can be tailored by varying the ratio of hard segments to soft segments and using different polyisocyanates and polyalcohols (figure 2) [28]. These components define the polymer structure. The soft segment determines the elasticity and flexibility of the TPU and is typically a polyetherdiol in light of its hydrolytic stability in biomedical applications [29]. The hard segments consist of an aromatic di-isocyanate, such as MDI, which gives the polymer its thermoplastic attributes.
Figure 2. The basic structure of linear thermoplastic polyether-urethanes with hard (MDI) and soft segments (polyether-diol). Reprinted from [30], Copyright (2022), with permission from Elsevier.
Download figure:
Standard image High-resolution imageA low-molecular-weight diol is also used as a chain-extending agent [28]. One of the key features of TPU is its thermoplastic nature, which allows it to be melt-processed and molded in various shapes and forms. When the ratio of soft and hard segments is high, the PU is more deformable and exhibits a rubber-like behaviour. The soft segments in the polymer structure provide flexibility and resilience, allowing the polymer to stretch and recover its original shape without permanent deformation. When the ratio of soft and hard segments is low, the PU is less deformable and exhibits a more complex and rigid behaviour.
The hard segments in the polymer structure provide strength and rigidity, limiting the polymer's ability to stretch. It is worth noting that the ratio of soft segments to hard segments can be adjusted to achieve the desired balance of properties [31, 32]. For example, a PU with an average ratio of soft segments to hard segments can exhibit a balance of flexibility and strength, making it suitable for various applications.
The polymerization of TPU uses aromatic bifunctional reagents diisocyanate (MDI) and a high-molecular-weight polyether-diol. The short chain-extending diol increases the polymer's molecular weight; e.g. Texin 986 uses MDI and a high-molecular-weight polyether-diol [33]. A short chain-extending diol increases the hard segment and the molecular weight of the TPU [34, 35]. In general, thermoplastic polyether-urethanes, which comprise the most implantable materials, have very high tensile strength, toughness, abrasion resistance, and resistance to degradation, in addition to biocompatibility, which has sustained their use as biomaterials [36]. The stability of thermoplastic polyether-urethanes depends on the polyol groups used in the synthesis [37]. TPUs are suitable for medical devices such as artificial joints, heart valves, and blood vessels. They are also used in coatings for medical implants, such as stents and artificial heart pumps, to reduce thrombosis [38, 39]. TPU can also be used in wound dressings, sutures, and TE applications [40]. Poly(ether urethanes) are used in blood-contacting applications, such as catheters, heart assist pumps and chambers for artificial hearts, vascular prostheses, drug delivery, and pacemaker lead insulation [24, 41, 42].
Poly(ether urethanes) are made from polyether polyols and are known for their excellent resistance to hydrolysis and good flexibility at low temperatures [43].
Poly(ester urethanes) are made from polyester polyols and are known for their good abrasion resistance, high tear strength, and excellent chemical resistance to oils and solvents. However, it is unstable towards enzymes and has a high degradation rate. Therefore, they can be utilized as bioabsorbable polymers [43].
Poly(carbonate urethanes) are made from a combination of polycarbonate and polyester or polyether polyols, known for their high strength, high temperature, and good chemical resistance [44, 45]. PUs are used due to their physiological compatibility, appropriate haemocompatibility, excellent in vivo stability over long implant periods, and excellent physical and mechanical properties [8, 46]. Polycarbonate urethanes have displayed significant potential as durable elastomers that remain stable over extended periods. They demonstrate exceptional resistance to processes such as hydrolysis, environmental stress cracking, and oxidation caused by metal ions [47]. Different polyol groups can affect the properties of the resulting material, such as its flexibility, toughness, and thermal stability. The choice of polyol groups can also affect the material's processing conditions and final properties [26, 48]. Table 2 shows the biomedical applications of various TPUs.
Table 2. Biomedical applications of poly-urethanes [49].
| Purpose | Examples | References |
|---|---|---|
| Polyether-urethane | ||
| Implants | Artificial heart, cardiac pacemaker leads, vascular tube prosthesis, breast implants, membrane for the reconstruction of the meniscus, materials to fix bones, implants for the reconstruction of facial bones | [49] |
| Materials with membrane properties | Adhesive materials, materials for drug-delivery-systems, inclusion membrane for the fixation of inner organs, dialyses-membrane, filtration of oxygen-adsorption-module, artificial skin | |
| Auxiliary material | Catheter, cannula, blood sack, wound dressing | |
| Polycarbonate | ||
| — | Biostable polycarbonate PU is better for vascular prostheses | [50] |
| — | Coatings for cardiovascular devices | [51] |
| Polyester | ||
| Foam | Used as a breast prosthesis coating | [52] |
| — | Polyester PU is suitable for vascular scaffolds | [50] |
| — | Cardiovascular stents | [53, 54] |
| Scaffold | As cardiac tissue model | [55] |
It has been shown that polycarbonate-based urethanes have better biostability and biocompatibility than other types of PU (e.g. polyester urethane) for biomedical applications, which were correlated to better and higher stability of polycarbonate's soft segments inside of the body [56]. Zhu et al have designed a system containing 4,4'-methylenebis (cyclohexyl isocyanate) (H12MDI), poly(1,6-hexanediol) carbonate diols (PCDL), 1,4-butanediol (BDO) and 1,6-hexamethylene diisocyanate (HDI) to synthesize polycarbonate-based urethanes and evaluated biocompatibility property using platelet adhesion and haemolytic tests. They reported that this composition could be applied as a biomaterial due to its excellent blood biocompatibility [57]. Previous reports showed that polycarbonate-based urethanes have higher biostability and oxidative stability than polyether urethanes, which caused them to be a better replacement for polyether urethanes for biomedical applications [29, 56]. They showed appropriate heat stability, mechanical properties, and hydrolytic stability; however, they are reported to undergo possible enzymatic degradation because of inflammatory cells for long-term in vivo investigations [58].
2.2. Evaluation of bioactivity and biocompatibility
Biocompatibility refers to the ability of a material to perform with an appropriate host response in a specific in vivo application. In other words, it means that a material does not cause any adverse non-physiological reaction when it comes into contact with living tissue or bodily fluids. As with all biomaterials, PU's biocompatibility can vary depending on the specific application and the type of tissue or fluid with which the material will come into contact. Therefore, biocompatible materials in one application may not be biocompatible in another one [59]. However, cytotoxicity is the minimum requirement for using a PU for medical applications [60]. PU's cytotoxicity has been extensively investigated, including in vivo and in vivo studies. For example, PU elastomer was previously evaluated for its blood biocompatibility [61, 62]. In another case study, mouse fibroblast cell lines and human saphenous-vein endothelial cells (HSVECs) were used for culture with various commercial PUs. The results showed that PU could provide sufficient conditions to colonize patient-derived HSVECs [63]. Since different PUs have been developed, it is vital to understand the relationship between biocompatibility and PU structure. Lyman and Picha reported that the biocompatibility of PUs depends on their configuration and morphology [64, 65].
Moreover, the relationship between soft and hard segments and the blood response has been reported in other investigations. They illustrated that the hard segment is widely thrombogenic [66, 67]. Two reasons were considered for this high thrombogenicity; the first was the high-level crystallinity of polymers, and the second one was that they demonstrate a strong and reasonable hydrogen bond surface. Cooper et al stated that surface mobility could be essential in interacting with polymers and biological systems [68]. In the late 1980s, many studies investigated chemical, structural and morphological modification in PUs to confirm their blood biocompatibility. Recently, some surface modifications and synthesis methods, such as grafting, chemical incorporation, and coating techniques, have been developed to increase the blood biocompatibility of PUs [69]. Incorporating and substituting nanoparticles is another way to improve PU biocompatibility [70–72]. In addition to the composition variation, ion beams and plasma techniques have been considered an appropriate way to enhance PU biocompatibility [73].
2.3. Biodegradability and biostability
Biostability refers to the ability of a material to resist degradation by microorganisms, such as bacteria and fungi. Biodegradability, on the other hand, refers to the ability of a material to be broken down by microorganisms over time. Some PU formulations can be biostable, meaning they are generally resistant to degradation by microorganisms (table 3). Certain PUs can be made biodegradable by incorporating biodegradable polyols into the polymer structure. These biodegradable PUs are typically made from polyester polyols modified to include groups that microorganisms can easily break down [74].
Table 3. Summary of some development of biostability of PU.
| Date | Outcome | References |
|---|---|---|
| 2009 | Poly(carbonate urethane)s have been studied as biostable elastomers for long-term implantable PU biomaterials. Micro-catheters made of poly(carbonate urethane) (Bionate®) and poly(ether urethane) (Pellethane®) were designed and tested to compare their long-term in vivo stability. The PU catheters were stretched and exposed to a hydrogen peroxide/cobalt chloride solution for up to 10 months. The results showed that the soft polycarbonate segment of the PU catheters was more resistant to oxidative degradation than the polyether soft segment. The study suggests that poly(carbonate urethane) could be appropriate for developing long-term implantable biomaterials. | [108] |
| 2013 | Silicon-based polyurethanes (PUs) are being investigated to improve the biostability of PUs due to silicon's high resistance to oxidation, temperatures, and water. Silicon-based thermoplastic PU nanocomposites were created using commercial ElastEonTM E5325, which showed improved biostability of the silicon-based PU nanocomposites against metal ion-induced oxidation, suggesting their potential use in long-term implantable biomaterials such as artificial intervertebral discs. | [109] |
| 2012 | To improve the biostability of polyether-PU, layered silicates with more excellent chemical resistance than native PU were incorporated. Cloisite® 30B (QACMMT), a commercially available and organically modified montmorillonite, was utilized in the research. QACMMT contains quaternary ammonium compounds (QAC), specifically methyl tallow bis-2-hydroxyethyl ammonium chloride, as an organic modifier. Upon the addition of QACMMT at loadings below 3 wt%, the biostability of the PU increased by up to 50%, primarily due to decreased material permeability. | [110] |
| 2022 | Segmented thermoplastic polyurethanes (STPUs) with varying hard-to-soft segment ratios were synthesized and characterized for use in shape memory polymers. Results showed that PUs with higher hard segment content underwent less degradation, with only 4%–5% mass loss for higher hard segment content samples versus 28% mass loss for lower hard segment content samples under 20% H2O2 conditions. This suggests that stronger interactions between chains increase material stability and enable the tuning of biostability based on the required degree of degradation. | [111] |
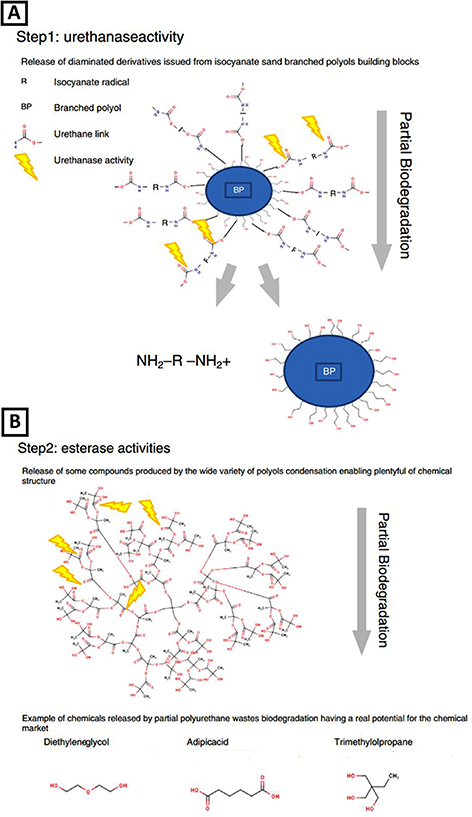
The degradation mechanism of biodegradable PUs typically involves the action of enzymes produced by microorganisms. The enzymatic degradation of PU is shown in figure 3. The enzymes break down the PU polymer by attacking the ester bonds in the polyol, causing the polymer to lose its mechanical properties and eventually break down into smaller molecules that microorganisms can further degrade [75]. Figure 4 illustrates PU biodegradation.
Figure 3. Main mechanisms of enzymatic degradation of PU. Reprinted from [10], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageFigure 4. PU biodegradation occurs in two steps via the activity of urethanases and esterases. Reprinted from [98], Copyright (2013), with permission from Elsevier.
Download figure:
Standard image High-resolution imageIt is worth noting that the biodegradation rate of PU polymers is affected by various factors, such as the type of polyol used, the environmental conditions (e.g. temperature, humidity, and the presence of other microorganisms), and the size and shape of the polymer. Therefore, the biodegradability of PU polymers is highly dependent on the specific conditions of the environment. PU can degrade through various mechanisms depending on the type of polyol used. Degradation mechanisms can co-occur, and their degradation rate is affected by environmental conditions. Therefore, choosing the appropriate PU type is essential depending on the intended application and environmental exposure conditions [10]. Some of the possible degradation mechanisms include:
Ester hydrolysis: PUs made from polyester polyols are vulnerable to hydrolysis, a chemical reaction that occurs when the water breaks down the ester bond in the polyol. Hydrolysis can cause the PU to weaken and lose its mechanical properties over time [10].
Ether oxidation: PUs made from polyether polyols are vulnerable to oxidation, which is a chemical reaction that occurs when the ether bond in the polyol is broken down by oxygen. This can cause the PU to become brittle and lose flexibility over time [76].
Heat-induced oxidation (HIO): is a degradation mechanism that can occur in polyurethanes (PUs) when exposed to high temperatures. HIO can cause the PU to become brittle and lose its mechanical properties, as well as a change in colour and surface appearance. During HIO, the polymeric chains in the PU can break down due to the presence of free radicals generated by the high temperature. This can lead to the formation of short-chain fragments, which can cause crosslinking between the polymer chains. While this crosslinking can increase the PU's thermal stability, it can also cause the material to become brittle and lose its mechanical properties. The brittle behaviour of PUs at high temperatures instead of melting is due to the nature of the polymer structure. PUs typically comprise soft and hard segments, resulting in a complex morphology that can affect the material's behaviour at high temperatures. The hard segment in the PU can act as a stabilizing agent, preventing the material from melting even at high temperatures, but when exposed to HIO, the hard segments can also degrade, causing the material to become brittle. While increasing the temperature can cause crosslinking in PUs, it can also lead to HIO, which can cause the material to become brittle and lose its mechanical properties. Understanding the underlying mechanisms of PU degradation at high temperatures is essential for developing PUs with improved thermal stability and better resistance to HIO [77].
Environmental stress cracking (ESC): is a phenomenon that can occur in PUs when exposed to certain chemicals and mechanical stress. ESC can cause PU to become brittle and lose its mechanical properties, resulting in cracks and failure of the implanted device. ESC is more commonly observed in polyether-based PUs (PEUs) than polyester-based PUs (PESs) due to ether linkages in the polymeric chain. When exposed to certain chemicals, these ether linkages are more susceptible to hydrolysis, forming cracks and fissures in the material. Mechanical stress, such as bending or flexing of the material, can further exacerbate ESC's effects by increasing the crack propagation rate. The synergistic effect of chemical exposure and mechanical stress can cause the PU to fail, leading to potential complications in medical device applications. ESC is a significant concern, as PUs are commonly used in implanted devices such as catheters, stents, and artificial heart valves. In these applications, the PU may be exposed to various chemicals in the body, such as blood, enzymes, and medications, which can lead to ESC. In conclusion, ESC is a phenomenon that can occur in PUs, especially in polyether-based PUs when exposed to certain chemicals and mechanical stress. Understanding the underlying mechanisms of ESC is essential for developing PUs with improved chemical and mechanical stress resistance, which can increase the safety and efficacy of implanted medical devices [78].
Enzymatic degradation: PUs are known to be biodegraded by certain microorganisms, particularly bacteria and fungi. Enzymes can cause PU to become weaker and lose its mechanical properties over time [76, 79, 80].
Carbonate degradation: PUs made from polycarbonate polyols are vulnerable to degradation caused by the presence of the carbonate group in the polyol, leading to a loss of strength and mechanical properties [10, 81, 82].
Biostability is another essential property for the long-term medical application of PU [36]. Polyester-based PU is known not to be resistant to hydrolysis. The ester-group starts to degrade after implantation, leading to strong inflammatory reactions. This problem was solved by introducing a hydrolysis-stable polyether-urethane [83] based on polyether. Hence, polyester-based PUs are no longer used to produce implants [36]. Generally, the hydrolytic degradation of polyether-urethanes in pure water is minimal. Nevertheless, the aqueous environment of the body, i.e. cations and anions, has a strong catalytic effect in hydrolysis. Polyether-urethanes and polycarbonate-urethanes are generally less susceptible to hydrolysis than polyester-based ones. However, they can degrade hydrolytically at high temperatures (>50 °C) in the presence of water. These conditions often exist in polymer processing methods like injection molding and extrusion [84] and should be avoided.
For this reason, most PUs available for medical applications are based on polycarbonate or polyether. However, the biodegradation property of PU has been widely investigated [8, 85, 86]. In this regard, some modifications have been reported to improve PU biostability.
One proposed modification is to replace the soft domains in the PU with materials that have improved stability, such as polysiloxane or polyolefins [87–89] since it has been reported that the soft segments had the most prominent effect on biostability [90]. Haugen et al found that polyether-urethane scaffold biostability was worsened with a higher gamma-irradiation dose [79, 91]. Cozzens et al [88] designed a new PU based on a mix of poly(tetramethylene oxide) (PTMO)/poly-isobutylene (PIB) and provided accelerated testing (H2O2 (20%) solution contained 0.1 (M) CoCl2 at 50 °C) to evaluate and analyse resistance against degradation of metal ions oxidative in vivo. The new composition showed considerable stability compared to the commercial types, like Pellethane 2363-80A and 2363-55D [88].
Additionally, surface modification [92], antioxidants [93], and nanoparticles [94, 95] were also introduced as methods for the improvement of PU stability. Notably, the biostability property is vital for long-term applications since biodegradation entails a loss of shape and mechanical properties. However, investigation of some scaffolds-based biodegradation in PU is also needed in TE, and control of the degradation rate is essential in some applications, such as drug delivery, scaffolds, and short-term implants [96, 97]. Many factors can affect PU's biostability, including mechanical properties, chemical structure, morphology, fabrication methods, and working condition [95]. Surface chemistry, the relative hydrophobicity, and hydrophilicity of polyether-urethanes have been found to influence the proteins adsorbed by the surface [37]. The first event in soft tissue interactions with polyether-urethane is the adsorption of water and protein onto the surface, followed by an acute inflammatory response characterized by polymorphonuclear leukocytes. The consequence of the adsorbed protein layer on the biomaterial is that cells never actually contact the material. After protein adsorption, an acute response is followed by chronic inflammation, which includes a wound-healing mechanism and foreign body reactions involving macrophages and fibroblasts [59]. Like many other biomaterials, polyether-urethanes elicit a foreign body response determined by multinucleated giant cells in contact with their surfaces, generally called ESC.
Additionally, researchers have found that polyether-urethanes often become encapsulated in a fibrous capsule [36, 99]. Ultimately, this influences the subsequent cell migration and implant function [100]. Mohanty et al [101] and other researchers [102] have found a relationship between pore size and the infiltration of macrophages into a polymer implant. The researchers found that a 5–10 µm pore inhibits cell infiltration in the bulk material, while larger pore sizes (200–300 µm) encourage cell infiltration [36]. Mohnty's work also indicates that a porous implant delays the incidence of capsule formation and contracture for about ten years. Haugen et al reported using porous PU as a gastro application to reduce gastroesophageal reflux diseases. Where Haugen et al developed a porous PU implant with supercritical CO2 [103, 104], where the porous structure was supposed to fixate around the oesophagus. Recently, in vivo experiments have shown that tissue ingrowth into a porous PU structure is only possible with plasma surface treatment, and untreated TPU resulted in massive fibrotic encapsulation [105].
Hence, chemical composition is the key factor [86]. ESC is also reported as one reason for PU's degradation [106]. Various works have investigated other mechanisms of PU degradation [5, 9, 97, 107].
In summary, over the last two decades, several chemistry approaches have been designed primarily focusing on decreasing or eliminating functional groups sensitive to oxidative degradation to improve biostable PUs. in vivo analysing and animal investigations have produced long-term biostability for clinical applications. While most approaches have helped design PU elastomers with improved resistance to oxidative degradation compared with conventional PU, their utility in long-term medical implants is yet to be assessed. The improved understanding of the relationship between PU chemical structure on properties and biostability will help synthetic polymer chemists design new materials to suit the needs of next-generation medical implants.
2.4. Shape memory property of PU
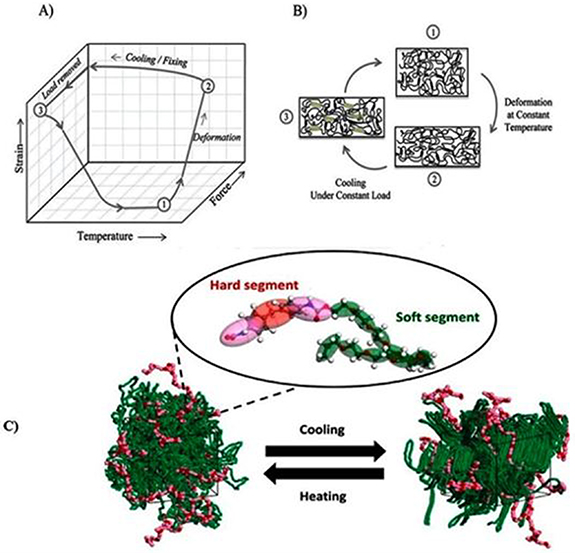
Smart materials known as shape memory polymers (SMPs) can remarkably alter and regain their shape in response to various stimuli [112]. The shape memory of polymers has been investigated previously [113]. Shape memory polyurethane (SMPU) is a TPU that exhibits shape memory properties. When SMPUs are heated above their glass transition temperature (Tg) but below their melting temperature (Tm), the material can be deformed into a temporary shape maintained by cooling it below the Tg. Soft segments in the PU structure are responsible for this temporary shape, as they allow the material to be deformed and maintain the temporary shape. Upon heating the material above the Tg, the hard segments in the PU structure help to recover the material's permanent shape. The hard segments are responsible for 'remembering' the permanent shape and help drive recovery [114, 115]. Tm is an essential parameter for processing SMPUs to achieve the desired permanent shape. During processing, the material is typically heated to a temperature above the Tm to facilitate the recovery of the permanent shape. The shape memory behaviour is achieved by adjusting the polymer structure's ratio between hard and soft segments. The hard segments provide the polymer with shape memory properties, while the soft segments provide flexibility and elasticity [116]. SMPU can be a good candidate for medical devices because of their flexibility in shape change in different areas [117, 118]. A specific chemical combination can allow a geometric shape-changing that makes them resistant to external factors like pH [119], light [120], and temperature [121], as shown in figure 5. This change is because the polymer's structure contains switching segments [122]. If the temperature exceeds the glass transition temperature (Tg), materials are in their rubbery-elastic form, which can deform easily in the desired shape. If the temperature falls below Tg, they become fixed without any deformation in shape. At this step, materials are rigid. For biomedical applications, Tg should be around the body temperature to allow the recovery of the permanent shape in vivo. This phenomenon occurs due to molecules' movement and structure [117]. This shape memory property can make these polymers an ideal material for medical devices because they can keep a specific shape during implantation or delivery and recover to their original shape. Interestingly, increasing the temperature above Tg can recover the materials' original, permanent shape. The development of SMPUs began 15 years ago [117]. The biomedical applications of PUs as SMPs include clot removal, vascular occlusion, and stenting [123, 124]. Also, it has been reported that SMPs can be used for cardiovascular implants [53], bone tissue engineering (BTE) applications [116], electromagnetic shielding, pressure bandages, and self-healing [125]. Also, range of dual bioactive electroactive shape memory PU elastomers by incorporating dopamine into a formulation has been synthesized based on citric acid, PCL, dopamine, and the electroactive aniline hexamer. These materials are suitable for soft tissue applications that require sensitivity to electrical like skeletal muscle regeneration [126]. A pH-sensitive PU was also designed.
Figure 5. (A) Shape memory cycle for SMPs, (i) starting at high temperature (point 1), (ii) reaching point 2 by deforming, (iii) specimen is cooled under a constant load, (iv) reaching point 3 by removing load and obtaining a fixed shape, (v) shape memory happens by reheating specimen and reaching point 1. (B) A molecular schematic for SMPs. (C) Shape memory of PU under heating and cooling process. (B) [134] John Wiley & Sons. [© 2016 Wiley Periodicals, Inc.]. (C) Reprinted from [135], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution imageIn this case, the shape memory of PU depended on pH variation instead of temperature variation. The mentioned PU was designed for drug delivery and TE applications [127–131]. It is worth noting that the shape-memory effect is highly dependent on the specific conditions of the environment, such as the temperature, humidity, and the type of polyol used [132]. De Nardo et al showed that SMP scaffolds could be obtained via solvent casting/particulate leaching of gelatin microspheres prepared via oil/water emulsion. Varying the gelatin microsphere size enabled easy control of scaffold morphology, pore size, and shape. Homogeneous spherical and interconnected pores have been achieved with the preservation of shape memory ability, with a recovery rate of up to 90%. Regardless of pore dimensions, MG63osteoblast-like cells were observed adhering and spreading onto the inner surface of the scaffolds obtained in in vivotests [118, 133].
In summary, using SMPUs in various applications demonstrates their versatility and importance. The shape memory effect can be driven by external forces such as electricity, temperature, electricity, and light. SMPs respond to external stimuli because of hard and soft segment domains. Desired properties of SMPUs can be achieved by cross-linking through ionic, covalent, and hydrogen bonding [128].
2.5. The antibacterial property of PU
Some types of PU have been designed to exhibit antibacterial properties. This can be achieved by incorporating antimicrobial agents, such as silver or zinc ions, into the polymer structure. These antimicrobial agents can kill or inhibit the growth of bacteria, fungi, and other microorganisms on the surface of PU devices [136]. The antimicrobial agents are incorporated into the polymer structure during the manufacturing process, and they are released slowly over time, providing long-lasting protection against microorganisms. The antimicrobial agent's release rate can be controlled by adjusting the concentration of the antimicrobial agents in the polymer and the molecular weight of the polymer [137]. Antibacterial PU can be used in many applications where controlling microorganisms' interaction with the PU structure is essential, such as in medical devices and food packaging. Like any other antimicrobial agent, the effectiveness of the antibacterial properties of PU can be influenced by many factors, such as the amount of antimicrobial agent used, the type of microorganism, and the environmental conditions [138]. Therefore, choosing the appropriate type of PU and antimicrobial agent is essential, depending on the intended application and the microorganisms needing control [139].
Kasi et al comprehensively collected these investigations. Different methods and materials have been reported to increase antibacterial properties [140]. Moreover, silver (Ag) nanoparticles, metal oxides and metals, nanomaterials based on carbon, synthetic polymeric materials based on PU, natural sources, and more have been reported [140]. In a different study, functionalized PU was applied as a magnesium (Mg) coating to enhance and improve antibacterial and corrosion resistance. The results showed considerable enhancement in corrosion resistance and excellent antibacterial activity [141]. Jiang et al designed amphiphilic poly(dimethylsiloxane) based on PUs, using carboxybetaine to obtain an antibacterial effectiveness of 97.7% [142]. Because PUs have remarkable properties, they have been used as a based substrate for other antibacterial agents, such as copper oxide (CuO) and chitosan [143, 144]. The effects of different agents, such as Ag, zinc oxide (ZnO) [145], and titanium dioxide (TiO2) nanoparticles loaded in a PU matrix, have been reviewed, and it has been reported that these nanoparticles can improve PUs' antibacterial activity [146]. Wang et al reviewed the antimicrobial PU coatings for different applications [147]. Also, Wang et al evaluated the antibacterial activity of PU and suggested different methods to design PUs' antibacterial properties for use in medical devices [136].
3. pH-responsive PUs
pH-responsive PUs are a type of PU that can change their properties in response to environmental pH changes. These PUs have pH-sensitive groups incorporated into their polymer backbone, which can respond to changes in pH by changing their conformation [148]. This can lead to changes in solubility, swelling, and mechanical properties. pH-responsive PUs have been investigated for many applications, such as drug delivery and TE [12]. In TE, pH-responsive PUs can mimic the natural pH changes in the body, such as in the stomach or gut. These PUs can be used to produce scaffolds that can change their properties in response to changes in pH, promoting cell growth and differentiation [149]. The properties of pH-responsive PUs can be tailored to specific applications by adjusting the pH-sensitive groups in the polymer structure and the pH range at which the PU will respond. For example, pH-responsive PUs can be used to control the release of drugs by changing their solubility at different pH levels [148]. In a neutral environment, the PU is insoluble, and the drug is not released, while in an acidic environment, the PU becomes more soluble and the drug can be released. pH-responsive PUs can be categorized into different fields, including (i) optical imaging, (ii) drug delivery, (iii) bioactuators and biosensors. pH-responsive PUs can provide a substrate for controlling targeted drug delivery and the release rate [150, 151]. pH-responsive PUs have primarily been investigated because of their optimal mechanical properties [152], easy synthesis [153], possible biodegradability, and biocompatibility. Most pH-responsive PUs for controlled and targeted drug delivery are based on crosslinked networks, like hydrogels and microgels. Notably, the hydrogel has a porous structure and can swell in water. The most-used polymers in this application are redox and dual pH-responsive PUs that maintain either ketal or disulfide crosslinkers. This structure can help release drugs in a controlled acid-type pH and protect them from the drugs (figures 6 and 7) [12]. Also, the nano-gels have a diselenide crosslink in their structure, granting excellent colloidal stability. The drug loading efficiency of 76.3% has been reported for nano-gels by Cheng et al [154]. Moreover, the compact structure of nano-gels in the core prevents the leakage of drugs. Since they can swell, they could accelerate the release process in the presence of pH 5.0 and a high concentration of H2O5 [12, 154]. Song et al designed a nanomicelle based on PU that contained some soft chains, including hydrophilic PEG, poly(neopentyl glycol adipate) diol (PNA-2000), 2-[N,N-bis (2-hydroxyethyl)] aminoethanesulfonic acid sodium salt (BES-Na) and 1,4-butanediol (BDO). Folic acid was embedded in micelles, and PU micelles were self-assembled, improving the micellar's stability. The results showed that the drug release was greater in an acidic environment compared with pH 7.4 [155]. The delivery of doxorubicin (DOX) loaded on responsive PU micelles occurred at pH 5.5, and less toxicity was observed in an in vivo environment [156].
Figure 6. (A) An example of protonation (at low pH) and deprotonation (at high pH) of an amphoteric copolymer. The carboxylic groups are labelled in purple, the amine groups are labelled in green; (B) an example of a polymer containing zwitterionic ions (both carboxylic group and amino group) with model drug loaded. At the isoelectric point, the acidic pendant group, basic pendant group, cations and anions remain in equilibrium. When the pH is decreased, the amino groups are protonated, and the polymer networks swell due to the electrostatic repulsion between the neighbouring positive groups, which induces water diffusion into the polymer network, releasing the incorporated drugs. When increasing the pH, the pendant carboxylic group ionized and deprotonated, and the polymer swelled due to the increase of electron charge density, which induced water diffusion into the polymer network, releasing the incorporated drug. Reproduced from [12]. CC BY 4.0.
Download figure:
Standard image High-resolution imageFigure 7. Schematic of drug release in a polymer containing an acid-labile linkage. The acid-labile linkage hydrolysed, and bond cleavage happened in an acidic pH environment, releasing loaded drugs. Reproduced from [12]. CC BY 4.0.
Download figure:
Standard image High-resolution imageAnother application of PU is in cancer therapy [148]. Tumours tend to grow in an acidic environment. The pH value of the bloodstream is about 7.4; however, the pH of the existing tumoural cells and the components of the endocytic cells is from 4 to 6. This difference makes it necessary to provide pH-targeted drug delivery systems. As a result, utilizing pH-sensitive biodegradable PU nanoparticles as a carrier for the delivery of anticancer drugs has been analysed both in vivo and in vivo with promising results [157].
While pH-responsive PUs have the potential for a wide range of applications, some drawbacks are associated with their use [158]. Some of the main drawbacks are briefly described here below.
- (1)Limited pH range: pH-responsive PUs typically have a limited pH range to respond. pH range can make it challenging to achieve the desired response over a wide range of pH conditions;
- (2)Inconsistent response: pH-responsive PUs can have an inconsistent response to changes in pH. The response can make it challenging to achieve a consistent and predictable release of drugs or to achieve consistent cell growth and differentiation on the scaffold;
- (3)Complex synthesis: Synthesis of pH-responsive PUs can be complex and challenging, requiring a high degree of control over the polymerization process [159],
- (4)Cost: The cost of pH-responsive PUs can be higher than conventional PUs due to the complexity of the synthesis process and the need for specialized equipment;
- (5)Toxicity: Some pH-sensitive groups used in pH-responsive PUs can be toxic to cells and negatively affect PU performance [159].
It is worth noting that pH-responsive PUs are still under development, and ongoing research aims to address these drawbacks and improve their performance and applicability in various fields [158].
In conclusion, pH-responsive PUs attracts attention in the pharmaceutical and biomedical industries. Although there is great potential in biomedical and drug delivery systems, such attempt is still needed to confirm their safety and effectiveness in biological systems. It seems that in most investigation on pH-responsive PU systems for biomedical and drug delivery applications, the stability, biodegradability, biocompatibility, and mechanical properties of the PU as the potential polymeric substrate for various potential drug delivery and biomaterials were not compared with other polymeric materials in more details. Therefore, in vitro and in vivo studies of pH-responsive PUs should be conducted more to guarantee the safety of PUs in in vivo environments for further clinical usage [12].
4. PUs as medical devices
PUs have been used as a material for various medical devices due to their unique properties, such as biocompatibility, flexibility, strength, and durability [160]. Some examples of medical devices made from PUs include [161] the following ones:
Surgical implants: PU can make artificial joints, such as spinal discs, heart valves, and other surgical implants. Some examples of PU valves are shown in figure 8. The flexibility and strength of PU make it an ideal material for these applications, as it can withstand high fatigue resistance and provide a long service life [162, 163].
Figure 8. Trileaflet PU valves developed by the Aachen group. (a) The Reul-Ghista trileaflet valve, (b) the Reul-Häussinger valve, and (c) the Helmholtz Institute valve. [172, 2011], reprinted by permission of the publisher (Taylor & Francis Ltd, www.tandfonline.com.).
Download figure:
Standard image High-resolution imageCatheters: PU can make catheters, which are thin, flexible tubes to remove bodily fluids or deliver drugs or other biomolecules. The flexibility and durability of some PUs make them ideal for catheters, as they can bend and twist without breaking and withstand repeated use [164].
Stents are small, mesh-like tubes with open, blocked or narrowed blood vessels. However, due to PU's appropriate flexibility and durability, examples of PU are ureteral stents commonly used in urologic disorders aiding urine flow [165, 166].
Wound dressings: PU can be used to make wound dressings that can protect and promote the healing of skin wounds, and the PU properties allow for conforming to the shape of the wound without breaking [167].
Artificial skin and tissue engineering (TE): PU's biocompatibility and flexibility can also be used for artificial skin and TE. It can be used to make scaffolds for TE and can be used to mimic the mechanical properties of natural skin.
It is worth noting that the medical use of PU requires rigorous testing and CE/FDA approval, as the safety and efficacy of the medical device are of paramount importance [168].
Cardiovascular disease is one of the leading causes of mortality worldwide. Heart valve replacements and cardiovascular procedures have recently increased [169, 170]. It has been reported that 82.6 million elderly people in the United States suffer from cardiovascular disease [169]. Autologous arteries and veins or vascular grafts (Dacron and Goretex), which are biostable, have been applied as prostheses. Synthetic vascular grafts are helpful for large and medium blood vessels, but they are not effective for small types (diameter < 6 mm) because they can cause many thrombotic difficulties [171].
Moreover, they lack sufficient biocompatibility and elastic properties [173]. Cardiac valves based on xenografts and synthetic materials such as ceramics, metals, and polymers have been utilized for many years. However, these materials are not considered ideal due to concerns regarding their haemocompatibility, long-term mechanical stability, and antithrombogenicity [174]. In light of this, although calcification has been an issue, PU has been recognized as an alternative that can provide good mechanical and biocompatibility properties for cardiovascular applications [118, 119]. PU is extensively investigated for in cardiovascular applications due to its properties of elasticity, high shear strength, durability, light weight, fatigue resistance, transparency, and, importantly, good biocompatibility, tolerance, and acceptance during the treatment and healing process, which allows unrestricted use in blood-contacting devices [175, 176]. PU has been investigated as a possible candidate for a medical device for cardiovascular applications, such as vascular prostheses, heart valves, and cardiac assistance devices. This is because, as mentioned in the previous sections, PU has perfect biocompatibility and blood compatibility [177, 178]. Also, PU has been used as an insulator in the structure of cardiac pacing leads, which are thin wires used to deliver electrical impulses to the heart to treat arrhythmias. However, some degradation problems are associated with using polyether urethanes as an insulator in cardiac pacing leads [110, 172, 179]. Although all components of polyether-urethane, including the soft segment, hard segment, and chain extenders, have the potential to be toxic either by themselves or as part of degradation products, there have not been any published reports linking the use of polyether-urethane implants or the degradation of polyether-urethane to cancer [36].
As PU is a reliable candidate for short-time application, many medical devices based on PU have been developed [180]. Previously, the applicability of PUs for small-diameter vascular applications has been reviewed. These applications can be divided into two different groups: biostable prostheses and biodegradable scaffolds. So, the requirements for these groups' long- and short-term applications are thoroughly explained previously [50].
Insulator failure: PU can degrade over time due to exposure to heat, chemicals, and microorganisms. Such failures can lead to a loss of mechanical properties, such as strength and flexibility, and can affect the performance of the cardiac pacing lead. Insulator failure is a major problem for the pacing leads and can lead to the lead breaking or malfunctioning, which may require replacement surgery.
Fracture: Although PUs have good mechanical properties, haemocompatibility, and biocompatibility, they can become brittle after prolonged mechanical load and can fracture when subject to mechanical stress and degrade during long-term applications [54], resulting in severe problems after implantation. Fractures can lead to a loss of electrical insulation, which can cause electrical leakage and lead improper heart pacing. It has been demonstrated that a coating containing antioxidants on the surface can decrease oxidative degradation [181]. Also, designing a vascular graft based on PU/glycosaminoglycans has shown promising results [182–184]. Moreover, PU-nanofibres are nontoxic materials that can provide an environment and substrate for the human umbilical cord vein endothelial cells [184]. Polymer from a family of degradable-polar hydrophobic ionic polyurethanes (D-PHI) was applied to produce multifunctional thin films based on PU that could prevent blood clotting and decrease the immune system response [185]. PUs' resistance against microbes prevents infection and decreases the risk of rejecting foreign materials [186, 187]. The tensile mechanical properties of different materials have been compared with the native heart tissue to fabricate heart valves. It has been reported that among the compared materials, PU and poly(glycolic acid) (PHA) had remarkable mechanical properties compared to the soft materials (figure 9) [188].
Figure 9. Comparing mechanical properties of different materials (TPU, native materials, PHA, Poly(glycerol sebacate))PGS(poly(hydroxyalkanoate) (PLA), fibrin and collagen, poly(glucolic acid) (PGA)) for fabrication of heart valves. Reprinted from [188], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageAllergic reactions: Some individuals may be allergic to PU, which can cause inflammation, itching, and other symptoms. This can be particularly problematic for long-term devices such as cardiac pacing leads. Blood biocompatibility is another main criterion restricting biomaterials useful for cardiovascular applications [175, 176]. Various surface modifications have been developed to increase blood biocompatibility. PUs can be subjected to chemical and physical changes. It has been reported that surface modification can change only the physical properties and does not influence bulk chemical properties [186, 189]. Altering the physical surface can enhance the PU's haemocompatibility without compromising its structured surface and bulk properties. This can be achieved through protein adsorption or platelet adhesion on the structured surfaces [190]. Calcification often limits the use of polyether-urethanes in cardiovascular applications [191]. in vivo and in vivo polyether-urethane calcification has been reported, and they are associated with stiffening, failure in flexure, and perforations [36, 192, 193]. Most of the effects of calcification on polyether-urethanes have been investigated concerning cardiovascular devices [36, 191]. Because PUs are excellent bioinert and biocompatible polymers, they have applications as thromboresistant coatings [194] to prevent blood clot formation [194]. Although PUs have advantages, their disadvantages, such as cost or unexpected effects on the activation of the complement group, should be considered [195]. PUs have also been reported as a substrate for cardiac stem cell treatment [196]. PUs have other cardiovascular applications, such as ventricular-assisting devices and pacemaker leads [110, 175, 197]. Previously, a series of elastomeric films featuring a surface micropattern have developed. These films are made from a combination of poly(glycerol sebacate) (PGS) and graphene (Gr). They exhibit sufficient mechanical strength (0.6 ± 0.1–3.2 ± 0.08 MPa) to withstand heartbeats, and the micropatterned structure also aligns with the natural anisotropy of the myocardium in both transverse and vertical orientations. Additionally, the incorporation of Gr enables these films to be conductive (up to 5.80 × 10−7 S m−1), facilitating the conduction of electrical signals between cardiomyocytes and the cardiac tissue. The obtained data showed that electroactive micropatterned anisotropic elastomer film can be used in cardiac TE [198].
Incompatibility with imaging techniques: Polyurethane (PU) can interfere with specific imaging techniques, such as magnetic resonance imaging (MRI), due to the susceptibility of PUs to generate artefacts in the images. PU-based medical devices behave similarly to natural tissue. This can make it difficult to differentiate between the PU and surrounding tissue on imaging scans, leading to image artefacts and reduced diagnostic accuracy. The reasons for this MRI-PU interface are related to the material's magnetic properties, including its susceptibility to magnetic fields, radiofrequency interference, and eddy currents. These properties can lead to image distortion, signal loss, and other artefacts in MRI scans. While PU has numerous advantages for medical devices, its interaction with specific imaging techniques, such as MRI, can lead to imaging artefacts and reduced diagnostic accuracy. As a result, understanding the limitations of PU in imaging can be crucial for developing appropriate medical devices and interpreting diagnostic imaging results [199].
In summary, although studies have reported that PUs suffer from severe problems when applied in blood-contacting devices implanted for long periods, they can be modified regarding chemical composition and surface characteristics to improve their mechanical properties and blood interaction [200]. These modifications enable the use of PUs in the design and manufacture of cardiovascular devices. Heart valves and heart patches among cardiovascular devices need materials with appropriate properties, such as strength and elastomeric mechanical behaviour, to tolerate the cardiac contractile tissue and support regeneration [201]. It is vital to design heart patches and valves to create a structure similar to muscle tissue. PUs are appropriate for cardiac applications because their biocompatibility and elastomeric behaviour enable them to resist cyclic heart stresses without plastic deformation or fatigue failure [1]. The PU structure can be modified to create anisotropic microstructures that may mimic the heart tissue function's varying pore sizes. This could promote cell colonization, cell migration, nutrient supply, and vascularization. However, efforts are still needed to bring PU-based cardiovascular devices to clinical application. More controlled, detailed, and deeper investigation in vivo and in vivo characterizations are needed to gain PU-based cardiac patches finally, and heart valves approval as medical devices [202].
4.1. PUs for drug delivery
PUs have been investigated as a material for drug delivery systems due to their unique features and have been used as PU-based hydrogels, PU-based nanoparticles, PU-based microparticles, and PU-based films and membranes [203]. For example, they can be used as a coating on implantable devices to control the release of drugs. The properties of PU can be modified to suit the specific drug delivery application by adjusting the polymer properties, such as porosity, mechanical properties, and degradation rate [204]. Controlled drug delivery has been an important issue in recent years because of its advantages, such as lower toxicity and less time required for injection [205, 206]. Also, selecting a carrier for the drug is critical because it can protect drugs from damage or loss or play an essential role in providing a targeted and smart release [207]. Carriers are applied to immobilize the active materials and substances in drug delivery preparation. They should have a balanced structure between hydrophobicity and hydrophilicity and good biocompatibility [208, 209]. The form of a carrier can vary based on the targeted application [210]. PUs are an attractive material for drug carriers and drug delivery systems because of many possible modifications and processing techniques that can be applied to control drug release systems. PU nanoparticles, scaffolds with various porosity and pore sizes or coatings, different compositions and hydrophilic properties of raw materials, type, and the ratio of hard and soft segments, crystallinity, and the crosslinking degree have the properties and forms that give the possibility to control speed and amount of drug released. Also, modified cross-linked PUs can behave as hydrogels, absorbing large amounts of water without dissolving, an essential quality for drug carriers [207].
Depending on the targeted application of PUs, they can be classified into two groups: biodegradable and bioinert. The first category is used in drug delivery [211]. PU scaffolds or stents have been recognized as a suitable carrier for drug delivery, and many investigations have been conducted. Moon et al loaded heparin-deoxycholic acid (DOCA) into a PU coating and reported that the percentage of loaded DOCA on the PU was more than 75% and that the released DOCA could prevent fibrin clot formation or adhesion of platelets on the film and layer surface [212]. Moura et al demonstrated the potential for drug delivery using PU-based implants by loading dexamethasone onto the material. Their findings showed that using PU for drug delivery could modulate the fibrosis and angiogenesis induced by the disc-shaped spongy and main inflammation components [213]. Other drugs in molecule shape have been investigated, such as 5-flurouracil and ibuprofen [214–216]. In addition to the molecular-shape drugs, rhBMP-2 is a protein based on recombinant human bone morphogenetics incorporated into scaffold-based PU and implanted for defects healing in the rat femoral bone. The results showed that the formation of bone increased after four weeks of implantation [217].
In another study, a PU that contained 5-aminosaheylic acid (5-ASA) was used, and results showed that the urethane group hydrolysis caused a release of drugs [218]. Also, Ghosh and Mandal reported on the release of ibuprofen based on PU structure [219]. It has been reported that drug incorporation does not considerably affect PU's biological and mechanical properties. Simmons et al reported that after using dexamethasone acetate in PU based on siloxane, there were no considerable differences in biocompatibility and biostability after six months of implantation in an animal model [220]. Drug delivery by PU-based carriers can be affected by some factors. For example, Da Silva et al investigated that the presence of poly(caprolactone) together with poly(ethylene glycol) as a soft segment in PU could enhance the release rate of dexamethasone acetate compared to PU with poly(caprolactone) alone. Furthermore, it was shown that the structure of PU was also involved in drug release behavior [221].
Furthermore, the use of ionic ligands has been shown to accelerate the release of drugs from PU-based carriers [222]. It has also been reported that the drugs' properties and functions can influence the drug release rate from the PU carrier [223]. This suggests that the drug itself plays an essential role in determining the release kinetics of the carrier. The factors related to the drugs' properties and functions, such as their molecular weight, hydrophobicity, and charge, can influence their interaction with the PU carrier, thereby affecting the drug release rate. For example, drugs with a higher molecular weight or greater hydrophobicity may have a slower release rate from the PU carrier, as they may be more strongly bound to the material.
Similarly, drugs with a higher charge may interact more strongly with ionic ligands, resulting in a faster release rate. Moreover, using ionic ligands can accelerate the release of drugs from a carrier base. E.g. Grigoreva reported that changing the PU structure (soft and hard segments) could make it possible to design composite PUs based on desired drug delivery. Another example; is the release of drugs, such as naltrexone, cefazolin, and piroxicam, on the base cross-linked PUs and dioxydine for linear PUs was shown to have an effect [224].
Gentile et al investigated a system based on ceramics scaffolds coated with gelatin. Then, they incorporated PU nanoparticles into the gelatin, loaded with indomethacin (IDMC), into the scaffold. Incorporated PU nanoparticles allowed a sustained IDMC release at about 65%–70% during the first week, and compressive modulus was increased [225]. Kolmas et al also used a system containing PU and HA to avoid cell invasion and the growth of bone tumours in the extracellular matrix (ECM). The system was designed to facilitate a prolonged release of bisphosphonates. The results showed that the release rate varied from 20% to 80%, indicating a possibility of controlling the release kinetics [226]. Guo et al worked on stent coatings based on biodegradable PU and were able to adjust the drug release [21, 227]. Polymer nanoparticles were analysed for improvement in cancer chemotherapy. Therefore, declining drug resistance and improving synergy in malignant lesions are necessary. Nanoparticles based on PU can co-encapsulate chemotherapeutic agents, such as doxocetal and doxorubicin hydrochloride [228]. Tissue adhesives based on PU can also be applied as a delivery system to release antibiotics and painkillers [171] slowly. Some types of PU that have been investigated so far are summarised in table 4 [229].
Table 4. The different types of PU and model drugs for the delivery system [229].
| Application | Drug used | Author | PU type | References |
|---|---|---|---|---|
| Gels | Crystal violet | Kohjiya et al | PU gels, were prepared from hydroxyl-terminated poly-(oxytetramethylene) and hydroxyl-terminated poly(oxyethylene)-b-poly(oxytetramethylene)-b-poly-(oxyethylene) | [230] |
| Azo-containing PU as drug-coating | Model drugs-hydrophilic in nature- | Yamaoka et al | A segmented PU containing azo aromatic groups in the main chain was synthesized by reaction of isophorone diisocyanate with a mixture of m,m´-di(hydroxymethyl)azobenzene, poly(ethylene glycol) (Mn = 2000), and 1,2-propanediol | [231] |
| Ibuprofen bearing PU | Ibuprofen | Ghosh and Mandal | Polyethylene glycol (PEG) based PU bearing non-steroidal ibuprofen drug and release characteristics of ibuprofen from this polymeric backbone were investigated. | [219] |
| Drug release was based on the degradation of ester linkages | ||||
| Scaffolds | Platelet-derived growth factor (PDGF) | Li et al | — | [232] |
| Scaffolds | Recombinant human bone morphogenetic protein (rhBMP) | Li et al | Polyester triols (900 Da) were prepared from a glycerol starter and a backbone comprising 60 wt% ε-caprolactone, 30 wt% glycolide, and 10 wt% d,l-lactide | [217] |
| In order to incorporate protein into PU scaffold, lyophilized protein powder, poly(lactic-co-glycolic acid)-large microspheres (PLGA-L), or small microspheres (PLGA-S) was added to the hardener component before mixing with the isocyanate to prepare the scaffolds | ||||
| Pressure-sensitive adhesives (PSAs) | Thiamazole, diclofenac sodium, ibuprofen | Chen et al | PEG-based PU pressure-sensitive | [211] |
| Nano-structured polymers | Cefamandole nafate | Crisante et al | A polyetherurethane acid was obtained from the condensation of methylene bis-phenyl-isocyanate (MDI, Polyscience Inc.), polypropylene oxide (PPO, number average molecular weight 1200 Fluka) and dihydroxymethyl-propionic acid (DHMPA) in a 2:1:1 stoichiometric ratio | [233] |
| Foams | DB-67 and doxorubicin | Sivak et al | PU foams constructed from lysine diisocyanate (LDI) and glycerol | [234] |
| PU intravaginal rings | Dapivirine and tenofovir | Johnson et al | — | [235] |
| Films | Chlorhexidine diacetate (CDA) | Huynh et al | Medical grade PU (3M Unitek, Cergy Pontoise, France) was used as a polymer to incorporate CDA | [236] |
| PU matrix containing albumin nanoparticles | Cefamandole nafate | Martinelli et al | Carboxylated PU | [237] |
| Nanoparticles | Adriamycin | Sun et al | Temperature-sensitive polymers consisting of PEG and l-lysine ester diisocyanate | [238] |
| Film as a stent covering | Gemcitabine | Shin et al | N,N-dimethylacetamide | [239] |
| (DMAc) and tetrahydrofuran (THF) | ||||
| Were reagent grade, and poly(carbonate urethane) ChronoFlex AL 85A was used | ||||
| pH- and temperature responsive PU (nanoparticles) | Doxorubicin | Wang et al | A series of temperature- and pH-responsive PUs based on hexamethylene diisocyanate (HDI) and 4,4'-diphenylmethane diisocyanate (MDI) were synthesized | [240] |
| Tablets | Diprophylline | Claeys et al | Mixtures of diprophylline (Dyph) and TPU (ratio: 50/50, 65/35 and 75/25 wt.%) were used | [241] |
| Thermoplastic PU | Metoprolol tartrate | Vervaet et al | TPU | [242] |
| Dual stimuli-responsive PU-based hydrogels | Ibuprofen | Laurano et al | A thermo- and pH-responsive hydrogel was prepared from a synthesized amphiphilic poly(ether urethane), exposing a considerable amount of –COOH groups (8.8 ± 0.9 nmol/gpolymer). | [243] |
In conclusion, PUs family is widely used in biomedical applications. According to the adjustable physical and chemical properties of PUs, it is possible to change the degree of crystallinity and their interaction with water. Since PUs have appropriate biocompatibility, low cytotoxicity, and good mechanical properties, they have the potential to overcome challenges in drug delivery systems [218, 219]. By further attempting to have more in vivo investigation, more applications, limitations, and challenges of PUs for controlled drug delivery can be found.
4.2. PU applications in tissue engineering (TE)
PU has been investigated as a biomaterial for various tissue TE applications due to its unique properties, such as biocompatibility, flexibility, strength, and durability. Scaffolds are critical to TE as they support cells to grow and differentiate into functional tissue. The properties of PU, such as biocompatibility, flexibility, and durability, make it an attractive material for scaffolds [244]. PU-based scaffolds can mimic the mechanical properties of natural tissue and can be designed to provide a suitable environment for cell growth and differentiation [245]. PU-based scaffolds have been developed for various TE applications, including skin, cartilage, bone, and nerve tissue. For example, PU-based scaffolds have been developed to repair skin for treating burns and wounds. These scaffolds can support cells to grow and differentiate into functional skin tissue [246]. PU-based scaffolds can also be used to repair cartilage and bone tissue. These scaffolds can support cells to grow and differentiate into functional cartilage and bone tissue [247]. PU-based scaffolds have also been used in nerve TE, as they can mimic the mechanical properties of the nerve and provide a suitable environment for the growth and differentiation of neural cells [248]. PU scaffolds can have a variety of morphologies depending on the manufacturing process and the specific application [60]. The morphology of a PU scaffold refers to the physical structure and shape of the scaffold. PU can be fabricated as a porous structure (figure 10), to improve and enhance proliferation, cell adhesion, and differentiation.
Download figure:
Standard image High-resolution imageSome typical morphologies of PU scaffolds include:
- (1)
- (2)Fibrous: PU scaffolds can also be processed in thin fibres and elongated structures that mimic natural tissue structures. Fibrous PU scaffolds can provide a suitable environment for cell growth and differentiation. They can be used in many TE applications, such as tubular structures for blood vessel regeneration, nerve guides for peripheral nerve regeneration, and cartilage TE constructs [248, 251].
- (3)Film/coating: PU scaffolds can also be made as a film. Film scaffolds can be used in many applications, such as wound dressings, cell culture substrates, or coating materials to modify the substrate's surface properties to provide an active environment for cell growth and differentiation or an additional layer of protection [141, 252–255].
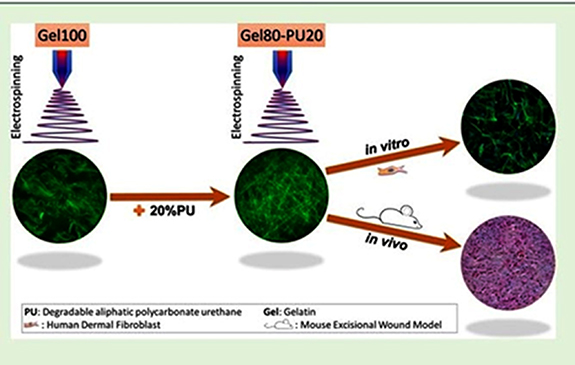
Figure 11 shows a hybrid gelatin-based electrospun scaffold that the use of biodegradable polycarbonate PU has fabricated. It is supposed that adding PU could enable a tailored degradation rate and an increased mechanical strength compared to electrospun gelatin. Adding 20% PU to gelatin scaffolds (gelatin 80− polycarbonate PU 20) significantly increased the scaffolds' yield strength, degradation resistance, and elongation. Gelatin 100 had an elastic modulus of 130 ± 10 kPa, whereas the addition of 20% PU enhanced the elastic modulus to 260 ± 11 kPa. Gelatin 80−PU 20 showed the greatest elongation at break (60.7 ± 4.8), while this parameter was 34.7 ± 7.7 for gelatin 100. In vivo studies using a mouse excisional wound biopsy grafted with the scaffolds demonstrated that the gelatin 80−PU 20 scaffold enables more significant cell infiltration than clinically established matrices.
Figure 11. Schematically PU−gelatin composite as scaffolds for A skin regeneration and repair application. Reprinted with permission from [256]. Copyright (2019) American Chemical Society.
Download figure:
Standard image High-resolution imageThe findings show that electrospun gelatin 80−PU 20 scaffolds can generate tissue substitutes and overcome some limitations of conventional wound care matrices [256].
Topography can control the behaviour of cells, such as proliferation and growth alignment. Moreover, the shape change (stimulus-regulated swelling and shrinking) benefits the release of encapsulated agents in a precisely controllable manner [257, 258]. Additionally, the scaffold should benefit from a targeted degradation rate, and this rate should be designed based on the new tissue formation rate. Modifying PU with ascorbic acid (vitamin C) can improve material properties for applications in soft tissue [259]. Shahrousvand et al also incorporated some iron oxide nanoparticles in PUs for TE applications [260]. Results indicated that the produced material had good potential in cell therapy applications. Li et al fabricated a scaffold containing hydroxyapatite (HA)/PU. Glyceride of castor oil (GCO) was applied to modify the soft part of PU. Finally, it was added to enhance the compressive strength, which increased when the HA particles were added.
Moreover, the HA particles were essential in bonding with the bone tissue and GCO-PU matrix interface [261]. HA and PU have been investigated more because HA can improve bioactivity, and PU can provide an excellent mechanical property [262]. Haryńska et al designed a 3D-printed filament based on PCL and PU for medical applications [14]. Also, thermoplastic-elastomer PU has been reviewed for its potential as a filament for FDM 3D printers [263]. Piotrowska-Kirschling conducted a study on the combination of chitosan and PU. It was reported that the presence of chitosan accelerates PU degradation. The combination of chitosan and PU was introduced as a highly biocompatible and bioactive combination that can be a good candidate in TE applications [264].
Additionally, the combination of chitosan and chitin with PU caused the creation of an applicable and influential composite for the biomedical field. The amino, carbamate, and acetamide groups (in chitosan, PU, and chitin, respectively) play a prominent role in making a material suitable for medical applications. In another investigation, collagen and PU were reviewed for TE application [265].
The use of PU for 3D-printed scaffolds is currently receiving much attention. The methods studied are fused filament fabrication (FFF), inkjet printing, stereolithography (SLA), and low-temperature deposition. Scaffolds based on poly(carbonate-urea) urethane enhance surface adherence and cell viability; therefore, they can become a polymer to improve laryngeal regeneration and reconstruction [266, 267]. In addition, elastomeric-PU can avoid shear forces between implant and bone, supporting osteogenic cells' proliferation in hard tissue. Regarding soft tissue, PU-based scaffolds can be applied to muscle regeneration, blood vessels, heart tissue, cartilage, and nerve [268].
A novel PU scaffold containing megni oil was used in TE due to its ability to enhance antithrombogenicity [269]. On the other hand, angiogenesis is another hot topic in the biomaterials field, and many researchers are trying to design a material that either inhibits or improves it. Colloidal aggregates and gels based on the mediated aggregation of cationic-PU particles have been applied to produce a matrix [270–272]. Notably, PU-based scaffolds have been established for the repair and regeneration of bone tissue based on osteogenic differentiation and proliferation [52, 273]. Membranes of PU and silk fibroin were designed and evaluated to mimic oral soft tissue [274]. The biological effect of these membranes was analysed regarding cell proliferation, cell adhesion, phosphatase activity, calcium content, and viability. These membranes seemed adequate for bone tissue regeneration [274, 275].
It is worth noting that the scaffold properties can be modified to suit the specific TE application by adjusting the polymer properties, such as porosity, mechanical properties, and degradation rate. Moreover, developing these scaffolds is still ongoing as the field of TE is rapidly advancing.
Thoughsome major challenges persist, the future of PU scaffolds is promising. The PU scaffolds in a specific form targeted for a specific organ or tissue can be investigated. Thus, for future recommendations, a multidisciplinary approach is still required for fully exploiting PU scaffolds, combined with computational approaches for designing patient-specific PUs TE scaffolds. As a result, more investigations are required to narrow the differences between the cell interactions of PUs scaffolds in the in vivo and in vivo environments [276].
4.3. PUs for wound healing
The wound-healing process involves regenerating epidermal and dermal tissues that heal the wound. Successful wound healing involves interaction between dermal and epidermal cells, angiogenesis, and ECM. These can be regulated by growth factors and cytokines [277]. It has been reported that an ideal wound dressing should have specific characteristics and properties, such as a high level of bacterial resistance to removing exogenous microorganisms, sufficient haemostatic properties, high fluid adsorption, specific mechanical properties, stability, the ability to be removed easily, cost-effectiveness and recyclability [278]. In this regard, Unnithan et al investigated the fabrication of nanofibre composites for wound dressing based on cellulose, PU, and zein with streptomycin sulfate. The results showed perfect cell attachment, antibacterial activity, hydrophilicity, and blood clotting [137]. Also, mats based on nanofibrous composites containing gelatine, chitosan, and SMPU were fabricated by electrospinning and post-treated with silver nitrate (AgNO3) solution. The obtained data illustrated that the produced composite could be a good candidate for wound dressing because it showed adequate antibacterial activity, an appropriate water vapour transmission rate, surface wettability, cytocompatibility, and haemostatic properties [279]. In another investigation, a combination of hydrogel and PU produced a composite for wound healing. The fabricated composite could accelerate wound healing and reduce the formation of scars [280]. PU compositions are selected for wound healing applications based on their chemical composition, shape, and functionalization [7, 281]. Transparent and thin PU films are primarily suitable for surface protection and wounds after an operation with less or no exudate secretion. These PUs are not permeable to water and bacteria but are permeable to gases. As a result, they can help wounds to breathe.
PU foams are absorbent and suitable for wounds with more exudate [281]. Salehi et al designed a nanofibre matrix based on polyurethane and hyaluronic acid (PU-HA). This nanofibre matrix was enriched with various concentrations of ethanolic extract of propolis (EEP) (figure 12, top panel).
Figure 12. Top panel. Schematic of synthesizing a scaffold containing PU, HA and EEP (modified). Lower Panel: Macroscopic photos of the wound healing process with three different specimens in 1, 7, 14, and 21 d (modified). Reprinted from [282], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageResults demonstrated that the PU-HA/1% EEP structure could be a good candidate for wound healing, with promising biocompatibility and antibacterial activity. It has been reported that PU in the composition accelerated the wound healing process (figure 12, lower panel) [282]. Also, lignin-based polyurethane foams loaded with Ag nanoparticles were investigated as a composite that showed good antibacterial activity in wound healing applications [283]. Even incorporating copper (Cu) in PU composites was reported to fabricate the nanofibrous composite for wound healing [284]. Another composition including PU for wound healing application has been introduced by Li et al [285]. A dressing material has been developed made of PU that possesses multiple functionalities. The designed material combines the electroactivity of aniline oligomer segments, the wettability of PEG segments, and the mechanical properties of PCL segments. The films exhibited favourable hydrophilic properties and swelling ratio, exceptional mechanical strength and shape memory capabilities, electroactivity, effective free radical scavenging capacity, non-adherent properties, and biocompatibility.
PUs have perfect properties for applications as pressure sensitive-adhesives (PSAs). These materials can provide appropriate adhesion to the base substrate, such as skin, with light pressure; as a result, they can be used in surgical dressing applications [286]. These materials' adhesion strength and breathability were improved by applying various crosslinkers [286]. Dressing foams based on PUs have good absorption of water and mechanical properties but less bioactivity and poor capability for healing. So, it has been suggested that incorporating and using bioactive nanoparticles like silica within the sol-gel process can improve the rate of wound closure and accelerate the regeneration of elastin fibre and collagen [287].
In summary, PUs as a wound dressing material have been commercially in the spotlight because of main characteristics such as biocompatibility, mechanical properties, elasticity, flexibility, and good oxygen/carbon dioxide permeability [288]. PUs can be a promising candidate for wound healing applications in the future. In addition, they expanded studies on the PUs to identify their limitations, challenges, advantages, and disadvantages for wound healing applications.
4.4. PUs for biosensors, and oral administration
PUs have been explored as a material for biosensors, devices that can detect and measure specific biomolecules such as glucose, proteins, and other biomolecules [289]. One of the key advantages of using PU in biosensors is its biocompatibility [290]. PU has good chemical stability and can withstand a wide range of pH and temperature conditions, making it a good biosensor choice [291, 292]. PU-based biosensors have been developed for various applications, including monitoring glucose levels in diabetes patients and detecting chemicals, toxins, and environmental pollutants. For example, PU-based glucose biosensors have been developed by immobilizing glucose oxidase enzymes onto a PU matrix, which can then be used to measure glucose levels in the blood [293].
Figure 13 shows a biosensor based on a nanofibre-based polydiacetylene (PDA) biosensor prepared with TPU to detect E. coli. 10,12-Pentacosadiynoic acid (PCDA) was used as a monomer for preparing PDA. In the electrospinning of PCDA and PU mixture solution, PCDA monomers were randomly distributed in the PU solution. PU-PDA nanofibre biosensors were prepared via electrospinning and tested with E. coli. The colour change in PU-PDA nanofibres began immediately when exposed to the E. coli concentration of approximately 9 × 108 CFU ml−1. The nanofibre mat changed colour in some spots and immediately changed to red after a few minutes [294].
Figure 13. A nanofibre-based polydiacetylene (PDA) biosensor was prepared with PU to detect Escherichia coli (modified) inspired by Bhattacharjee et al (2020). Adapted from [294] with permission from the Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageAnother example is using PU-based biosensors for detecting environmental pollutants such as heavy metals, pesticides, and other toxins. These biosensors are typically based on immobilizing a specific enzyme or antibody onto a PU matrix, which can then detect the presence of a specific pollutant. A suitable biosensor should have some desired properties, including specificity, accuracy, stability, a wide range of analytical capabilities, low cost, and portability [295]. Many kinds of biosensors have been introduced to date [296], and many kinds of polymers (e.g. polyaniline, poly(vinylchloride), polypyrrole, and cellulose acetate) have been investigated as the substrate for the fabrication of biosensors [297, 298]. However, this application has widely used PU due to its appropriate biocompatibility, flexibility, cost-effectiveness, and easy processability [292, 299]. The key challenge in this route is the low solubility of hydrophobic drugs. Also, components of drugs should keep their active state until they reach the absorption site [300, 301]. In order to achieve targeted drug absorption at a specific site, polymers can be coated onto the surface of drugs or mixed and linked with the drug to prevent potential degradation [231].
4.5. Drawbacks of PU in medical applications
While PU has many advantages and has been used in many medical applications, some drawbacks must be considered when using it in medical devices. Some of the drawbacks of the medical application of PU include [60, 163, 302, 303]:
Degradation over time: PU can degrade over time due to factors such as exposure to heat, chemicals, and microorganisms. This can lead to a loss of mechanical properties, such as strength and flexibility, and can affect the performance of the medical device [60, 163, 303, 304].
Allergic reactions: Some individuals may be allergic to PU, which can cause inflammation, itching, and other symptoms. This can be particularly problematic for long-term surgical implants [304, 305].
Incompatibility with imaging techniques: PU can interfere with specific imaging techniques, such as MRI, as it can cause image artefacts. This can make it challenging to diagnose and treat certain medical conditions accurately [306].
Sterilization issues: Sterilization problems are not unique to PU and can be challenging for many other polymers and materials used in medical devices, especially those with porous structures. Porous structures can harbour bacteria and other microorganisms, making them difficult to sterilize effectively. Furthermore, specific sterilization methods, such as gamma irradiation or ethylene oxide gas, can degrade or damage the polymer structure, reducing mechanical properties or potential toxicity. Therefore, careful consideration must be given to the choice of sterilization method and its potential effects on the polymer structure and device performance [79, 91, 307].
Cost: PU can be more expensive to manufacture than other medical device materials, such as polyethylene or polypropylene, due to the higher cost of raw materials and the more complex manufacturing processes involved.
PU requires specific chemical precursors, including isocyanates, polyols, and chain extenders, which can be more expensive than the raw materials used to produce other polymers. The production of PU also requires specialized equipment and more stringent manufacturing conditions, which can increase the overall cost of the process.
These drawbacks can vary depending on the specific type of PU used and the specific application. Therefore, it is essential to choose the appropriate PU type and consider the specific risks and benefits when using it in a medical application [302].
5. Conclusion and future perspective
- PU has already proven to be a versatile biomaterial with a wide range of applications in the medical field. Its unique properties, such as biocompatibility, flexibility, strength, and durability, make it an attractive option for many medical applications. Our findings from this review are as follows: PU family is rapidly advancing, and new developments are constantly being made. Therefore, it is expected that PU will continue to play an essential role in the medical field in the future. The future of polymer biomaterials is promising due to their advantages, including ease of processing. With its various properties, PU continues to have an essential role among polymers.
- The biostability of PU can potentially expand medical applications, including long- and short-term needs. Alternatively, controlled biodegradable PUs would eliminate the need for additional surgical procedures to remove the device. Examinations have shown that clinical applications can benefit from the long-term biostability achieved. While numerous approaches have been employed to enhance the resistance of polyurethane elastomers, compared to conventional PUs, against oxidative degradation, their suitability for extended medical implant usage remains to be assessed. Enhancing the comprehension of the interplay between PU chemical structure, properties, and biostability will enable synthetic polymer chemists to develop novel materials that cater to the demands of next-generation medical implants.
- Shape-memory PUs have the potential to be used in a wide range of medical applications, such as in biomedical devices, actuators, and robotics, where the ability to control the shape of the material is essential. The versatility and significance of shape memory SMPUs are evident in their diverse applications. External forces, including electricity, temperature, and light can trigger the shape memory effect in SMPUs. This response to external stimuli is due to the presence of both hard and soft segment domains in SMPUs. By employing cross-linking mechanisms such as ionic, covalent, and hydrogen bonding, it is possible to attain the desired properties in SMPUs.
- Using pH-responsive PUs has garnered attention in the pharmaceutical and biomedical fields. While there is significant potential in biomedical applications and drug delivery systems, further investigation is necessary to confirm their safety and efficacy in biological systems. It appears that in most studies focusing on pH-responsive PU systems for biomedical and drug delivery purposes, a detailed comparison of the stability, biodegradability, biocompatibility, and mechanical properties of PUs as potential polymeric substrates with other polymeric materials is lacking. Hence, more in vivo and in vivo studies on pH-responsive PUs must be conducted to ensure their safety in vivo and facilitate their future clinical use.
- While PUs face challenges when used in long-term blood-contacting devices, studies have shown that their mechanical properties and blood interaction can be improved through modifications in chemical composition and surface characteristics. This allows for the utilization of PUs in the development of cardiovascular devices, including heart valves and heart patches. These devices require materials with suitable properties such as strength and elastomeric behaviour to withstand cardiac contractile tissue and facilitate regeneration. Designing heart patches and valves that resemble muscle tissue structure is crucial. PUs are well-suited for cardiac applications due to their biocompatibility and ability to withstand cyclic heart stresses without deformation or fatigue failure. However, further efforts are required to bring PU-based cardiovascular devices into clinical use. Comprehensive and controlled in vivo and in vivo investigations are necessary for approval for PU-based cardiac patches and heart valves as medical devices.
Development of new PU-based hydrogels that absorb large amounts of water, making them ideal for wound dressings, drug delivery, and TE. PUs have gained attention as wound dressing materials due to their desirable properties, including biocompatibility, mechanical strength, elasticity, flexibility, and effective oxygen and carbon dioxide permeability. These characteristics make PUs a promising option for wound healing applications. But further research is needed to fully understand the limitations, challenges, advantages, and disadvantages of PUs in the context of wound healing.
Acknowledgments
We acknowledge financial support from the Baltic Research Programme Project No. EEA-RESEARCH-85 'Waste-to-resource: eggshells as a source for next generation biomaterials for bone regeneration (EGGSHELL)' under the EEA Grant of Iceland, Liechtenstein and Norway No. EEZ/BPP/VIAA/2021/1Eggshell, Research Council of Norway, 'MISFAITH' Grant Nos. 331752 and Horizon 2020, 'BIOMATDB', Grant No. 101058779.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Data access statement
Data access upon request to the corresponding author.
Conflict of interest
No conflict of interest reported.